Back to Journals » Research and Reports in Urology » Volume 10
Prostatic artery embolization versus transurethral resection of the prostate in the treatment of benign prostatic hyperplasia: protocol for a non-inferiority clinical trial
Authors Napal Lecumberri S, Insausti Gorbea I, Sáez de Ocariz García A, Solchaga Álvarez S, Cebrián Lostal JL, Monreal Beortegui R, Giral Villalta PJ, Urtasun Grijalba F
Received 7 April 2017
Accepted for publication 4 September 2017
Published 13 February 2018 Volume 2018:10 Pages 17—22
DOI https://doi.org/10.2147/RRU.S139086
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Saturnino Napal Lecumberri,1 Iñigo Insausti Gorbea,2 Ana Sáez de Ocáriz García,2 Saioa Solchaga Álvarez,2 José Luis Cebrián Lostal,1 Raquel Monreal Beortegui,2 Pedro José Giral Villalta,1 Fermín Urtasun Grijalba2
1Servicio de Urología, 2Sección de Radiología Vascular Intervencionista, Complejo Hospitalario de Navarra, Pamplona, Navarra, Spain
Background: Benign prostatic hyperplasia (BPH) is a prevalent disease associated with lower urinary tract symptoms (LUTS). The standard of care for moderate-to-severe LUTS unresponsive to pharmacological treatment is the transurethral resection of the prostate (TURP). However, this intervention is not exempt from complications. Prostatic artery embolization (PAE) has been described as a new, effective and safe procedure for the treatment of LUTS secondary to BPH. To date, only one clinical trial has been published on the use of PAE for LUTS, but the study was methodologically flawed in terms of safety monitoring. Therefore, well-designed clinical studies are required to compare the efficacy and safety of both techniques in the treatment of LUTS secondary to BPH.
Methods and design: This was a prospective, randomized, non-inferiority clinical trial comparing efficacy and safety of PAE and TURP in the treatment of BPH-related LUTS. A total of 60 patients diagnosed with BPH with obstructive moderate or severe LUTS refractory to medical therapy and candidates for TURP were randomized to either PAE or TURP. The presence and severity of LUTS were assessed using the validated Spanish version of the International Prostate Symptom Score (IPSS). Primary end points included improvement in maximum urinary flow rate (Qmax) as measured at baseline and 1 year after the intervention. Improvement in IPSS as measured at baseline and after the intervention, reduction in prostate volume, no deterioration or improvement of sexual function (International Index of Erectile Function [IIEF]), reduction in PSA and PVR, satisfaction of the patient with the operation and adverse events occurring during the study were secondary outcome measures.
Discussion: The aim of this clinical study was to investigate whether PAE is a valid therapeutic option for LUTS that is not inferior to TURP in terms of efficacy and safety. This study also helped to define the profile of candidates for PAE and analyzed the benefits and complications associated with this new technique.
Keywords: benign prostatic hyperplasia, lower urinary tract symptoms, transurethral resection of the prostate, prostatic artery embolization, clinical trial
Background
Benign prostatic hyperplasia (BPH) affects ~50% of males aged >60 years.1 The presence of BPH is associated with lower urinary tract symptoms (LUTS). LUTS secondary to BPH include nocturia, urinary frequency and urgency, urinary incontinence, difficulty with urinating, weak urinary stream, post-void dribbling and a sensation of incomplete bladder emptying.2,3 LUTS are the most common urological problems in men; they are the primary reason for visits to the urologist and have a major impact on patients’ quality of life (QoL).
The treatment of choice for LUTS secondary to BPH is drug therapy,4 based on alpha-blockers, 5-alpha reductase inhibitors and anticholinergic agents when the main symptom is irritation. Drugs need to be administered for life, and although both groups of drugs have been demonstrated to initially improve LUTS, they often fail in the medium to long term. In addition, the continuous use of these agents causes side effects, which are less frequent and better tolerated in the case of anticholinergic agents. In addition, 5-alpha reductase can affect sexual function and thus diminish the patient’s QoL.5
Transurethral resection of the prostate (TURP) is currently the “Gold Standard” treatment for patients with moderate-to-severe LUTS who are unresponsive to medical treatment and have prostate volumes <80 mL (in regular practice, this volume may be slightly higher).6 However, this technique is associated with complications, such as episodes of hematuria (1%) and clot retention (4.3%), and can occasionally require reintervention (0.2%).7,8 Notably, BPH essentially affects men aged >60 years with concomitant diseases, which increases the risk for complications and serious adverse events associated with highly invasive surgical techniques. Therefore, it is necessary that minimally invasive techniques that are equally or more effective than TURP for LUTS are developed. New techniques should reduce the occurrence of side effects and complications such as bleeding, which is the most serious complication of TURP.
In recent years, new therapeutic alternatives to TURP have been developed, such as plasmakinetic bipolar resection, holmium laser enucleation of the prostate (HoLEP), thulium laser resection, high-intensity ultrasound, transurethral ethanol ablation, hot water-induced thermotherapy or transurethral laser coagulation. However, although these new techniques cause milder side effects than TURP, they seem to be less effective, and some authors suggest that the search for new alternatives should continue. Therefore, the authors suggest that the search should continue further for new alternatives.9
In this context, prostatic artery embolization (PAE) is an innovative minimally invasive procedure that has been demonstrated to be as safe and effective in the treatment of BPH-associated LUTS as TURP.10–13
PAE was first described by Lang et al14 in 1979 as a treatment option of massive bleeding secondary to prostatectomy or prostatic biopsy. In 2000, DeMeritt et al10 observed a reduction in prostate size and an improvement in LUTS as an incidental effect of PAE administered for hematuria.
To date, only one randomized clinical trial15 has been published comparing the efficacy and safety of PAE vs. TURP for BPH,15 showing similar outcomes for both techniques. However, the study was refuted, as the type and incidence of complications associated with these two techniques were inconsistent with those published to date.16 Therefore, well-designed clinical studies are required to compare the efficacy and safety of both techniques in the treatment of LUTS secondary to BPH.
A non-inferiority randomized clinical trial was performed to assess the efficacy and safety of PAE vs. TURP in the treatment of BPH-related LUTS. The secondary objective was determining whether arterial embolization preserves sexual function and improves the QoL of patients as compared to transurethral resection.
Methods and design
Study design and location
A prospective, randomized, non-inferiority clinical trial was conducted at the Urology and Vascular Interventional Radiology Services of the Navarra Hospital Complex in Spain.
Study population and recruitment
Patients were selected from urology clinics by the principal investigator. The subjects who met all inclusion and none of the exclusion criteria (Table 1) were invited to participate in the study; then, they were appropriately explained the purposes, methods, objectives and possible risks associated with the study. A patient information sheet (PIS) and an informed consent form (ICF) were subsequently administered. Patients were given a telephone number, if they needed to contact the principal investigator to raise any questions and request more information on the study. For patients deciding to take part in the study, an inclusion visit was arranged and written informed consent obtained.
Study randomization
Simple, no-replacement randomization was performed in balanced blocks in a 1:1 ratio. According to this method, patients were randomized in blocks to ensure that the same number of patients were assigned to each treatment group (TURP or PAE), thus guaranteeing a balance in sample size across treatments over time. In this study, patients were randomized in blocks of six. In this case, for a sample size of 60 patients, 10 blocks were generated. For the first randomization, the principal investigator randomly selected a number from a “table or random numbers” to allocate the first block. From there, using the table of random numbers, the consecutive sequence of blocks was determined, generating patients’ allocation sequence.
Study procedures
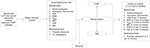
An inclusion visit was scheduled for patients who decided to participate in the study. Patients were provided with an ICF and underwent various examinations (Figure 1). The patients who were eventually selected were randomized to one of the two treatment groups 2 weeks before the intervention. Patients were informed of the group they have been assigned to and of the date of intervention. A description of the procedure was also provided. A specific consent form either for TURP or PAE was handed to participants, and a coagulation test was requested if no recent tests were available.
All patients were instructed to abandon their BPH medication the day of the intervention for the TURP group and 4 weeks after the intervention for the PAE group. Patients in the PAE group received antibiotic prophylaxis (ciprofloxacin 500 mg twice a day) and proton pump inhibitors (omeprazole 20 mg once a day) starting 2 hours before the intervention until 7 days after the procedure. The patients in the TURP group received a single preoperative dose of ceftriaxone 1 g. Patients were admitted on the day of the intervention.
TURP
Under local/regional anesthesia, a resector was introduced through the urethra to extract a section of the benign tumor, which was subsequently sent to the pathology service for analysis. The TURP system consisted of an electric generator – a resectoscope – which incorporated an electrode, a telescope, an inner and outer sheath and a light guide cable. The active and return electrodes were inserted into the resectoscope at the site of the operation, eliminating the need for a patient return electrode. The surgeon used endoscopic imaging to guide electrode assembly through the urethra to the prostate. The electrode was then used to resect and coagulate prostate tissue, and fluid was used to flush the bladder free of excess prostate tissue and blood. Electrodes were available in different sizes and shapes (described as loop, button and roller) for cutting or coagulation at the surgeon’s choice. Generally, a loop was used to repeatedly remove small chippings to create a wide channel though the prostate, and a roller or button may be used to achieve hemostasis. Prostatic chippings were flushed out before inserting a urethral urinary catheter at the end of the procedure.17
The patient stayed a few hours in the recovery unit and then remained in hospital until the hematuria caused by the surgery has cleared and normal micturation has returned after removal of the catheter; both therapeutic targets were usually achieved in ~4 days after the intervention.
PAE
Under local anesthetic in the inguinal area, a 5-F introducer (Radifocus, Terumo, Japan) was placed by retrograde femoral puncture. Using a 5-F hydrophilic catheter (Roberts Uterine Catheter; Cook Medical, Bloomington, IN, USA) or Cobra 1 catheter (Terumo, Tokyo, Japan), the left hypogastric artery was catheterized, reaching its anterior division. Then, coaxial with this catheter, a 2.8 F, 2.4 F or 2.0 F microcatheter (Progreat; Terumo) or 2.4 F Renegade microcatheter (Boston Scientific, Marlborough, MA, USA) was introduced to selectively catheterize the prostatic artery. If there was distal bifurcation of the prostate artery, the microcatheter was placed proximal to the bifurcation. If there was no bifurcation, the microcatheter was placed as distally as possible. Once the prostate artery had been catheterized, 200 µg of nitroglycerin diluted in saline was injected to prevent vasospasm and to increase the diameter of the artery to facilitate distal catheterization. If anastomoses with other pelvic arteries occurred (vesical, rectal, internal pudendal and accessory pudendal), proximal closure of the anastomoses was performed using coils to avoid unwanted embolization. Embolization was performed with 300–500 µm polyvinyl alcohol microspheres (Bead Block BTG plc, Farnham, Surrey, UK). Embolization of the left prostatic arteries was completed when slow flow or stasis was observed, with disruption of arterial flow and opacification of the prostate gland. The right hypogastric artery was then catheterized, and the right prostatic arteries were embolized in the same way as that described for the contralateral side. Patients were discharged the day after the intervention, except in case of pain or other complications.
Study outcome measures
Characteristics and timing of visits
Follow-up control of the parameters shown in Figure 1 took place 1, 3, 6 and 12 months after the procedure.
Primary and secondary end points
The primary end point was the improvement in maximum urinary flow rate (Qmax) 1 year after the intervention with respect to baseline.
Secondary end points were improved International Prostate Symptom Score (IPSS) measured at baseline and after the intervention; patient QoL, reduction in prostate volume, no deterioration or improvement in sexual function (International Index of Erectile Function [IIEF]), reduction in PSA and PVR, satisfaction of the patient with the operation and adverse events related to study procedure.
Statistics, study sample size and power calculation
Differences between the PAE group and the TURP group in the mean increase in maximum urinary flow rate (Qmax) 1 year after the intervention were assessed. Assuming a confidence level of 95% and standard deviation of 5 mL/second for the increased Qmax, with a power of 80%, a total of 25 patients were required per group to test the null hypothesis of non-inferiority (d: -0.5 mL/second). Differences of 2 mL/second in Qmax between groups were considered as clinically relevant. Assuming a percentage of loss to follow-up of 10%, the required number of patients per group was 30. Sample size calculations were carried out using the R statistical software package “TrialSize”.
Qualitative variables were expressed as mean values of frequencies and percentages, whereas quantitative variables were expressed as measures of central tendency (mean and median) with measures of dispersion (standard deviation and interquartile range). The normality of quantitative data was assessed by the Kolmogorov–Smirnov test. Student’s t-test or Mann–Whitney U test was used wherever appropriate to compare the mean values of quantitative variables (Qmax improvement, reduction in prostatic volume, improvement in IPSS, improvement in QoL, improvement in IIEF, reduction in PSA and PVR, etc.). c2 or Fisher’s test was used to compare qualitative variables as appropriate. SPSS software package, version 20, was used for the analyses.
Regulatory issues
Ethical approval
This clinical study had been approved by the local ethics committee (Comité Ético de Investigación Clínica de Navarra, Gobierno de Navarra, Departamento de Salud) and was undertaken according to the protocol and the principles established in the latest version of the Declaration of Helsinki,18 respecting the standards of Good Clinical Practice CPMP/ICH/135/95,19 and in accordance with the European and Spanish applicable current laws.
Quality control, quality assurance and confidentiality
All patient data were treated confidentially; they were recorded in the data collection logbook (DCL) and kept at the disposal of the pertinent health authority.
The principal investigator was responsible for the quality of the data logged in the DCL, which contained a complete and exact record of the patient data obtained during the study.
A study monitor was in charge of checking that the information contained in the DCLs reflected the data recorded in the patients’ clinical records. This verification procedure of the original data was ongoing and was undertaken paying due respect to patients’ confidentiality.
Missing data
Analysis of the primary end point was undertaken with all the randomized patients who had attended at least one follow-up visit (1, 3, 6 and 12 months after intervention).
Safety
All adverse events and serious adverse events, which might be related to the study procedures, were collected, investigated and notified by the investigators during the study period, in accordance with the procedures described in the “Guidelines on medical devices. Clinical investigations: Serious adverse event reporting under Directives 90/385/EEC and 93/42/EEC”.20 The sponsor notified all serious and unexpected adverse events to the ethics committee and the regulatory authorities.
Discussion
The aim of this study was to test the non-inferiority of PAE vs. TURP for LUTS in terms of safety and efficacy. This study also helped to define the profile of best candidates for PAE and analyzed the benefits and complications associated with this new technique.
However, it should be taken into account that the study had certain limitations such as its single-center design, the small patient sample and the medium-term follow-up period; in addition, prostate size was measured by ultrasound rather than by prostate magnetic resonance (MR) imaging; this study was limited to patients aged >60 years, and the inclusion of patients was not based on prostate size but on the urologist’s selection of candidates to be randomized to TURP or PAE.
Acknowledgments
We thank Berta Ibañez, Arkaitz Galbete and Ferran Capdevilla at the Navarrabiomed, Arturo Fueyo at the Biocompatibles UK Ltd, Dr. Vicente Grasa Lanau, Dr Francisco Lameiro Couso at Navarra Hospital Complex and Meisys. Biocompatibles UK Ltd provided part of the study funding.
Disclosure
The authors report no conflicts of interest in this work.
References
SEMERGEN. Documentos Clínicos SEMERGEN – Urología. Edicomplet; 2008, Madrid, Spain. | ||
Roehrborn CG. Male lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH). Med Clin North Am. 2011;95(1):87–100. | ||
Macey MR, Raynor MC. Medical and surgical treatment modalities for lower urinary tract symptoms in the male patient secondary to benign prostatic hyperplasia: a review. Semin Intervent Radiol. 2016;33(3):217–223. | ||
Michel MC, Mehlburger L, Bressel HU, Schumacher H, Schäfers RF, Goepel M. Tamsulosin treatment of 19,365 patients with lower urinary tract symptoms: does co-morbidity alter tolerability? J Urol. 1998;160(3 pt 1):784–791. | ||
Albisinni S, Biaou I, Marcelis Q, Aoun F, De Nunzio C, Roumeguère T. New medical treatments for lower urinary tract symptoms due to benign prostatic hyperplasia and future perspectives. BMC Urol. 2016; 16(1):58. | ||
Pinheiro LC, Martins Pisco J. Treatment of benign prostatic hyperplasia. Tech Vasc Interv Radiol. 2012;15(4):256–260. | ||
Sønksen J, Barber NJ, Speakman MJ, et al. Prospective, randomized, multinational study of prostatic urethral lift versus transurethral resection of the prostate: 12-month results from the BPH6 study. Eur Urol. 2015;68(4):643–652. | ||
Ahyai SA, Gilling P, Kaplan SA, et al. Meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic enlargement. Eur Urol. 2010;58:384. | ||
Lourenco T, Armstrong N, N’Dow J, et al. Systematic review and economic modelling of effectiveness and cost utility of surgical treatments for men with benign prostatic enlargement. Health Technol Assess. 2008;12(35):iii,ix–x,1–146,169–515. | ||
DeMeritt JS, Elmasri FF, Esposito MP, Rosenberg GS. Relief of benign prostatic hyperplasia-related bladder outlet obstruction after transarterial polyvinyl alcohol prostate embolization. J Vasc Interv Radiol. 2000;11(6):767–770. | ||
Carnevale FC, Antunes AA, da Motta Leal Filho JM, et al. Prostatic artery embolization as a primary treatment for benign prostatic hyperplasia: preliminary results in two patients. Cardiovasc Intervent Radiol. 2010;33(2):355–361. | ||
Pisco JM, Rio Tinto H, Campos Pinheiro L, et al. Embolisation of prostatic arteries as treatment of moderate to severe lower urinary symptoms (LUTS) secondary to benign hyperplasia: results of short- and mid-term follow-up. Eur Radiol. 2013;23(9):2561–2572. | ||
McWilliams JP, Kuo MD, Rose SC, et al. Society of Interventional Radiology position statement: prostate artery embolization for treatment of benign disease of the prostate. J Vasc Interv Radiol. 2014;25(9):1349–1351. | ||
Lang EK, Deutsch JS, Goodman JR, Barnett TF, Lanasa JA, Duplessis GH. Transcatheter embolization of hypogastric branch arteries in the management of intractable bladder hemorrhage. J Urol. 1979;121(1):30–36. | ||
Gao YA, Huang Y, Zhang R, et al. Benign prostatic hyperplasia: prostatic arterial embolization versus transurethral resection of the prostate – a prospective, randomized, and controlled clinical trial. Radiology. 2014;270(3):920–928. | ||
Bilhim T, Bagla S, Sapoval M, Carnevale FC, Salem R, Golzarian J. Prostatic arterial embolization versus transurethral resection of the prostate for benign prostatic hyperplasia. Radiology. 2015;276(1):310–311. | ||
Medical Technologies Guidance. The TURP System for Thransurethral Resection of the Prostate. NICE; 2015, London, UK. | ||
World Medical Association. Declaration of Helsinki. World Medical Association; 1964, Helsinki, Finland. | ||
Good Clinical Practice Guidelines. International Conference of Harmonization. 1996, Geneva, Switzerland. | ||
Guidelines on Medical Devices. Clinical Investigations: Serious Adverse Event Reporting under Directives 90/385/EEC and 93/42/ECC. Brussels, Belgium: European Commission; 2015. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.