Back to Journals » Patient Preference and Adherence » Volume 14
Real-World Observational Study on the Characteristics and Treatment Patterns of Allergic Asthma Patients Receiving Omalizumab in Canada
Authors Lee JK, Amin S, Erdmann M, Kukaswadia A , Ivanovic J, Fischer A, Gendron A
Received 4 February 2020
Accepted for publication 10 March 2020
Published 7 April 2020 Volume 2020:14 Pages 725—735
DOI https://doi.org/10.2147/PPA.S248324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jason K Lee,1 Suvina Amin,2 Michelle Erdmann,3 Atif Kukaswadia,4 Jelena Ivanovic,5 Aren Fischer,4 Alain Gendron6
1Clinical Immunology and Allergy, Internal Medicine, Evidence Based Medical Educator Inc. and Urticaria Canada, Toronto, ON, Canada; 2AstraZeneca, Gaithersburg, MD, USA; 3AstraZeneca, Mississauga, ON, Canada; 4IQVIA, Toronto, ON, Canada; 5IQVIA, Kanata, ON, Canada; 6AstraZeneca, Mississauga, Ontario, Canada and Department of Medicine, Université de Montréal, Montreal, QC, Canada
Correspondence: Jason K Lee
Clinical Immunology and Allergy, Internal Medicine, Evidence Based Medical Educator Inc., Toronto, ON, Canada
Tel +1 647-352-2554
Email [email protected]
Background: Omalizumab is a treatment option for pediatric and adult patients with moderate to severe allergic asthma poorly controlled with standard inhaled therapies. Clinical trials and observational studies have demonstrated the efficacy of omalizumab. There is limited real-world evidence on the characteristics and treatment patterns of Canadian asthma patients receiving omalizumab.
Objective: We profiled Canadian omalizumab users to estimate time to omalizumab discontinuation and to assess changes in concurrent medication usage before, during, and after therapy.
Methods: This was a retrospective, observational, cohort study that analyzed data from Canadian prescription claims databases. An algorithm was used to select naïve users of omalizumab with an inferred diagnosis of GINA 5-asthma who made a claim for omalizumab from February 1, 2007, to June 2, 2015. Demographic and baseline characteristics were assessed at index. Outcomes examined over the analysis period included (i) daily omalizumab dose per patient and per claim; (ii) omalizumab discontinuation (defined as ≥ 100-day gap in making omalizumab claims) and its potential predictors (ie, age, sex, province of residence, drug insurer; assessed by Cox Proportional Hazards Model); and (iii) for patients who discontinued omalizumab, changes in concurrent medication usage before, during, and 6 months after omalizumab usage.
Results: The final study cohort consisted of 1160 patients (mean age: 45.8 ± 15.2 years; 64.7% female). During the first year of omalizumab therapy, 29.5% of patients discontinued treatment. The singular characteristic that predicted omalizumab discontinuation with statistical significance was age group (20‒34 years vs 12‒19 years; hazard ratio 1.75, 95% confidence interval 1.11– 2.76; P< 0.05). There were significant reductions in the use of some concurrent inhaled and oral asthma medications during and/or after omalizumab use (P< 0.05).
Conclusion: Nearly one-third of patients who initiated omalizumab in Canada for refractory, moderate to severe allergic asthma discontinued treatment during the first year.
Keywords: allergic asthma, omalizumab, real-world, observational study, claims database, Canadian asthma patients
Plain Language Summary
Clinical trials and real-world observational studies have demonstrated the efficacy and safety of omalizumab in patients with refractory moderate to severe allergic asthma. This is the first real-world study to profile Canadian asthma patients receiving omalizumab, their rates of treatment discontinuation and concurrent medication use before, during and after omalizumab therapy. Nearly half (47.3%) of the study cohort discontinued omalizumab treatment within 2 years. The use of some, but not all, concomitant inhaled and oral asthma medications was lower during and after omalizumab use. This study provides insights about discontinuation trends for omalizumab and other asthma therapies after starting omalizumab, which has both public planning and policy implications and offers clinicians guidance about expectations for adherence and medication changes for patients who start omalizumab.
Introduction
Asthma is a heterogeneous respiratory disease characterized by chronic airway inflammation1 and is associated with significant morbidity and mortality.2 In 2014, 8.1% of Canadians aged 12 and older, roughly 2.4 million people, reported being diagnosed with asthma by a health professional—a rate which has remained fairly constant since 2001.3,4 Approximately 5% to 10% of the asthmatic population suffers from severe asthma.5,6 While most uncontrolled asthma can be managed by applying best practices for management, many patients with severe asthma fail to achieve effective control, even when such strategies are applied.6 Since the cost of asthma is strongly correlated with disease severity among other factors (eg, age, comorbidities),7 patients with severe asthma are substantial users of health care resources.6,8,9
Immunoglobulin E (IgE) plays a central role in the development of allergic diseases.2,10 It is estimated that greater than 50% of patients with severe asthma have allergic, IgE-mediated asthma11 and can experience acute signs and symptoms of asthma within minutes of exposure to associated allergens.12 There is strong evidence that IgE can influence the pathology of allergic asthma.12
Omalizumab is a humanized monoclonal IgG antibody that binds to and inhibits circulating IgE to block the immune system’s response to allergen exposure.13 Omalizumab decreases allergic airway inflammation by reducing the expression of high-affinity IgE receptors on basophils and reducing their release of histamine.13 In 2004, Health Canada approved omalizumab for use in adult and pediatric patients (≥6 years) with moderate to severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen, and whose symptoms are inadequately controlled with inhaled corticosteroids (ICS).14 Omalizumab thus represents a treatment option for pediatric and adult patients with moderate to severe allergic asthma whose asthma is poorly controlled with ICS and inhaled long-acting β2-agonists (LABA).10 Both the Global Initiative for Asthma (GINA)1 and the Canadian Thoracic Society (CTS)6 acknowledge the role of targeted therapeutics, such as omalizumab, for patients with moderate to severe refractory asthma. This should be distinguished from uncontrolled asthma, which is commonly associated with poor medication adherence, improper inhaler technique, untreated comorbidities, and ongoing exposure to sensitizing or irritant agents in home or work environments.1,6,15,16
A systematic review of 21 randomized controlled trials (RCTs) (N=5975) examined the effects of subcutaneous omalizumab versus placebo in the treatment of chronic allergic asthma in adults and children.2 For individuals with moderate to severe asthma, omalizumab, when given as an adjunct to stable ICS therapy, significantly reduced asthma exacerbations [odds ratio (OR) 0.50, 95% confidence interval (CI) 0.42–0.60; seven studies, n=2889 patients] and hospitalizations [OR 0.16, 95% CI 0.06–0.42; four studies, n=1824 patients] compared to placebo. For patients with moderate to severe asthma who were given omalizumab during steroid-tapering phases, omalizumab was significantly more effective than placebo in increasing the number of participants who were able to completely withdraw their ICS [OR 2.67, 95% CI 2.10–3.39; three studies, n=1388 participants]. The authors also noted that treatment with omalizumab versus placebo improved asthma symptom scores and quality of life in both steroid-stable and steroid-reduction phases.2
The results of real-world studies conducted in patients with moderate to severe allergic asthma in Canada,17 Japan,18 Israel,19 and Europe20–27 corroborate the favorable findings of clinical trials and confirm the effectiveness of omalizumab in reducing exacerbations,17,18,20,22-27 emergency room visits,19,23 hospitalizations24 and improving asthma symptoms and control,17,22,23,25,26 and quality of life.17,20,22,23 Real-world studies also reported a reduction in the need for oral corticosteroid (OCS) therapy with omalizumab treatment in patients with moderate to severe allergic asthma.17–21,24,25,27 A systematic review of 24 real-world studies (N=4117) conducted across 32 countries on the short- and long-term effects of omalizumab in the treatment of severe allergic asthma confirms the above-mentioned benefits.28
To date, there is limited real-world evidence on Canadian asthma patients receiving omalizumab. The objectives of this study were three-fold: (i) to describe the demographic profile of Canadian asthma patients receiving omalizumab; (ii) to measure time to omalizumab discontinuation; and (iii) to assess changes in concurrent medication use before, during, and after omalizumab therapy in the subgroup of patients who discontinued omalizumab.
Materials and Methods
Sample Selection
This was a retrospective, observational, cohort study using data from Canadian prescription claim databases (IQVIA). The IQVIA claims database collects prescription information from both private (Canada-wide) and public (Ontario and Quebec) drug insurers, comprising 70% of prescription claims submitted to national private drug plans, 100% of prescription claims submitted to the Ontario Drug Benefit Plan, and prescription claims from a 20% random sample of the Régie de l’assurance maladie du Québec population. These databases have been previously described.29–31
The algorithm used for the current study was similar to one used in a previous study of severe eosinophilic asthma in primary care in Canada.32 The study period is described in Figure 1. Patient index date was defined as the date of the patient’s first omalizumab claim. Patients were included in the study if they met the following inclusion criteria: (i) patient had a claim for omalizumab from February 1, 2007 to June 2, 2015; (ii) patient was ≥12 and ≤105 years of age at the index date; (iii) patient was naïve to omalizumab and had no claims for omalizumab in the 12 months preceding the index date; (iv) patient had ≥1 claim for any prescription drug 12 to 24 months preceding their index date and 6 to 12 months following omalizumab usage to ensure they were active and remained active in the claims database, respectively; and (v) patient met the criteria for an inferred asthma diagnosis. Institutional Review Board approval was not required since this is a prescription claims-level study using anonymized data.
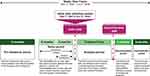 |
Figure 1 Schematic representation of the study time frame with the purpose of each time period. |
Asthma diagnosis was inferred if a patient met all of the following criteria: (i) patient had ≥2 prescription claims that have an asthma indication in the 12 months preceding the index date that were for drugs other than short-acting β2-agonists (SABA) (eg, claims made for ICS, ICS plus leukotriene receptor antagonist [LTRA], LTRA, ICS plus LABA, or ICS plus LABA plus LTRA); (ii) omalizumab was not prescribed by a dermatologist; and (iii) the omalizumab claim was accompanied by other respiratory drug claims. Patients were excluded from the study if there was an inferred diagnosis of: (i) chronic obstructive pulmonary disease (COPD) (eg, claims made for only long-acting muscarinic antagonists [LAMA], LAMA plus LABA, LABA, phosphodiesterase-4 inhibitor [PDE4], LAMA plus LABA plus ICS, or a beta-blocker); (ii) acute or mild asthma (eg, >75% of asthma claims were for rescue medications or there were fewer than three SABA claims); (iii) chronic idiopathic urticaria (eg, claim for omalizumab made after approval of this indication on August 26, 2014 and either by a dermatologist or there were no prior respiratory drug claims); (iv) asthma COPD overlap or undefined diagnosis; or (v) fewer than 3 months of drug plan history and thus insufficient information to establish a diagnosis.
Measures
Patient follow-up was conducted until the first of the following events: (i) omalizumab discontinuation (defined as a 100-day gap in patient making omalizumab claims; 100 days was selected as a conservative time gap since omalizumab’s half-life is 26 days);14 (ii) the end of the claim selection period (June 2, 2015); or (iii) 3 years after treatment initiation with omalizumab. Following omalizumab exposure and discontinuation, patients were followed for 6 months to measure treatment patterns (months 1–6 post-discontinuation) and for an additional 6 months to ensure they were still active in the drug plan (months 7–12 post-discontinuation).
Demographic and baseline characteristics including age, sex, province of residence, and drug insurer were assessed at index. The following outcomes were examined over the analysis period: (i) daily omalizumab dose per patient and per claim; (ii) omalizumab persistence and its potential predictors (ie, age, sex, province of residence, drug insurer); and (iii) for patients who discontinued omalizumab, changes in concurrent medication usage before, during, and 6 months after omalizumab usage.
The daily dosage of omalizumab was calculated in two ways. First calculation method: dosage of omalizumab per day per patient = (total number of vials claimed/number of days between the index and discontinuation dates) x 150 mg/vial. This was based on the average amount of omalizumab (in mg) claimed per day per patient during exposure to omalizumab and it was the average of all patients. This method accounted for real-world dosage increase and compliance. Second calculation method: dosage of omalizumab per day per claim = (number of vials per claim x 150 mg/vial)/days’ supply for the claim. This was based on the average amount of omalizumab (in mg) claimed per day and was based on the average of all claims. This method did not account for dosage increase and compliance. Implausible days of omalizumab supply were removed by standardizing the per-claim calculation to a 28-day supply and excluding claims with a supply of <14 days. Although it is possible that there was a physician directive overriding dosing frequency, it would be rare for a patient to receive omalizumab outside of this framework.
Persistence (ie, time to treatment discontinuation) was determined based on survival analyses (unadjusted Kaplan-Meier survival curves). Patients were categorized as either “events” (ie, omalizumab discontinuation) or “censored” (ie, the patient reached the end of the study or was on treatment for 3 years).
The concurrent medication assessment only included patients who discontinued omalizumab. The concurrent medication classes considered were: (1) SABA; (2) ICS; (3) ICS plus LABA; (4) LABA; (5) LAMA; (6) LTRA; (7) OCS; and (8) xanthine.
Statistics
The number and percent of patients within each category were computed (ie, age group, sex, province of residence, drug insurer). For continuous variables, the mean (standard deviation) and median (interquartile range) were determined.
Survival was defined as the cumulative probability of not discontinuing omalizumab during the specified time interval, after censored observations were excluded. The Cox Proportional Hazards Model, which estimated a hazard ratio (HR) and 95% CI, was used to evaluate the statistical significance of potential predictors of persistence.
The assessment of the population receiving concurrent asthma medications (in percentage and by class) before, during and after receiving omalizumab used the McNemar’s test to evaluate statistical differences between the time points (ie, before vs during; during vs after; and before vs after).
All analyses were conducted using Excel 2016 (Microsoft Corp, Redmond, WA) and SAS version 9.3 (SAS Institute, Cary, NC). P-values <0.05 were considered statistically significant.
Results
Profile of Omalizumab Users
A final cohort of 1160 patients met the pre-defined inclusion criteria; these patients represented 22.6% of the 5126 patients who had made a claim for omalizumab during the study period (Figure 2). Patient characteristics at the index date are summarized in Table 1. The majority of the cohort were female, living in Ontario or Quebec, with private insurance coverage, and their mean age was 45.8 ± 15.2 years. The daily dosage of omalizumab was 16.19 mg/day/patient/claim and 20.06 mg/day/claim. The majority of patients (68.9% [n=799]) claimed the same number of omalizumab vials per claim; 20.8% (n=241) had claims for two different vial amounts and 10.3% (n=119) had claims for ≥3 different vial amounts.
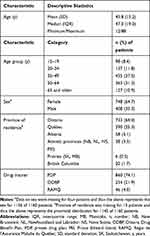 |
Table 1 Characteristics of the Study Cohort (N=1160) at Their Index Date |
 |
Figure 2 Order and results of applying the algorithm’s inclusion and exclusion criteria in the IQVIA claims database to construct the study cohort. |
Omalizumab Persistence and Discontinuation
Omalizumab persistence was assessed as shown in Figure 3. In terms of overall persistence, 70.5% (n=818), 52.7% (n=611) and 47.0% (n=545) of patients in the study cohort remained on omalizumab at 12, 24 and 36 months, respectively. During the first year of omalizumab therapy, 29.5% (n=342) of patients discontinued treatment with an additional 17.8% (n=206) and 5.7% (n=66) discontinuing treatment between 12 to 24 months and 24 to 36 months, respectively. Thus, almost half of the study cohort (47.3%; n=549) discontinued omalizumab treatment in the first 24 months. Sex, type of drug insurer, or province of residence were not significant predictors of omalizumab discontinuation (Table 2). Persistence in the 20–34 age group was significantly lower than the reference group (ie, patients aged 12–19 years; P=0.02). No significant differences were observed between the other age categories (35–64 years and 65 years of age and older) and the reference group. Overall, the 20–34 age group had the greatest discontinuation rates (ie, least persistence) of all the age groups.
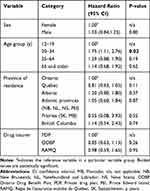 |
Table 2 Analysis of Potential Predictors of Omalizumab Discontinuation Over a 3-Year Period Using the Cox Proportional Hazards Model (Multivariable Regression Method) |
 |
Figure 3 Kaplan–Meier curve of omalizumab persistence and discontinuation. |
Changes in Concurrent Medications Following Omalizumab Discontinuation
Of patients who discontinued omalizumab therapy (n=433), the percentage who were using concurrent asthma medications before, during and after omalizumab is summarized in Table 3. During all three time periods, more than half (54.3–67.0%) had claims for a SABA and/or ICS plus LABA; 44.8–59.1% had claims for an OCS; 19.2–39.7% had claims for an ICS, LAMA, and/or LTRA; and, fewer than 7% of patients had claims for xanthine and/or LABA (Table 3). There were no significant changes in the percentage of patients using SABA, LAMA, LABA, or xanthine before, during, after omalizumab therapy (Table 4). Significantly fewer patients used the following medications during and/or after omalizumab therapy versus before omalizumab treatment: ICS plus LABA, ICS, LTRA, or OCS.
 |
Table 3 Use of Concurrent Medications Before, During, and After Omalizumab Usage for Patients Who Discontinued Omalizumab (n=433) |
 |
Table 4 Changes in the Percentage of Patients Using Concurrent Medications Before, During, and After Omalizumab Therapy for Patients Who Discontinued Omalizumab (n=433) Evaluated Using McNemar’s Test |
Discussion
This is the first real-world study to profile Canadian patients receiving omalizumab and their rates of treatment discontinuation and concurrent medication usage. According to this study, the mean age of omalizumab patients was 45.8 years, which suggests a working-age population. There were nearly twice as many females as males with omalizumab claims. During a period of 2 years, almost half of the study cohort (47.3%) discontinued omalizumab. There was an incremental decrease in the rate of discontinuation year over year, with the highest likelihood of discontinuation during the first year of treatment (29.5%), whereas by the third year, only 5.7% of patients discontinued treatment. Sex, type of drug insurer, and province of residence were not significant predictors of omalizumab discontinuation. After initiation of omalizumab, the use of ICS, ICS plus LABA, LTRA, and/or OCS significantly decreased (P<0.05). SABA and LAMA use remained unchanged. Patients aged 20–34 years had a significantly greater risk of discontinuing omalizumab than the reference group (patients aged 12–19 years) and, overall, the 20–34 age group had the greatest discontinuation rate of all the age groups. Level of asthma control, comorbidities, and exposure to allergy triggers were not accounted for in this claims analysis, all of which are plausible confounders of higher discontinuation rates observed in this age group.33 Changes in employment status, benefits, and improvements in lifestyle commonly occur in this age group.
Other observational analyses of patients newly initiated on omalizumab for the treatment of allergic asthma similarly report a higher percentage of females than males.34,35 Epidemiological data indicate that asthma prevalence, severity, exacerbation rate, hospitalizations, and mortality are greater in adult women than men overall.36 The reasons for this difference are unknown but have been linked to immunological and hormonal factors, and/or to differences in responses to environmental or occupational exposures;37–39 the latter is a noteworthy confounder given that omalizumab is indicated for allergic asthma.
The reasons for discontinuing omalizumab could not be determined within the context and design of this study. Nonetheless, the rates reported in this study are comparable to a real-world analysis of patients using omalizumab in a United States claims database,34 wherein discontinuations were reported in 32.1% and 52.9% of a subset of omalizumab patients (n=970; 61.8% female; mean age 44.7 ± 15.7 years) after 1 and 2 years of treatment, respectively. These findings support the observation that patients are most likely to discontinue omalizumab in the first year after initiation, whereas if they continue treatment for 2 years, they have a lesser likelihood of discontinuing in subsequent years. There are myriad potential reasons for discontinuation, including physician and patient perception of lack or loss of response to and/or efficacy of omalizumab; loss of insurance coverage; treatment-limiting side effects; enrolling in a clinical trial for another therapeutic; spontaneous or non-pharmacologic improvement in asthma control; addition of novel or alternative controller therapies; and patient decision.
It is unclear if omalizumab has disease-modifying properties in asthma and as such, the optimal duration of therapy has not been established. The benefits and/or risks of continuing or discontinuing omalizumab therapy in patients with allergic asthma have been investigated in RCTs40 and observational studies.41,42 In the XPORT trial, adults with moderate to severe allergic asthma who were receiving omalizumab for approximately 5 years were randomized to placebo (n=88) or continuation of omalizumab therapy (n=88) for 1 year.40 Patients who continued omalizumab therapy had a significantly greater likelihood of remaining free of exacerbations (67.0%) compared to those who switched to placebo (47.7%) (absolute difference 19.3% [95% CI, 5.0–33.6%]). They also had an increased time to the first exacerbation (HR 0.49; 95% CI, 0.28–0.86) and improved asthma control as measured by the Asthma Control Test score (P=0.02) and the Asthma Control Questionnaire score (P=0.004).40 Nearly half of patients in the placebo group did not experience an exacerbation. The authors concluded that omalizumab could provide a sustained benefit after it is discontinued; however, the extent to which these benefits could persist is unclear.40 In their multicentre, observational, retrospective study, Molimard et al investigated the time to loss of asthma control after omalizumab discontinuation in 61 patients (age 6–82 years) with severe allergic asthma; omalizumab treatment duration ranged from 2.5 to 59.5 months (mean 22.7 ± 13.1 months).41 Loss of asthma control occurred in 34 patients (55.7%) after a median time of 13.0 months (mean 20.4 ± 2.6 months; 95% CI, 8.3–28.1); omalizumab was re-initiated in 20 of these 34 patients (58.8%). There was no correlation between the time to loss of control and duration of omalizumab treatment or dosage used.41 In an open prospective study where 49 patients discontinued omalizumab treatment after 6 years of therapy, 14% (n=7), 25% (n=12), and 35% (n=17) experienced a loss of asthma control within the first 6, 12, or 24 months of discontinuation, respectively.42 These data suggest that the benefits of omalizumab may not persist in the long term after discontinuation of therapy. During a 9-year period of retrospective data analysis of patients newly initiated on omalizumab living in the US (N=1564; 61.8% female; mean age 44.9 ± 15.7 years), 38.0% of patients who discontinued omalizumab had to re-initiate omalizumab therapy due to loss of asthma control.34
Changes in the use of concurrent medications following omalizumab treatment in this study mirror the findings of real-world retrospective34 and prospective35 analyses of concurrent medication use by asthma patients newly initiated on omalizumab (N=1564)34 or with moderate to severe allergic asthma (N=549; 64.5% female; mean age 44.3 ± 16.0 years).35 Similar trends were observed in these other studies with respect to the use of ICS,34,35 ICS plus LABA,34 leukotriene modifiers,34,35 OCS,34 and LAMA.34 The authors suggested that the observed reductions in concurrent medication use might reflect improved asthma symptoms34 or overall clinical improvement in asthma control.35 However, unlike Ke et al who reported significant decreases in LABA and inhaled SABA (as a “rescue” medication),34 following omalizumab treatment initiation, use of these medications was not significantly altered in the present study. Chen et al also reported a sizable decrease in “regular” SABA use (“rescue” SABA use was not assessed).35 Since our study was a retrospective claims analysis, the reasons for discontinuing concurrent medications could not be ascertained, but it seems plausible that improved asthma symptoms and control, as suggested by Ke et al34 and by Chen et al,35 respectively, could potentially explain these observations.
The strengths of this retrospective observational analysis include: (i) the inclusion of a large, geographically dispersed population representing all provinces in Canada; (ii) the retrieval of prescription claims information made through public and private drug insurers, the latter with national representation; and (iii) a long selection period (approximately 8 years), which allowed for an investigation into the potential predictors of omalizumab treatment discontinuation.
Limitations of this retrospective observational analysis include: (i) the use of an algorithm to infer an asthma diagnosis that was based on respiratory drug claims that are not coded for research purposes and thus may lack accuracy; (ii) claiming a prescription does not guarantee the drug is actually used by the patient; (iii) the lack of clinical information, such as comorbidities, asthma control, and exposure to allergy triggers, all of which are potential confounders of omalizumab treatment discontinuation and concurrent medication use; (iv) the inability to ascertain reasons for discontinuation of omalizumab and/or concurrent asthma medications; and (v) the low representation of public drug providers, particularly from provinces other than Ontario and Quebec.
Reasons for discontinuation of omalizumab should be examined by future research studies. Potential implications of this study include the importance of engaging patients in a detailed discussion about treatment options, particularly given the emergence of new asthma therapies. Such discussions are critical for maintaining good continuity of care and for fostering a strong therapeutic alliance.
Conclusions
This real-world analysis of Canadian prescription claims information indicates that a notable percentage of patients discontinue omalizumab in their first (29.5%) or second (17.5%) year of treatment. Patients aged 20–34 years had a significantly greater likelihood of discontinuing omalizumab than did patients aged 12–19 years. Older age groups, sex, type of drug insurer, and province of residence were not significant predictors of omalizumab discontinuation. An analysis of concurrent medication use for the subgroup of patients who discontinued omalizumab revealed reductions in the use of concurrent medications following initiation of omalizumab therapy, namely decreases in the use of ICS, ICS plus LABA, LTRA, and/or OCS.
Abbreviations
CI, confidence interval; COPD, chronic obstructive pulmonary disease; CTS, Canadian Thoracic Society; GINA, Global Initiative for Asthma; HR, hazard ratio; ICS, inhaled corticosteroid; Ig, immunoglobulin; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; LTRA, leukotriene receptor antagonist; PDE4, phosphodiesterase-4 inhibitor; OCS, oral corticosteroid; OR, odds ratio; RCT, randomized controlled trial; SABA, short-acting β2-agonist; SD, standard deviation.
Ethics Approval
Institutional Review Board approval was not required since this is a prescription claims-level study using anonymized data.
Acknowledgments
The authors wish to acknowledge Ardeane Healthcare Solutions and medical writer, Christina Clark, for their assistance in the preparation of this paper. An abstract of this paper was presented at the 2018 Canadian Society of Allergy and Clinical Immunology Annual Scientific Meeting as a poster presentation. The poster’s abstract was published in the Proceedings of the Canadian Society of Allergy and Clinical Immunology Annual Scientific Meeting 2018. Allergy Asthma Clin Immunol 2019;15 (Suppl 1):A6 (available at https://aacijournal.biomedcentral.com/articles/10.1186/s13223-019-0322-9).
Author Contributions
All authors were involved in the conception and design of the study. Atif Kukaswadia, Jelena Ivanovic and Aren Fischer were responsible for the acquisition and analysis of data. All authors contributed to the interpretation of data, revised the manuscript for important intellectual content, approved the final version to be published, and agreed to be accountable for all aspects of this work.
Funding
This work was supported by AstraZeneca. AstraZeneca participated in the design of the study, interpretation of data, review, and approval of this publication. Data and statistical analysis, and medical writing and editorial support were funded by AstraZeneca. Data and statistical analysis support were provided by Driss Oraichi, PhD, IQVIA, and Shane Golden, MSc, IQVIA. Medical writing and editorial support were provided by Christina Clark, Ardeane Healthcare Solutions.
Disclosure
Jason K. Lee is affiliated with Evidence Based Medical Educator Inc and Urticaria Canada. He reports personal fees from AstraZeneca during the conduct of this study, and outside of this submitted work. He also reports grants from GlaxoSmithKline and Shire; grants and personal fees from Novartis, Sanofi, Genzyme, Genentech, Roche, and Pediapharm; personal fees from ALK, Aralez, Merck and Trudell Medical; and grants, personal and application development fees from Stallergens. Atif Kukaswadia and Jelena Ivanovic are current employees of IQVIA, while Aren Fischer, is a former IQVIA employee, all of whom collaborated on this study as consultants paid by AstraZeneca. Suvina Amin, Michelle Erdmann, and Alain Gendron, are employees of AstraZeneca. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2018. Available from: https://www.ginasthma.org.
2. Normansell R, Walker S, Milan SJ, Walters EH, Nair P. Omalizumab for asthma in adults and children (Review). Cochrane Database Syst Rev. 2014;1:CD003559.
3. Asthma. Statistics Canada; 2015. Available from: https://www150.statcan.gc.ca/n1/pub/82-625-x/2015001/article/14179-eng.htm.
4. Health indicators, annual estimates, 2003–2014. Statistics Canada; 2018. Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310045101.
5. Lougheed MD, Lemiere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Resp J. 2012;19:127–164.
6. FitzGerald JM, Lemiere C, Lougheed MD, et al. Recognition and management of severe asthma: a Canadian Thoracic Society position statement. Can J Resp Crit Care Sleep Med. 2017;1:199–221.
7. Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulm Med. 2009;9:24. doi:10.1186/1471-2466-9-24
8. Ehteshami-Afshar S, FitzGerald JM, Doyle-Waaters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20:11–23. doi:10.5588/ijtld.15.0472
9. Barnes PJ. Severe asthma: advances in current management and future therapy. J Allergy Clin Immunol. 2012;129:48–59. doi:10.1016/j.jaci.2011.11.006
10. Thomson NC, Chaudhuri R. Omalizumab: clinical use for the management of asthma. Clin Med Insights Circ Resp Pulm Med. 2012;6:27–40.
11. ENFUMOSA (European Network for Understanding Mechanisms of Severe Asthma). The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22:470–477. doi:10.1183/09031936.03.00261903
12. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi:10.1038/nm.2755
13. Pelaia G, Vatrella A, Maselli R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov. 2012;11:958–972. doi:10.1038/nrd3792
14. Product Monograph: Xolair® (omalizumab). Novartis Pharmaceutics Canada Inc.; 2017. Available from: https://www.novartis.ca/sites/www.novartis.ca/files/xolair_scrip_e.pdf.
15. Heaney LG, Brightling CE, Menzies-Gow A, Stevenson M, Niven RM; on behalf of the British Thoracic Society Difficult Asthma Network. Refractory asthma in the UK: cross-sectional findings from a UK multicentre registry. Thorax. 2010;65:787–794. doi:10.1136/thx.2010.137414
16. Bel EH, Sousa A, Fleming L, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI). Thorax. 2011;66:910–917. doi:10.1136/thx.2010.153643
17. Bhutani M, Yang WH, Hebert J, de Takacsy F, Stril J-L. The real world effect of omalizumab add on therapy for patients with moderate to severe allergic asthma: the ASTERIX Observational study. PLoS One. 2017;12:e0183869. doi:10.1371/journal.pone.0183869
18. Adachi M, Kozawa M, Yoshisue H, et al. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med. 2018;141:56–63. doi:10.1016/j.rmed.2018.06.021
19. Rottem M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: real-life experience in Israel. J Asthma. 2012;49:78–82. doi:10.3109/02770903.2011.637598
20. Barnes N, Menzies-Gow A, Mansur AH, et al. Effectiveness of Omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013;50:529–536. doi:10.3109/02770903.2013.790419
21. Braunstahl G-J, Chlumsky J, Peachey G, Maykut R, Chen C-W. Reduction in oral corticosteroid use in patients with severe allergic (IgE-mediated) asthma receiving omalizumab in a real-world setting. Clin Translat Allergy. 2013;3(Suppl 1):P13. doi:10.1186/2045-7022-3-S1-P13
22. Brusselle G, Michils A, Louis R, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009;103:1633–1642. doi:10.1016/j.rmed.2009.06.014
23. Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Respir Med. 2009;103:1725–1731. doi:10.1016/j.rmed.2009.05.002
24. Molimard M, Buhl R, Niven R, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med. 2010;104:1381–1385. doi:10.1016/j.rmed.2010.06.001
25. Pelaia C, Calabrese C, Barbuto S, et al. Omalizumab lowers asthma exacerbations, oral corticosteroid intake and blood eosinophils: results of a 5-year single-center observational study. Pulm Pharmacol Ther. 2019;54:25–30. doi:10.1016/j.pupt.2018.11.002
26. Tzortzaki EG, Georgiou A, Kampas D, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther. 2012;25:77–82. doi:10.1016/j.pupt.2011.11.004
27. Vennera MD, Llano De LP, Bardagi S, et al. Omalizumab therapy in severe asthma: experience from the Spanish registry—Some new approaches. J Asthma. 2012;49:416–422. doi:10.3109/02770903.2012.668255
28. Abraham I, Alhossan A, Lee CS, Kutbi H, MacDonald K. ‘Real-life’ effectiveness studies of omalizumab in adult patients with severe allergic asthma: systematic review. Allergy. 2016;71:593–610. doi:10.1111/all.12815
29. Wagg A, Franks B, Ramos B, Berner T. Persistence and adherence with the new beta-3 receptor agonist, mirabegron, versus antimuscarinics in overactive bladder: early experience in Canada. Can Urol Assoc J. 2015;9:343–350. doi:10.5489/cuaj.3098
30. Bhoi P, Bessette L, Bell MJ, Tkaczyk C, Nantel F, Maslova K. Adherence and dosing interval of subcutaneous antitumour necrosis factor biologics among patients with inflammatory arthritis: analysis from a Canadian administrative database. BMJ Open. 2017;7:e015872. doi:10.1136/bmjopen-2017-015872
31. Blais A-S, Bergeron M, Nadeau G, Ramsay S, Bolduc S. Anticholinergic use in children: persistence and patterns of therapy. Can Urol Assoc J. 2016;10:137–140. doi:10.5489/cuaj.3527
32. Husereau D, Goodfield J, Leigh R, Borrelli R, Cloutier M, Gendron A. Severe, eosinophilic asthma in primary care in Canada: longitudinal study of the clinical burden and economic impact based on linked electronic medical record data. Allergy Asthma Clin Immunol. 2018;14:15. doi:10.1186/s13223-018-0241-1
33. Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118:899–904. doi:10.1016/j.jaci.2006.07.002
34. Ke X, Kavati A, Wertz D, et al. Real-world clinical characteristics, treatment patterns, and exacerbations in US patients with asthma newly treated with Omalizumab. Clin Ther. 2018;40:1140–1158. doi:10.1016/j.clinthera.2018.05.014
35. Chen H, Eisner MD, Haselkorn T, Trzaskoma B. Concomitant asthma medications in moderate-to-severe allergic asthma treated with omalizumab. Respir Med. 2013;107:60–67. doi:10.1016/j.rmed.2012.09.008
36. Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep. 2015;15:28. doi:10.1007/s11882-015-0528-y
37. Almqvist C, Worm M, Leynaert B. working group of GA2LEN WP 2.5 gender. Impact of gender on asthma in childhood and adolescence: a GA2LEN review. Allergy. 2008;63:47–57. doi:10.1111/j.1398-9995.2007.01524.x
38. Vink NM, Postma DS, Schouten JP, Rosmalen JGM, Boezen HM. Gender differences in asthma development and remission during transition through puberty: the TRacking Adolescents’ Individual Lives Survey (TRAILS) study. J Allergy Clin Immunol. 2010;126(3):498–504. doi:10.1016/j.jaci.2010.06.018
39. Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep. 2007;7:143–150. doi:10.1007/s11882-007-0012-4
40. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162–169. doi:10.1016/j.jaci.2016.08.054
41. Molimard M, Mala L, Bourdeix I, Le Gros V. Observational study in severe asthmatic patients after discontinuation of omalizumab for good asthma control. Respir Med. 2014;108:571–576. doi:10.1016/j.rmed.2014.02.003
42. del Carmen Vennera M, Sabadell C, Picado C, et al. Duration of the efficacy of omalizumab after treatment discontinuation in ‘real life’ severe asthma. Thorax. 2018;73:782–784. doi:10.1136/thoraxjnl-2017-210017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
