Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Protective Effect of Asacol in Combination with Pantoprazole in Ulcerative Colitis Patients Who Defecate Asacol Tablets Intactly: A Clinical Trial Study
Authors Bashiri H, Bozorgomid A
Received 29 September 2019
Accepted for publication 14 January 2020
Published 24 January 2020 Volume 2020:13 Pages 47—51
DOI https://doi.org/10.2147/CEG.S225675
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Everson L.A. Artifon
Homayoon Bashiri,1,2 Arezoo Bozorgomid1
1Infectious Diseases Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran; 2Department of Internal Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
Correspondence: Arezoo Bozorgomid
Infectious Diseases Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
Tel +989188728269
Email [email protected]
Purpose: Mesalazine formulations are the drug of choice in the treatment of ulcerative colitis (UC). They are released at alkaline pH in order to deliver 5-aminosalicylic acid to the colon. The colonic pH is significantly lower in UC patients than in normal patients. This study was conducted for the first time to evaluate the clinical efficacy of co-administration of pantoprazole and Asacol in the treatment of ulcerative colitis patients who excrete intact Asacol tablets in the feces.
Patients and Methods: Thirty patients with mild-to-moderate active ulcerative colitis who reported passing intact Asacol tablets in stools received oral Asacol plus pantoprazole for 2 weeks. The demographic characteristics of the patients and the body mass index were collected through interviews. For each patient, the stool frequency, visible blood, and presence of intact Asacol tablets in the stool were compared before and pantoprazole treatment.
Results: There was a significant difference in the stool frequency (number of daily stools) before and after pantoprazole treatment (mean ± sd, 6.06 ± 1.04 vs 1.5± 0.5; P< 0.001). In addition, pantoprazole administration statistically reduced visible blood in the stool (100%; P< 0.001). Co-administration of pantoprazole and Asacol was effective in all age groups and both sexes. None of the patients reported the presence of intact Asacol tablets in their stools.
Conclusions: Co-administration of pantoprazole and Asacol would be useful for symptom management UC patients that excrete intact Asacol tablets in their feces through increasing the gastric pH and releasing the maximum concentration of the drug in the proximal gastrointestinal tract.
Keywords: Asacol, pantoprazole, treatment, ulcerative colitis, pH
Introduction
Ulcerative colitis (UC) is a subcategory of inflammatory bowel disease (IBD) which causes rectal bleeding, diarrhea, abdominal pain, tenesmus and fever.1,2 It most commonly affects people between 15 and 30 years of age but can occur in any age group and in both sexes.3 The etiology and pathophysiology of UC are still unclear and depend on a number of factors such as environmental factors, genetic, reactive oxygen species and gastrointestinal infections.4
Mesalazine formulations (Asacol, Pentasa, Salofalk, Mesasal, Claversal) are the drug of choice for induction and maintenance of remission in ulcerative colitis.5 The current treatment of UC with delayed-release formulations of mesalazine includes pH-sensitive polymers that dissolve at a specific pH.6 Asacol is a sulfa-free, delayed-release mesalazine formulation consisting of a core of 5-aminosalicylic acid (5-ASA) within an acrylic resin coat (Eudragit-S). Coat dissolution occurs at pH 7 or above, generally in the distal small intestine and colon.7
The intra-colonic pH in patients with active UC may be decreased to acidic pH values, which raises the possibility of incomplete dissolution of pH-dependent release tablets and reduces their efficacy.2,8 Low absorption and resistance to mesalamine in UC patients are associated with a fivefold increase in the risk of colorectal cancer and a decreased quality of life.4,9 Hence, it is necessary to search for effective co-therapies to increase the release 5-ASA in UC patients who excrete intact Asacol tablets in the feces.
It has been recently reported that a combination of mesalamine and omeprazole as a proton-pump inhibitor (PPIs) accelerates mucosal healing in UC patients.10 Pantoprazole also belongs to the family of proton-pump inhibitors that inhibit the activity of H + - K + - ATP enzyme to block the secretion of gastric acid and increase the pH of the gastric acid significantly. It is approved by the FDA as a prescription drug and is widely used for treating gastroesophageal reflux and peptic ulcer disease.11
In view of the above, the main objective of our pilot study, which was done for the first time, was to evaluate the possible effectiveness of pantoprazole combined with Asacol for symptom management in UC patients who excrete intact Asacol tablets in the feces.
Materials and Methods
This clinical trial study was carried out in patients with mild-to-moderate UC referred to Imam Reza Hospital (a referral center affiliated with Kermanshah University of Medical Sciences), Kermanshah, west of Iran from March 2017 to December 2017. The inclusion criteria were a definite diagnosis of mild to moderate UC and, Mayo score of 4–10 at enrollment, presence intact Asacol tablets in the stools and age 18–60 years. The exclusion criteria were the use of blood pressure medicines, underlying systemic diseases, pregnancy, adverse reaction to Asacol and patients with severe UC (Mayo score >10). All the participants written consent prior to the study and the protocol was approved by the Kermanshah University of Medical Sciences Ethics Committee (IRCT2017012227761N3).
Based on the previous study that elevated PH of 1 to 7 could enhance the release of 5-ASA from the Asacol tablets from 70% to 100%,12 with a confidence level of 95%, and at power 90%, the minimum sample size required was 25 subjects, which was increased to 30 to allow dropouts.
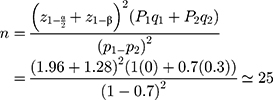
The study participants were diagnosed with ulcerative colitis based on clinical, histological, radiological and colonoscopic criteria. The patients had a history of passing intact Asacol tablets in their stools. The participants received a 2-week course of oral Asacol (0.4–0.8 g/d) three times daily plus pantoprazole 40 g/d once daily. The demographic characteristics of patients were collected through interviews. Moreover, the body mass index (BMI) was calculated by dividing the body weight (in kilograms) by the square of the height (in meters). All patients were asked to record their stool frequency, visible blood, and intact Asacol tablets in their stools.
Statistical analysis was performed with the SPSS software v.16. For continuous variables, mean and standard deviation were calculated. Categorical variables are expressed as frequencies and percentages. To compare continuous variables in patients before and after treatment with pantoprazole, Wilcoxon test was used and comparisons between categorical variables were performed using McNemar test. A P value < 0.005 was considered statistically significant.
Results
Of 30 patients, 11 (36.7%) were male and 19 (63.3%) were female. The participants were 18 to 59 years with a mean age of 40.9±11.04 years. The BMI of the patients ranged from 19.48 to 29.34 (mean ± sd; 25.46 ± 2.55). While a BMI in the range of 18.5–24.9 and over 25 is considered healthy and obese or overweight, respectively. There was a significant difference in the stool frequency before and after pantoprazole treatment (mean ± sd, 6.06 ± 1.04 vs 1.5± 0.5; P<0.001). Co-administration of pantoprazole reduced visible blood in the stool significantly (100%; P<0.001). There was a significant difference in the stool frequency (number of daily stools) before and after pantoprazole treatment (mean ± sd, 6.06 ± 1.04 vs 1.5± 0.5; P<0.001). Pantoprazole administration also statistically reduced visible blood in the stool (100%; P<0.001). There was a significant difference in the stool frequency before and after pantoprazole treatment in UC patients with a normal BMI as well as obese or overweight subjects, mean ± sd, 6± 1.11 vs 1.66± 0.5 and 6.09± 1.04 vs 1.42± 0.5, respectively. None of the patients reported the presence of intact Asacol tablets in their stools after treatment with pantoprazole. The demographic and clinical characteristics of 30 UC patients before and after pantoprazole treatment are summarized in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of 30 Patients with UC Following Treatment with Pantoprazole |
Discussion
More than 1.5 million and 2 million people suffer from UC in the North America and Europe, respectively. About 4.98–7.71 new UC cases in 100,000 population were detected in Iran from 1990 to 2016.13–15 In our study, regarding the clinical characteristics of patients, the administration of pantoprazole was related with the improvement of rectal bleeding, stool frequency, and general well-being. These results are in favor of the hypothesized protective effects of Asacol in combination with pantoprazole, on symptoms in UC patients. In vivo studies have shown that PPI has anti-inflammatory, anti-oxidative, anti-mutagenic activities and cancer-preventive role against colitis-induced carcinogenesis.16,17 However, the therapeutic efficacy of pantoprazole may not be solely due to its anti-inflammatory properties.
Mesalazine formulations are the drug of choice for the treatment of UC. They are released at alkaline pH to deliver 5-aminosalicylic acid to the colon.18 The advantages of colon-targeted drug delivery systems are their near-normal pH and longer transit time.19 However, while the pH in the colonic lumen ranges from pH 6.8 to 7.2 (proximal to distal colon), this can significantly vary in active UC patients from pH 5.5 to 2.3.20 Raimundo et al reported decreases in colonic luminal pH to less than 4.7 in UC patients.21 In a further recent study, Fallingborg et al reported lower colonic luminal pH (ranging between pH 2.3 and 3.4) in three of six patients with active ulcerative colitis.22
In a study by Abinusawa et al, while no 5-ASA was released by Asacol formulations at pH 6.0, the complete release of 5-ASA from the Asacol MR and Asacol HD formulations was observed at pH 6.8 after 4 and 2 hrs, respectively.23 Therefore, pH changes can affect the release of compounds from pH-dependant release coatings, which may result in passing intact Asacol in the stool. Colonic acidification in UC is partly due to reduced mucosal bicarbonate secretion, increased mucosal, bacterial lactate production, impaired absorption, and metabolism of short-chain fatty acids.8 Therefore, due to decreased colonic luminal pH in UC patients, it is necessary to search for effective co-therapies to increase the release of 5-ASA in patients who excrete intact Asacol tablets in the feces.
Pantoprazole, like other proton-pump inhibitors, is the most potent gastric acid suppressant because of its ability to inhibit the proton-pump H+-K+- ATPase.11 Therefore, one important mechanism of pantoprazole in the treatment of UC is probably related to acid suppression leading to a significant increase in the gastric pH and 5-ASA release from pH-dependent formulations in the alkaline environment of the stomach.
A recent clinical study found that co-administration of mesalazine and omeprazole in the treatment of ulcerative colitis not only significantly increased the therapeutic effect compared to the control group but also decreased the time of disappearing colitis symptom and reduced the recurrence after treatment.10 The researchers suggested that the possible mechanism of omeprazole in the treatment of UC may be related to the physicochemical properties of omeprazole, which is similar to metronidazole. However, Wiltink et al studied the effect of famotidine as an H2-antagonist on the absorption of different mesalamine formulations (Asacol, Salofalk, and Pentasa) based on the acetylmesalazine assay in the urine and finally the authors unexpectedly observed lower absorption of Asacol in combination with famotidine.24
However, pantoprazole may be more effective than omeprazole in UC patients, because of its higher bioavailability and more plasma elimination half-life (hours).25 Pharmacodynamic studies of omeprazole have shown an increasing trend toward reactivity with some drugs. Thus, using Pantoprazole, a PPI with low potential to inhibit CYP2C19, may be considered a safer treatment option.26 Furthermore, some studies have reported the antimicrobial activity of PPIs against several species of clinical pathogens such as Heliobacter pylori, Acinetobacter baumannii through accelerating mucosal repair.27,28
To our knowledge, this is the first study of the use of pantoprazole a proton-pump inhibitor, for the treatment of UC. Despite its strengths, our study had limitations. The number of UC patients was too small and there was no control group. Furthermore, the follow-up duration was not long enough to assess the long-term effects and complications. Further, larger studies considering more parameters are needed for clarifying the effectiveness of pantoprazole in UC patients. The present results suggest that pantoprazole in combination with Asacol may be a new therapeutic strategy that could prevent UC progression in patients who excrete intact Asacol tablets in the feces.
Ethics Approval and Consent to Participate
All the participants written informed consent prior to the study and this study was conducted in accordance with the Declaration of Helsinki. The protocol was approved by the Kermanshah University of Medical Sciences Ethics Committee (IRCT2017012227761N3).
Data Sharing Statement
The data sets used and/or analyzed during this study are available from the corresponding author on reasonable request and were received permission for use by the Kermanshah University of Medical Sciences Ethics Committee.
Acknowledgments
The authors want to thank their colleagues in Imam Reza Therapeutic Educational hospital of Kermanshah, Iran for their contribution to the patient’s diagnosis. We also extend our thanks to clinical research development center of Imam Reza Hospital affiliated to Kermanshah University of Medical Sciences for their kind support. This study received financial support from Kermanshah University of Medical Sciences, Iran (Grant Number. 95611).
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Chaparro M, Gisbert JP. Maintenance therapy options for ulcerative colitis. Expert Opin Pharmacother. 2016;17(10):1339–1349. doi:10.1080/14656566.2016.1187132
2. Newton A, Kumar N. IBD–impact of colonic pH, onset of action and other factors in modern therapeutic approach. Interdiscip J Microinflammation. 2014;1:116. doi:10.4172/ijm.1000116
3. Awaad AS, El-Meligy RM, Soliman GA. Natural products in treatment of ulcerative colitis and peptic ulcer. J Saudi Chem Soc. 2013;17(1):101–124. doi:10.1016/j.jscs.2012.03.002
4. Sairenji T, Collins KL, Evans DV. An update on inflammatory bowel disease. Prim Care. 2017;44(4):673–692. doi:10.1016/j.pop.2017.07.010
5. Cohen RD, Woseth DM, Thisted RA, Hanauer SB. A meta-analysis and overview of the literature on treatment options for left-sided ulcerative colitis and ulcerative proctitis. Am J Gastroenterol. 2000;95(5):1263–1276. doi:10.1111/j.1572-0241.2000.01940.x
6. Ye B, van Langenberg DR. Mesalazine preparations for the treatment of ulcerative colitis: are all created equal? World World J Gastrointest Pharmacol Ther. 2015;6(4):137–144. doi:10.4292/wjgpt.v6.i4.137
7. Ham M, Moss AC. Mesalamine in the treatment and maintenance of remission of ulcerative colitis. Expert Rev Clin Pharmacol. 2012z;5(2):113–123. doi:10.1586/ecp.12.2
8. Nugent S, Kumar D, Rampton D, Evans D. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001;48(4):571–577. doi:10.1136/gut.48.4.571
9. Testa A, Castiglione F, Nardone OM, Colombo GL. Adherence in ulcerative colitis: an overview. Patient Prefer Adherence. 2017;11:297–303. doi:10.2147/PPA
10. Zheng G. A study on the curative effect of omeprazole in the treatment of ulcerative colitis. Int J Clin Exp Med. 2017;1(3):17.
11. Bardou M, Martin J. Pantoprazole: from drug metabolism to clinical relevance. Expert Opin Drug Metab Toxicol. 2008;4(4):471–483. doi:10.1517/17425255.4.4.471
12. Dong Y, Zhou Z, Ding H, Zhang S. Preparation and properties of a pH sensitive carrier based on three kinds of polymer blend to control the release of 5-amino salicylic acid. Pharm Dev Technol. 2014;19(8):960–967. doi:10.3109/10837450.2013.846372
13. Malekzadeh MM, Vahedi H, Gohari K, et al. Emerging epidemic of inflammatory bowel disease in a middle income country: a nation-wide study from Iran. Arch Iran Med. 2016;19(1):2–15.
14. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. doi:10.1016/S0140-6736(17)32448-0
15. Zobeiri M, Bashiri H, Askari L, et al. Epidemiologic characteristics of patients with inflammatory bowel disease in Kermanshah, Iran. Middle East J Dig Dis. 2017;9(3):164–169. doi:10.15171/mejdd.2017.68
16. Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54(11):2312–2317. doi:10.1007/s10620-009-0951-9
17. Kim YJ
18. Hauso Q, Christian Martinsen T, Waldum H. 5-Aminosalicylic acid, a specific drug for ulcerative colitis. Scand J Gastroenterol. 2015;50(8):933–941. doi:10.3109/00365521.2015.1018937
19. Verma S, Kumar V, Mishra DN, Singh SK. Colon targeted drug delivery: current and novel perspectives. Int J Pharm Sci Res. 2012;3(5):1274–1284.
20. Hua S, Marks E, Schneider JJ, Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11(5):1117–1132. doi:10.1016/j.nano.2015.02.018
21. Raimundo AH, Evans DF, Rogers J, Silk DBA. Gastrointestinal pH profiles in ulcerative colitis. Gastroenterology. 1992;102:A681.
22. Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment Pharmacol Ther. 1989;3(6):605–613. doi:10.1111/j.1365-2036.1989.tb00254.x
23. Abinusawa A
24. Wiltink EH, Mulder CJ, Stolk LM, Rietbroek R, Verbeek C, Tytgat GN. Absorption of oral mesalazine-containing preparations and the influence of famotidine on the absorption. Scand J Gastroenterol. 1990;25(6):579–584. doi:10.3109/00365529009095533
25. Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash). 2000;40(1):
26. Ferreiro JL, Ueno M, Tomasello SD, et al. Pharmacodynamic evaluation of pantoprazole therapy on clopidogrel effects: results of a prospective, randomized, crossover study. Circ Cardiovasc Interv. 2011;4(3):273–279. doi:10.1161/CIRCINTERVENTIONS.110.960997
27. Suzuki H, Miyazawa M, Nagahashi S, et al. Rabeprazole treatment attenuated Helicobacter pylori‐associated gastric mucosal lesion formation in Mongolian gerbils. J Gastroenterol Hepatol. 2003;18(7):787–795. doi:10.1046/j.1440-1746.2003.03038.x
28. Yang Y, Chua KL. Assessment of the effect of efflux pump inhibitors on in vitro antimicrobial susceptibility of multidrug-resistant Acinetobacter baumannii. Int J Antimicrob Agents. 2013;42(3):283–284. doi:10.1016/j.ijantimicag.2013.05.011
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
