Back to Journals » Clinical Ophthalmology » Volume 12
Randomized comparative trial of diode laser transscleral cyclophotocoagulation versus Ahmed glaucoma valve for neovascular glaucoma in Chinese – a pilot study
Authors Choy BN, Lai JS, Yeung JC , Chan JC
Received 28 September 2018
Accepted for publication 15 November 2018
Published 7 December 2018 Volume 2018:12 Pages 2545—2552
DOI https://doi.org/10.2147/OPTH.S188999
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Bonnie Nga Kwan Choy, Jimmy Shiu Ming Lai, Jane Chun Chun Yeung, Jonathan Cheuk Hung Chan
Department of Ophthalmology, University of Hong Kong, Hong Kong, People’s Republic of China
Purpose: This is a pilot study to compare the effectiveness and safety of diode laser transscleral cyclophotocoagulation (TSCP) with the Ahmed glaucoma valve (AGV) in the management of neovascular glaucoma (NVG).
Methods: Eyes with NVG and an IOP greater than 21 mmHg on maximal medications, without previous glaucoma surgery or cyclodestruction, were randomized for either TSCP or AGV implantation. These eyes were followed for at least 6 months and analyzed with respect to their visual outcome, IOP, number of glaucoma medications required, and related complications.
Results: Twenty eyes (eight TSCP and 12 AGV) of 19 subjects with a follow-up duration of greater than 6 months were recruited. Mean follow-up duration was 28.5±17.9 and 31.0±15.4 months for the TSCP and AGV groups, respectively (P=0.80). IOP was successfully controlled in 86% of the eyes for both interventions. By including preservation or improvement of visual acuity as additional criteria for overall success, success decreased to 63% for TSCP and 42% for AGV, although the difference was not statistically significant (P=0.65). Eyes that had TSCP had fewer complications and required less subsequent procedures, compared to those that underwent AGV implantation.
Conclusion: Both procedures were equally effective in controlling the IOP and reducing glaucoma medications in NVG. However, eyes with AGV implant tended to have higher rates of visual loss and complications, as well as requiring more postoperative procedures, than eyes that were treated with TSCP, although the difference was not statistically significant.
Keywords: tube shunts, glaucoma drainage devices, glaucoma implants, cyclodiode, rubeotic glaucoma
Introduction
Neovascular glaucoma (NVG) is a devastating disease that often results in blindness despite aggressive management. Although the IOP may be normalized after adequate retinal ablation in early cases, it is unlikely to be controlled with medical treatment alone for established cases, and swift progression to total blindness usually occurs without further intervention. Numerous procedures have been tried for managing raised IOP in NVG, but no consensus exists for the most effective and safest procedure,1 especially since randomized controlled trials for NVG are few, at least outside China or not published in Chinese.2
Common surgical interventions for NVG include trabeculectomy with antimetabolites, aqueous shunt implantation, and diode laser cyclophotocoagulation. Since previous studies in our predominantly Chinese population already show lower success of trabeculectomy with mitomycin-C in mostly primary glaucoma subjects compared to reports involving predominantly Caucasian subjects,3,4 it is expected that success in Chinese NVG eyes will likely be even lower.
Another local study involving the AGV shows comparable success to published studies in other populations.5 Although NVG cases still fared poorly in this study, its 45% success rate (with a mean follow-up duration of 21.8 months) was comparable to another NVG study using the Baerveldt Glaucoma Implant (50% success with a mean follow-up of 15.7 months).6
Diode laser transscleral cyclophotocoagulation (TSCP) is also frequently used in NVG, where the reported rates of successful IOP control range from 60% at 2 years7 to 50%–56% at 3 years8,9 and 35% at 6 years postoperatively.9 TSCP also has fewer complications compared to older cyclodestructive procedures.1 Among reports published in English, we found only one from Turkey which compared TSCP with AGV in NVG.10 No difference in success was found between the two interventions from this report, but their generalizability to a non-Turkish population is uncertain, nor was it specified whether subjects with previous glaucoma surgery or cyclodestruction procedure were recruited or excluded. Although the use of anti-vascular endothelial growth factor (anti-VEGF) agents in NVG has become increasingly popular in many centers worldwide, it has yet to be widely adopted into the treatment protocol for NVG due to their cost, off-label use (for NVG), as well as insufficient data on efficacy.11
This randomized, prospective, pilot study aims to compare the efficacy and safety of TSCP with AGV in the treatment of medically uncontrolled IOP in Chinese NVG eyes with no previous glaucoma surgery or cyclodestructive procedures. Anti-VEGF use was not examined in this study, as it is currently not available for use in NVG in Hong Kong’s public health sector (including the recruiting center). The results from this exploratory trial facilitate the design and sample size calculation of any subsequent large clinical studies on NVG, especially in a predominantly East Asian population.
Patients and methods
Subjects were recruited from the Ophthalmology Outpatient Clinic, United Christian Hospital, Hong Kong. The study was approved by the hospital’s institutional review board and adhered to the tenets of the Declaration of Helsinki. Consecutive patients satisfying the inclusion/exclusion criteria were invited to join as subjects for a period of at least 6 months. Inclusion criteria included a clinical diagnosis of NVG from ocular ischemia, IOP greater than 21 mmHg despite maximal medications, over 18 years of age, and willingness to give informed consent. Eyes with no light perception (NLP) or previous history of glaucoma surgery or cyclodestruction were excluded.
After obtaining written informed consent from each participating patient, their best-corrected visual acuity (BCVA), IOP, and number of glaucoma medications used were recorded as baseline data. Each suitable eye was then randomized, using a computer-generated random number table, to receive either TSCP (even numbers) or AGV (odd numbers). Preoperative panretinal photocoagulation (PRP) was performed whenever fundal view is adequate, or as soon as possible postoperatively if or when the fundal view improves.
TSCP subjects received treatment under regional anesthesia (retrobulbar or peribulbar block with 2% lidocaine) in the Outpatient Department. Diode laser with an wavelength of 810 nm was delivered using the G-probe (OcuLight SLx; IRIS Medical Instruments, Mountain View, CA, USA) for 270° around the limbus with six shots per quadrant (total 18 shots). Duration was set to be 2 seconds and initial power to be 1.75 W. A “pop”-titrated protocol was used to adjust laser power stepwise throughout the procedure in 250 mW increments, such that it was decreased after every two consecutive audible shots (“pop”) and increased after every two consecutive silent shots. Postoperatively, prednisolone acetate 1% every 4 hours and atropine 1% twice daily eye drops were prescribed. Both drops were gradually tapered off as clinically indicated. Subjects initially continued all glaucoma medications after TSCP, which were later adjusted according to the IOP during follow-up visits.
AGV subjects underwent the procedure in the operating theater under either regional anesthesia (retrobulbar or peribulbar block with 2% lidocaine) or general anesthesia. A fornix-based conjunctival flap was made in one quadrant, preferably superotemporal. The AGV (Model S2; New World Medical Inc., Rancho Cucamonga, CA, USA) was primed by injecting balanced salt solution into the lumen of its tube until it flowed out of the exit opening over the plate, followed by implant placement beneath the conjunctival flap at 8–10 mm posterior to the limbus with scleral fixation using 9–0 nylon sutures. The tube of the implant was trimmed to the desired length with its bevel facing anteriorly. Anterior chamber paracentesis with injection of a viscoelastic (1% sodium hyaluronate) was followed by the creation of a scleral tunnel with a 23-G hypodermic needle at 1–2 mm posterior to the limbus. The tube was inserted into the anterior chamber through the scleral tunnel, parallel to the iris, and held flush to the sclera with a 9/0 nylon cross suture. The external part of the tube near the entry site was covered with either a donor corneoscleral graft or with a partial thickness scleral flap created before the sclerotomy. The graft or flap was sutured to the sclera with 10/0 nylon sutures and the conjunctiva closed with 8/0 polyglactin sutures. Postoperatively, prednisolone acetate 1% and levofloxacin 0.5% eye drops every 4 hours were given for at least 6 weeks, then tapered off according to clinical response. All previous glaucoma medications were stopped postoperatively and resumed as required later.
In the TSCP group, the laser procedure was repeated as necessary, spaced at least 4 weeks apart, until either the IOP was 21 mmHg or below, or a total of five treatments had been given. Uncontrolled IOP requiring any additional, non-assigned interventions was counted as failure. Additional procedures in relation to the implanted AGV (for example, clearing tube blockage, repair of implant exposure, excision of encapsulated bleb, and repositioning of implant) were not counted as failure but documented as complications. Similarly, any TSCP-related conjunctival burn or scleral thinning, with or without need for additional procedures, was recorded as a complication.
Outcome analysis
Visual acuity
The BCVA of each eye at their final visit was compared to its baseline level. Changes in the final BCVA were classified as “worsened”, “static”, or “improved” when compared to baseline BCVA. Changes of one line of Snellen visual acuity or less was defined as static, whereas greater changes were defined as being worsened or improved accordingly. For BCVA not better than count fingers (CF), this was defined as improved if it changed from light perception only (LP) to hand movement (HM) or better or from HM to CF or better. The reverse applied for worsened visual acuity.
IOP and number of glaucoma medications
IOP was measured with a Goldmann applanation tonometer at baseline and subsequent follow-ups. Successful IOP control was defined as a final visit IOP not higher than 21 mmHg, with or without medication, and no hypotony-related maculopathy or choroidal detachment.
Overall success rate
The subject’s desire for continuing IOP-lowering treatment in the recruited eye is influenced by the visual potential of that eye. Success based on IOP normalization alone fails to account for those subjects who deviate from their assigned protocol by declining further intervention despite elevated IOP (if asymptomatic), due to the loss of visual potential in the study eye. To account for this, overall success is defined as not only IOP normalization but also preservation or improvement of BCVA.
Complications
Any intraoperative and post-operative complications were recorded.
Statistical analyses
The database was maintained and managed using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA). All statistical analyses were performed using SPSS software version 16.0 (SPSS Inc., Chicago, IL, USA). For descriptive statistics, continuous variables were expressed as mean ± SD (range), whereas categorical variables were presented as frequencies with percentage. Continuous variables including age, follow-up duration, baseline, and final IOP were tested for normal distribution with the Kolmogorov–Smirnov test. Test of significance was done using Student’s t-test to compare the mean values of normally distributed variables: independent t-test for differences between the two study groups and paired t-test for changes of baseline to final IOP. Non-parametric tests such as Mann–Whitney and Wilcoxon signed-rank tests were used whenever the variables were not normally distributed. Chi-square test or Fisher’s exact test was used to compare the proportions of subjects with visual stability or success. A P-value of ≤0.05 was considered statistically significant. For outcome measures, the proportion estimates of successful IOP control and overall success, expressed in percentages, were computed for the two study groups. All treatment-related complications of each subject are reported descriptively if they occurred. Kaplan–Meier (K–M) survival analysis with the logrank test was conducted to compute and compare the K–M estimate of overall success for the two treatment groups. HR was calculated to test whether the RR between the two treatment groups is constant through time.
Results
A total of 22 eyes in 21 subjects with a mean age of 62.2±11.8 years (range 39–79) were recruited over a period of 2 years. Nine eyes in eight subjects with a mean age of 61.3±13.5 years (range 39–79) were randomized to the TSCP group, whereas the AGV group had 13 eyes in 13 subjects with a mean age of 62.8±11.0 years (range 41–75). The demographics in both groups were comparable with no statistically significant difference (Table 1). Two subjects, one in each group (with one study eye each), had a follow-up duration of less than 6 months and were excluded, leaving a total of 20 eyes (eight TSCP and 12 AGV) for further analysis.
  | Table 1 Demographics of study subjects |
The most frequent cause of NVG was proliferative diabetic retinopathy, followed by central retinal vein occlusion, then central retinal artery occlusion and ocular ischemic syndrome (Table 2). Preoperative PRP was performed in 16 eyes (73%). For the remaining four eyes, one had previous posterior vitrectomy with adequate endolaser PRP performed, while poor fundal view from corneal edema or vitreous hemorrhage precluded preoperative PRP in the remaining three eyes. Nine eyes (41%) had undergone previous cataract surgery (eight cataract extraction alone and one combined cataract extraction and posterior vitrectomy). The mean follow-up duration was 25.5±16.29 months (range 7–51) for the TSCP group and 29.08±11.91 months (range 8–52) for the AGV group. There were no significant differences in the abovementioned findings for the two study groups.
In the TSCP group, the mean number of treatment sessions was 2.7±1.4 (range 1–5). One eye failed with IOP remaining uncontrolled after five sessions of TSCP, and AGV was eventually required. In the AGV group, four eyes had combined phacoemulsification and AGV (as cataract was also present), whereas nine eyes had AGV alone (four phakic eyes and five pseudophakic eyes). There was one failed AGV case with recurrent implant exposure despite multiple repairs, which eventually required implant removal at 2 months postoperatively. This eye subsequently required two sessions of TSCP (at 3 and 4 months) for uncontrolled IOP.
wVisual acuity
The baseline BCVA ranged from HM to 0.2 in the TSCP group and from LP to 0.2 in the AGV group. As mentioned, two eyes (one from each group) had failed due to uncontrolled IOP despite receiving assigned intervention, subsequently requiring non-assigned intervention of the other study arm and therefore excluded from this part of the analysis. The remaining 18 eyes (seven in the TSCP group and 11 in the AGV group) had only received intervention from their assigned arm of the study (Table 3). For the final BCVA in the TSCP group (n=7), two had worsened, three remained static, and two had improved compared to baseline. For the AGV group (n=11), six had worsened, three remained static, and two had improved BCVA. The difference in BCVA changes between the two groups was not statistically significant (P=0.38).
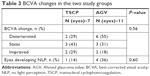  | Table 3 BCVA changes in the two study groups |
Of the eight eyes with visual deterioration (two TSCP and six AGV), one from the TSCP group developed NLP at 4 months, whereas four from the AGV group developed NLP at 2, 5, 8, and 17 months, respectively. Although more eyes in the AGV group developed NLP compared to the TSCP group (4 of 11 vs 1 of 7, 36% vs 14%), this was not statistically significant (P=0.60).
IOP and number of glaucoma medications
For this part of the analysis, two eyes were excluded for having received interventions from both arms of the study (and hence counted as failure for all subsequent analyses of success rate), and six eyes were excluded because the subjects decline further intervention according to their assigned study protocol due to the loss of visual potential in the study eye (Table 4). Of these six eyes, five had developed NLP (one TSCP and four AGV), and one (AGV) had worsened from HM to LP.
  | Table 4 Baseline and final mean IOP, number of glaucoma medications, and mean follow-up duration |
In the TSCP group, the mean baseline IOP was 42.5±13.9 mmHg (range 32–64), mean final IOP was 15.2±6.6 mmHg (range 5–21), and mean follow-up duration was 28.5±17.9 months (range 7–51). For the AGV group, the mean baseline IOP was 41.5±11.5 mmHg (range 30–55), mean final IOP was 14.7±4.2 mmHg (range 8–21), and mean FU duration was 31.0±15.4 months (range 8–52). The mean final IOP for both study groups was significantly lower than their mean baseline IOP (P<0.01 in both groups). Comparing the two study groups, there was no significant difference in the mean baseline IOP (P=0.90) or final IOP (P=0.88).
The mean number of glaucoma medications for both groups as a whole was significantly reduced from baseline of 3.0±1.1 to the final visit of 1.1±1.2 (P<0.01), giving a mean reduction of 1.9±1.7 medications. In the TSCP group, the mean number of medications was reduced from 3.2±0.8 to 1.7±1.4 (mean reduction 1.5±0.8, P<0.01), whereas for the AGV group, it was reduced from 2.8±1.5 to 0.5±0.8 (mean reduction 2.3±2.3, P=0.05). There was no significant difference in the mean baseline number (P=0.63) or final number (P=0.11) of glaucoma medications between the two study groups.
Overall success rate
Twenty of the 22 eyes originally recruited had a follow-up duration of at least 6 months and were included in this analysis (Table 5). If IOP control was the only success criterion, excluding the six eyes that declined further IOP-lowering intervention, both TSCP and AGV were successful at controlling the IOP in six of seven eyes, giving a success rate of 86%. When BCVA preservation or improvement was added as a success criterion, overall success was achieved by five of eight (63%) eyes in the TSCP group and five of 12 (42%) eyes in the AGV group, with no significant difference (P=0.65). Worsened BCVA accounted for six of the seven AGV failures, and five of these eyes subsequently declined further intervention for elevated IOP due to limited visual potential. K–M survival analysis (Figure 1) demonstrated no significant differences between TSCP and AGV groups in determining the overall success rate (P=0.42). Compared to TSCP, AGV implantation had an insignificantly lower overall success rate (HR 1.72; 95% CI 0.45, 6.67).
Complications
All significant treatment-related complications occurred in the AGV group (Table 6). Complications in eyes that had received both TSCP and AGV implant are counted as complications resulting from the initially assigned intervention. There were two cases of implant exposure requiring surgical repair, with one eventually having removal of the implant (this eye subsequently redeveloped raised IOP requiring TSCP and was counted as an AGV failure). One eye was complicated by intraoperative bleeding causing vitreous hemorrhage and hyphema and later developed corneal decompensation. Two eyes had overfiltration after AGV implant. One resolved spontaneously within 3 weeks. The other eye had recurrent flat anterior chamber despite multiple reformations with viscoelastic and subsequently underwent phacoemulsification with intraocular lens implantation for cataract progression at 1 month after AGV implantation. This subject subsequently declined further intervention for raised IOP, at 3 months after the initial AGV procedure, as vision had deteriorated to LP (from CFs) and the eye was painless. One bleb encapsulation occurred at 4 months postoperatively, which required excision. Three eyes (in three subjects) developed phthisis bulbi during follow-up, all of which had proliferative diabetic retinopathy and lost light perception before declining further intervention for their vitreoretinal and/or glaucoma conditions, except for two eyes that underwent TSCP for pain control. Three subjects died from medical diseases unrelated to their AGV at 11, 38, and 48 months.
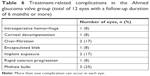  | Table 6 Treatment-related complications in the Ahmed glaucoma valve group (total of 12 eyes with a follow-up duration of 6 months or more) |
For the TSCP group, no eyes developed any significant complications (including phthisis bulbi) from cyclophotocoagulation, whereas one subject died of medical disease unrelated to TSCP at 24 months after entering the study.
Discussion
Our pilot study compared TSCP and AGV for the treatment of medically uncontrolled IOP in NVG without previous history of glaucoma surgery or cyclodestruction, with respect to visual outcome, control of IOP, reduction of glaucoma medications, and any related complications. Visual acuity deteriorated in a higher proportion of the eyes in the AGV group than the TSCP group, and the incidence of developing NLP was also higher in the AGV group. However, possibly due to the small sample size, the difference in visual outcome between the two groups was not statistically significant.
For the six eyes with very limited or no visual potential (LP or NLP), the subject’s motivation for further IOP reduction intrinsically affects the success rate for IOP control, as any treatment used is mainly for symptom control only. After excluding these six eyes for IOP analysis, both interventions were equally effective in achieving successful IOP control in the majority of eyes.
Potential severe complications, including phthisis bulbi, have been reported for both interventions.5,7,9,12,13 In this study, the AGV group had four eyes losing light perception and three eyes developing phthisis bulbi, compared to one eye losing light perception and none developing phthisis bulbi in the TSCP group. There was also more complications and post-operative surgical procedures from AGV implantation than TSCP in our group of Chinese NVG eyes, which is comparable to a retrospective study on a similar population.13
This may be the first randomized prospective study comparing the effectiveness and safety of TSCP and AGV as the initial surgical intervention for NVG with medically uncontrolled IOP, as a previous similar study did not specify whether the study treatment (TSCP or AGV) was the initial surgical intervention for uncontrolled IOP.10 In that study, conducted on a Turkish population, almost a quarter of their TSCP cases were lost to follow-up compared to none in the AGV group, thus affecting the interpretation of their results. This pilot study is also the first such study conducted on an East Asian (Chinese) population.
The limitation of our pilot study includes the relatively small sample size (related to the rarity of NVG within our local population) and also not incorporating the use of anti-VEGF for our subjects.
The small sample size of this pilot study may have contributed to the difference in overall success (TSCP 63% and AGV 42%) not reaching statistical significance, as post hoc analysis shows a low statistical power of 14.1% for the study. According to the results of our pilot study, 178 subjects (89 per group) are required to achieve an 80% power for determining a significant difference in the overall success rate. If the primary aim is to detect a significant difference in preserving or improving visual acuity (TSCP 75% and AGV 50%), then a study with 80% power would require 116 subjects (58 per group). Given the rarity of NVG in most developed regions (where access to TSCP and glaucoma implants are readily available), it is likely that only large, multi-center trials will have sufficient subjects to conduct an adequately powered study.
Although mainly approved for use in age-related macular degeneration and diabetic macular edema, anti-VEGF agents have been increasingly used in recent years to treat retinal ischemia. It has been shown to be beneficial in NVG when used alone14,15 and in conjunction with filtration surgery.16–20 However, as mentioned, anti-VEGF treatment is currently not widely utilized (or available) for the management of NVG in Hong Kong due to its off-label use (for NVG) and high cost. Therefore, both TSCP and AGV remain our current first-line interventions for controlling IOP in NVG eyes that failed to respond adequately to panretinal laser photocoagulation and glaucoma medications. Regarding cyclophotocoagulation, newer methods are becoming available for the treatment of NVG, for example, long duration burn TSCP,21 micropulse TSCP,22,23 and endoscopic cyclophotocoagulation.24 These newer methods of cyclophotocoagulation all aim to reduce post-operative inflammation and decrease complication. However, more research is required to confirm whether they are better treatment options for NVG, and in the cases of micropulse TSCP and endoscopic cyclophotocoagulation, availability and cost are additional factors limiting their adoption in many centers.
Conclusion
In this prospective, randomized comparative pilot study, both TSCP and AGV were equally effective in reducing IOP and glaucoma medications in NVG with no previous glaucoma surgery or cyclodestruction. When preservation or improvement of visual acuity is included as an additional criterion for overall success, both interventions did not perform as well. Although no significant difference in overall success was found between the two groups, there was a trend for higher overall success and better visual outcome for TSCP compared to AGV (63% vs 42% and 75% versus 50%, respectively). In addition, eyes that underwent TSCP had fewer complications and required less subsequent procedures (apart from repeated TSCP) than those which had the AGV implant. A larger, likely multi-centered, study is needed to confirm whether TSCP has a similar or better outcome for treating medically uncontrolled IOP in NVG. For now, maintaining visual function appears to be the major limiting factor for overall success.
Author contributions
Chan JC had substantial involvement with the study concept and design. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Sivak-Callcott JA, O’Day DM, Gass JD, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology. 2001;108(10):1767–1776. | ||
Dong Z, Gong J, Liao R, Xu S. Effectiveness of multiple therapeutic strategies in neovascular glaucoma patients: A PRISMA-compliant network meta-analysis. Medicine (Baltimore). 2018;97(14):e9897. | ||
Ng PW, Yeung BY, Yick DW, Yu CB, Lam DS. Fornix-based trabeculectomy with Wise’s suture technique in Chinese patients. Ophthalmology. 2000;107(12):2310–2313. | ||
Tham CC, Lai JS, Poon AS, Lai TY, Lam DS. Results of trabeculectomy with adjunctive intraoperative mitomycin C in Chinese patients with glaucoma. Ophthalmic Surg Lasers Imaging. 2006;37(1):33–41. | ||
Lai JS, Poon AS, Chua JK, Tham CC, Leung AT, Lam DS. Efficacy and safety of the Ahmed glaucoma valve implant in Chinese eyes with complicated glaucoma. Br J Ophthalmol. 2000;84(7):718–721. | ||
Sidoti PA, Dunphy TR, Baerveldt G, et al. Experience with the Baerveldt glaucoma implant in treating neovascular glaucoma. Ophthalmology. 1995;102(7):1107–1118. | ||
Nabili S, Kirkness CM. Trans-scleral diode laser cyclophoto-coagulation in the treatment of diabetic neovascular glaucoma. Eye (Lond). 2004;18(4):352–356. | ||
Oguri A, Takahashi E, Tomita G, Yamamoto T, Jikihara S, Kitazawa Y. Transscleral cyclophotocoagulation with the diode laser for neovascular glaucoma. Ophthalmic Surg Lasers. 1998;29(9):722–727. | ||
Delgado MF, Dickens CJ, Iwach AG, et al. Long-term results of noncontact neodymium:yttrium-aluminum-garnet cyclophotocoagulation in neovascular glaucoma. Ophthalmology. 2003;110(5):895–899. | ||
Yildirim N, Yalvac IS, Sahin A, Ozer A, Bozca T. A comparative study between diode laser cyclophotocoagulation and the Ahmed glaucoma valve implant in neovascular glaucoma: a long-term follow-up. J Glaucoma. 2009;18(3):192–196. | ||
Simha A, Braganza A, Abraham L, Samuel P, Lindsley K. Anti-vascular endothelial growth factor for neovascular glaucoma. Cochrane Database Syst Rev. 2013;10:CD007920. | ||
Iliev ME, Gerber S. Long-term outcome of trans-scleral diode laser cyclophotocoagulation in refractory glaucoma. Br J Ophthalmol. 2007;91(12):1631–1635. | ||
Liao N, Li C, Jiang H, Fang A, Zhou S, Wang Q. Neovascular glaucoma: a retrospective review from a tertiary center in China. BMC Ophthalmol. 2016;16:14. | ||
Aref AA. Current management of glaucoma and vascular occlusive disease. Curr Opin Ophthalmol. 2016;27(2):140–145. | ||
Iliev ME, Domig D, Wolf-Schnurrbursch U, Wolf S, Sarra GM. Intravitreal bevacizumab (Avastin) in the treatment of neovascular glaucoma. Am J Ophthalmol. 2006;142(6):1054–1056. | ||
Arcieri ES, Paula JS, Jorge R, et al. Efficacy and safety of intravitreal bevacizumab in eyes with neovascular glaucoma undergoing Ahmed glaucoma valve implantation: 2-year follow-up. Acta Ophthalmol. 2015;93(1):e1–e6. | ||
Sahyoun M, Azar G, Khoueir Z, et al. Long-term results of Ahmed glaucoma valve in association with intravitreal bevacizumab in neovascular glaucoma. J Glaucoma. 2015;24(5):383–388. | ||
Kang JY, Nam KY, Lee SJ, Lee SU. The effect of intravitreal bevacizumab injection before Ahmed valve implantation in patients with neovascular glaucoma. Int Ophthalmol. 2014;34(4):793–799. | ||
Sevim MS, Buttanri IB, Kugu S, Serin D, Sevim S. Effect of intravitreal bevacizumab injection before Ahmed glaucoma valve implantation in neovascular glaucoma. Ophthalmologica. 2013;229(2):94–100. | ||
Ghosh S, Singh D, Ruddle JB, Shiu M, Coote MA, Crowston JG. Combined diode laser cyclophotocoagulation and intravitreal bevacizumab (Avastin) in neovascular glaucoma. Clin Exp Ophthalmol. 2010;38(4):353–357. | ||
Alzuhairy S, Albahlal A, Aljadaan I, et al. Intraocular pressure outcomes following transscleral diode cyclophotocoagulation using long and short duration burns. J Glaucoma. 2016;25(9):e782–e786. | ||
Gavris MM, Olteanu I, Kantor E, Mateescu R, Belicioiu R. IRIDEX MicroPulse P3: innovative cyclophotocoagulation. Rom J Ophthalmol. 2017;61(2):107–111. | ||
Tan AM, Chockalingam M, Aquino MC, Lim ZI, See JL, Chew PT. Micropulse transscleral diode laser cyclophotocoagulation in the treatment of refractory glaucoma. Clin Exp Ophthalmol. 2010;38(3):266–272. | ||
Yip LW, Yong SO, Earnest A, Ji J, Lim BA. Endoscopic cyclophotocoagulation for the treatment of glaucoma: an Asian experience. Clin Exp Ophthalmol. 2009;37(7):692–697. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



