Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 12
Prevalence rates of histopathologic subtypes associated with steroid resistance in childhood nephrotic syndrome in Sub-Saharan Africa: a systematic review
Authors Uwaezuoke SN , Ndu IK , Mbanefo NR
Received 2 March 2019
Accepted for publication 22 May 2019
Published 8 July 2019 Volume 2019:12 Pages 167—176
DOI https://doi.org/10.2147/IJNRD.S207372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Samuel N Uwaezuoke,1 Ikenna K Ndu,2 Ngozi R Mbanefo1
1Pediatric Nephrology Firm, Department of Pediatrics, College of Medicine, University of Nigeria Nsukka/University of Nigeria Teaching Hospital, Ituku-Ozalla, Enugu, Nigeria; 2Department of Pediatrics, Enugu State University Teaching Hospital, Enugu, Nigeria
Introduction: The prevalence rates of the common histopathologic subtypes of childhood nephrotic syndrome associated with steroid resistance appear to be changing globally. In Sub Saharan Africa (SSA), the trend is similar over the past few decades.
Aim: This systematic review aims to determine the current prevalence rates of the histopathologic subtypes associated with childhood steroid-resistant nephrotic syndrome (SRNS) in SSA.
Methods: A search of the PubMed, Google and African Journals Online databases was conducted from January to December 2018 using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow-chart to identify relevant articles which met the aim of the systematic review. A qualitative synthesis and descriptive analysis of the extracted data were then conducted. The mean values for the prevalence rates of the reported histopathologic subtypes were calculated. A meta-analysis was not done due to few numbers of studies reviewed. The review is registered with PROSPERO, number CRD42018111916.
Results: In the West African sub-region, the currently reported histopathologic subtypes associated with childhood nephrotic syndrome are focal segmental glomerulosclerosis (FSGS), minimal-change nephropathy (MCN), membrano-proliferative glomerulonephritis (MPGN), membranous nephropathy (MN) and mesangial proliferative glomerulonephritis (MesPGN). The picture is the same in South Africa. More importantly, the predominant histopathologic lesions associated with steroid resistance are FSGS (West Africa) and MCN/FSGS (South Africa), with mean prevalence rates of 57.2% and 36.1% respectively.
Conclusion: The prevalence of FSGS is currently high in childhood nephrotic syndrome in SSA. This histopathologic subtype remains the commonest lesion associated with SRNS in this part of the globe.
Keywords: steroid-resistant nephrotic syndrome, children, histopathologic subtypes, focal segmental glomerulosclerosis, prevalence, Sub-Saharan Africa
Introduction
Childhood nephrotic syndrome consists of the tetrad of “nephrotic-range” proteinuria (urine protein excretion of more than 40 mg/m2/hour), hypoalbuminemia, generalized edema, and hyperlipidemia. It is the most common manifestation of glomerular disease in children.1 Childhood nephrotic syndrome is now broadly classified into congenital, infantile, idiopathic and secondary forms.2 Congenital nephrotic syndrome is seen in the first 3 months of life while the infantile form occurs from 3 to 12 months. Secondary nephrotic syndrome is caused by extra-renal diseases such as systemic lupus erythematosus (SLE), quartan malaria, hepatitis B & C etc. When the syndrome does not fit into any of these forms, it is referred to as idiopathic nephrotic syndrome.2 Although the common variants of idiopathic nephrotic syndrome consist of membrano-proliferative glomerulonephritis (MPGN), membranous nephropathy (MN), and mesangial proliferative glomerulonephritis (MesPGN), the major histopathologic subtypes are minimal change nephropathy (MCN) and focal segmental glomerulosclerosis (FSGS). In fact, MCN and FSGS are thought to occupy the extreme ends of the same disease spectrum. For instance, their electron microscopy findings are similar whereas their light microscopy findings are different.3 Interestingly, FSGS has been further classified into primary (idiopathic) FSGS and secondary FSGS.4 Whereas the former remains a diagnosis of exclusion, the latter is made up of virus/parasite-associated forms (such as HIV1, Plasmodium, Schistosoma mansoni), drug-induced forms, forms mediated by adaptive functional-structural responses and familial/genetic forms.5–10
In the Western world, majority of pre-adolescent children reportedly have MCN on histology: the presumed most common histopathologic subtype in childhood.11,12 Children with MCN frequently achieve remission with oral corticosteroids and thus have the steroid-sensitive nephrotic syndrome (SSNS), which has a good prognosis.13 In contrast, FSGS is characterized by poor response to corticosteroids and subsequent progression to end-stage kidney disease (ESKD).14 Only 20% of patients with FSGS may achieve remission with oral corticosteroids,14 and thus majority who do not respond to these drugs are identified as having the steroid-resistant nephrotic syndrome (SRNS). In spite of the initial steroid-response rate of about 95% in SSNS, frequent relapses can occur in 20–60% of the patients with subsequent development of steroid dependence.15 Therefore, the major challenges in the treatment of SSNS include frequent relapses, steroid dependence and possible steroid resistance.16
Although both MCN and FSGS are regarded as more prevalent lesions in children globally,1 within Sub-Saharan Africa (SSA) there are regional differences in the prevalence of other histopathologic subtypes and a change in their relative frequencies reported over the years.17–20 For instance, a recent study in western Nigeria noted a change in the predominant histopathologic subtype from Quartan Malarial Nephropathy (QMN) five decades ago to MPGN three decades ago, and to FSGS till date.18 In addition, FSGS was found to be the most common histopathologic subtype associated with SRNS.18 In northern Nigeria, the documented predominant subtypes were QMN approximately two decades ago,21 and FSGS about four years ago.22 A recent study from eastern Nigeria, however, reported the predominant subtypes as MCN, FSGN, and MPGN.23 Elsewhere in the West African sub-region, other authors similarly observed a predominance of FSGS and MCN in Ghana about thirteen years ago.19 In Southern Africa, MCN, FSGS and membranous nephropathy (MN) were more frequently reported in the Republic of South Africa in that order about two decades ago.20 However, another report by the same authors in a retrospective study which extended to 2004 (about fifteen years ago) showed that MCN and FSGS ranked high in the common histopathologic subtypes found to be steroid resistant.24
Because of the changing trend in the prevalent histopathologic subtypes associated with steroid resistance, a systematic review of the literature (published within the last two decades) was conducted to establish their current prevalence rates in SSA. With the discovery of genetic forms of FSGS and the ongoing genetic-based research in this region, data on prevalence rates of these histopathologic subtypes may change in future and may necessitate a follow-up systematic review.
Methods
Literature search strategy and inclusion criteria
A search of the PubMed, Google and African Journals Online databases was conducted from January to December 2018 using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart to identify relevant articles which met the aim of the systematic review. A combination of relevant terms and names of the sub-regions within SSA (West Africa, East Africa, Central Africa, and Southern Africa) was used. Terms used for “steroid-resistant nephrotic syndrome” included “idiopathic nephrotic syndrome”, “steroid-unresponsiveness” and “frequent relapses”. For “children,” terms like “preschool child”, “school-aged child”, “adolescent” and “pediatric” were entered for the search. Articles included in the review were either cross-sectional prospective studies or retrospective studies of children and adolescents aged 1–18 years who were residents in the countries within SSA. In addition, the eligible studies (published in English language) were conducted from January 1997 to December 2017. Case reports, Letters to the Editor and Review articles were however excluded. These bibliographic database searches were supplemented by scrutinizing references of the selected original articles in order to identify additional sources of data.
Data extraction and analysis
The following data were extracted from the original articles that fulfilled the inclusion criteria: first author’s name, year of publication, period of study, country of study, study design, sample size, age range of subjects and their mean age, the histopathologic subtypes and their prevalence rates, the administered immunosuppressive medications, as well as the patterns of remission. The methodological quality of each selected article was evaluated using the Newcastle-Ottawa quality assessment scale (adapted for cross-sectional studies).25 The assessment scale involves the assignment of stars (as a measure of quality) based on 3 domains: (i) selection of study groups (maximum 5 stars), (ii) comparability of groups (maximum 2 stars), and (iii) ascertainment of outcome (maximum 3 stars). A cross-sectional study was rated low, moderate or high quality if ≤3 stars, 4–7 stars and ≥8 stars respectively were assigned. Any inter-rater variability of the study quality was resolved by consensus between two of the authors (SNU and IKN). A qualitative synthesis and descriptive analysis of the extracted data were then conducted. The mean values for the prevalence rates of the histopathologic subtypes were calculated. A meta-analysis was not done due to the few numbers of studies reviewed.
Results
Using the bibliographic databases (PubMed, Google, and AJOL), a total of 27 full-text articles were identified. After removal of duplicates, 24 articles were left (Figure 1). Screening of these articles led to the exclusion of 6 articles unrelated to the topic under review. Eighteen (18) full-text articles were then assessed for eligibility. A total of 10 articles which comprised review articles, case reports and letters to the Editor were further excluded leaving behind a total of 8 articles/studies for the review. The methodological quality and findings of each of the 8 studies are summarized in Table 1. All eight studies were cross-sectional and retrospective. Five of them were conducted in the West African sub-region: four emanating from Nigeria18,22,26,27 and one from Ghana.19 In Central Africa, one study was conducted in the Democratic Republic of Congo,28 while two studies were conducted in the Republic of South Africa.20,24
 |
Table 1 Steroid-resistant nephrotic syndrome (SRNS) in children in Sub-Saharan Africa: summary of findings |
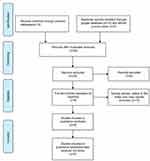 |
Figure 1 Steroid-resistant nephrotic syndrome (SRNS) in children in Sub-Saharan Africa: PRISMA flow chart on literature search and outcome. |
Characteristics and findings of the studies
West African sub-region
The methodological quality of the study by Asinobi et al in western Nigeria18 is rated moderate (7 stars). There were two study groups: pretreatment biopsy-era group- from 1997 to 2001 and posttreatment biopsy-era group- from 2006 to 2013. All nephrotic syndrome patients in the former group (n=58) were biopsied before treatment while in the latter group (n=106), patients were biopsied after treatment based on specific indications namely; steroid resistance, steroid dependence, frequent relapses, secondary nephrotic syndrome and atypical presentation such as gross hematuria, azotemia, hypertension, and intermittent gross proteinuria. However, 78 patients had successful biopsies out of 106 patients in the posttreatment biopsy-era group: giving a biopsy success rate of about 73.6%. The age range of patients in the two study groups was 21/2 to 16 years with a calculated mean age of 8.9±4.0 years for the pretreatment biopsy-era group and 8.3±3.7 years for the posttreatment biopsy-era group. In both groups, the overall prevalence rates for the subtypes determined on light microscopy were 39.8% for FSGS, 20.5% for MPGN, 19.2% for MCN and 20.5% for others. Specifically, the prevalence rates of the histopathologic subtypes seen in the post-treatment biopsy era were reported as 47.6% for FSGS, 19% for MPGN and 19% for MCN. Thirty patients who had SRNS were distributed in the following order of frequency with respect to histopathologic subtypes: FSGS (60%), MPGN (27%), MCN (3%) and others (10%). Second or third-line immunosuppressive drugs (such as cyclosporine A, mycophenolate mofetil, and rituximab) were administered to these patients.
For the study by Obiagwu et al in northern Nigeria,22 the methodological quality is rated low (3 stars). The period covered by this retrospective study was not stated by the authors. Only 20 patients who had renal biopsy were included in the study. The age range of the patients was 2.5–13 years with a peak age of 7–8 years and a mean age of 8.3±3.0 years. Of the 11 patients with SRNS, the following histopathologic subtypes on light microscopy and their frequencies were reported: FSGS (54.5%), MCN (27.3%), MN (9.1%) and MPGN (9.1%). However, in the study population (n=20), the frequencies of these histopathologic subtypes were noted as follows: FSGS (40%), MCN (20%), MPGN (15%), MN (15%) and others (10%). The patients received only steroids as the authors did not indicate any other immunosuppressive drugs.
The methodological quality of another study in western Nigeria by Olowu et al26 is rated high (8 stars). The authors conducted a retrospective study of 23 patients (aged between 2–16 years) diagnosed with SRNS (between January 2001 and December 2007). Their clinicopathological features were described, and the outcome was determined based on response to the authors’ treatment protocols. The mean age of their patients at diagnosis was 8.3±3.5 years (age range =2.1–13 years). The histopathologic lesions on light microscopy and their prevalence rates were reported as follows: MPGN (43.5%), FSGS (39.1%), MesPGN (8.7%), MCN (4.35%) and MN (4.35%). Patients were exposed to three treatment protocols viz routine treatment protocol (intravenous dexamethasone plus intravenous cyclophosphamide as induction therapy and oral prednisolone plus oral cyclophosphamide as follow-up therapy); add-on routine treatment (addition of lisinopril or spironolactone in the absence of complete remission 3 months after follow-up therapy of the routine treatment protocol); and non-routine treatment (oral prednisolone plus either lisinopril or oral cyclophosphamide). The overall treatment response was 90.45% (complete remission =57.12% and partial remission =33.33%).
The last Nigerian study under review was also conducted in the western part of the country by Ladapo et al,27 and its quality is assessed as moderate (5-star rating). It was a retrospective review of the short-term treatment outcomes of children with SRNS on cyclosporine compared to previous outcomes with other immunosuppressive drugs such as cyclophosphamide. Out of the 103 children with nephrotic syndrome diagnosed from 2009 to 2014, 25 patients (24.3%) who had steroid resistance (SRNS) were assessed by the authors. The patients were aged between 0.6−15.2 years with a median age of 8.8 years. Among 10 of these children who were successfully biopsied, the frequency of the histopathologic lesions on light microscopy was reported as follows: FSGS (70%), MCN (20%) and MPGN (10%). Remarkably, the complete remission rate for cyclosporine was 70% in comparison with 40% for cyclophosphamide.
Outside Nigeria, another study in the West African sub-region conducted in Ghana by Doe et al19 is rated as a low-quality study (3 stars). A total of 32 patients, aged 1–18 years (median =12 years) and treated from 2000 to 2003, were retrospectively studied. Thirteen of them were successfully biopsied with these histopathologic lesions on light microscopy: FSGS (76.9%), and MCN (15.4%). There was a 31.25% response rate of patients with SRNS to administered immunosuppressive drugs (intravenous methylprednisolone pulses or oral cyclophosphamide).
The calculated mean prevalence rates of MCN, FSGS, MPGN and MN among children with SRNS are 14.0%, 57.2%, 22.4%, and 6.73% respectively (Table 1).
Central Africa
In the Democratic Republic of Congo, Aloni et al28 conducted another retrospective study whose quality is adjudged low (3 stars). The authors described the clinical profile and management of children with SRNS in a setting reportedly bedeviled with a dearth of laboratory facilities and pharmaceutical supplies. Out of a total of 62 children (aged 1.5–13 years) diagnosed with nephrotic syndrome and seen from 1983 to 2008, 16 (25.8%) had SRNS. The histopathologic subtypes were however not stated.
Southern Africa
Bhimma et al in the Republic of South Africa published 2 retrospective studies in 199720 and 2006.24 In the first study acclaimed as the largest reported series of nephrotic syndrome among children in Africa (rated moderate quality with 5 stars), the authors retrospectively reviewed their 20 year-experience with 636 children diagnosed with nephrotic syndrome. These children who were categorized into 3 racial groups namely Blacks, Indians and Colored. Among the black children (n=306), the histopathologic subtypes were MN (40%), MCN (56.3%) and FSGS (28.3%): all cases of FSGS being steroid-resistant. For Indian children (n=307), the authors noted biopsy-proven MCN (46.8%), 94.8% of which were steroid-sensitive; and FSGS (20.6%), 95.6% of which were steroid-resistant. The histopathologic subtypes among colored children (n=23) were FSGS (43.5%), MN (26.1%) and biopsy-proven steroid-sensitive MCN (21.7%). In the second study whose quality is rated high (8 stars), a retrospective review of 223 children with SRNS managed from 1976–2004 (aged 1–16 years) was conducted by the same authors. A comparison of the clinical and biochemical characteristics, as well as the outcome of four treatment groups was made. These histopathologic subtypes namely MCN (36.1%), FSGS (36.1%), and MesPGN (8.2%) were assigned to treatment groups comprising oral cyclophosphamide plus prednisolone (n=90), oral prednisolone alternating with oral methylprednisolone plus oral cyclophosphamide (n=117), alternate-day oral prednisolone plus 3 doses of alternate-day intravenous methylprednisolone plus monthly intravenous cyclophosphamide (n=10) and oral cyclosporine (n=6). Interestingly, there was a significant difference in response to the treatment regimens between Indian and black children. Sixty-nine percent of biopsied Indian children achieved complete remission: 72.5% of them to oral cyclophosphamide plus prednisolone. In contrast, 20.2% of biopsied black children achieved complete remission but none to oral cyclophosphamide plus prednisolone. However, 80% of non-biopsied Indian children achieved a complete remission to this treatment regimen.
Discussion
In this systematic review of 8 studies from SSA, FSGS was consistently on top of the list of histopathologic subtypes associated with SRNS in black children.18,19,22–24,26,27 In nephrotic children, steroid resistance generally appears to be the hallmark of FSGS as a renal biopsy is usually performed in the event of treatment failure with corticosteroids. The prevalence of FSGS, relative to other histopathologic subtypes, seems to be increasing globally, especially in the Western world and Asia.29–33 For instance in Nigeria, the most frequent cause of nephrotic syndrome has changed from QMN (in the 1960s) to MPGN (in the 1980s) to FSGS (till date).18 Specifically, FSGS is the most prevalent histopathologic subtype among children with nephrotic syndrome in Nigeria,18,22,26,27 and in Ghana as well.19 On the other hand, QMN is becoming extinct or non-existent in Nigeria according to a prospective study conducted a decade ago.34 A recent review underscores this finding which indicates that a transition has probably occurred from QMN through MPGN to FSGS in Nigeria.35 Although the factors responsible for the increased prevalence of FSGS are not yet clearly understood, it may be due to improved diagnosis, as well as the absolute rise in the incidence of adaptive FSGS worsened by obesity and chronic inflammation.36 For instance, improved expertise in renal biopsies may have been contributory to this rising prevalence rate, given that FSGS lesions may easily be missed if cortical glomeruli are biopsied, instead of targeting deep juxtamedullary glomeruli as well as obtaining a large glomerular yield.1,4,36–39 Recently, FSGS has been categorized into six forms: primary or idiopathic FSGS, adaptive FSGS (the two common forms), high-penetrance genetic FSGS, viral-mediated FSGS, and medication-associated FSGS (the three less common forms) as well as the newly identified APOL1-associated FSGS.40,41 Within these FSGS categories, emerging evidence indicates that the link with APOL1gene identifies a group most likely to end up with ESKD.40
Among children with SRNS in this systematic review, the calculated mean FSGS prevalence rate of about 57.2% (based on the figures reported within the West African sub-region) is consistent with the rates of 42.0–72.0% noted among African-American children with SRNS in the United States.42,43 This is not surprising as the black race is considered a risk factor for FSGS because of the prevalent APOL1 gene in individuals with sub-Saharan ancestry.44,45 Specifically, there is a rapid progression of FSGS to ESKD in black children when compared to other racial groups.46,47 This underscores the poor prognosis associated with this histopathologic subtype, especially in black children: more so with the likelihood of APOL 1-associated FSGS occurring in this racial group. Elsewhere in North Africa, a single-center retrospective study in Egypt however reported a comparatively low FSGS prevalence rate of 7–20% of all pediatric patients with nephrotic syndrome.48 Interestingly, despite the high rate of steroid resistance in this series, the authors reported a good overall renal-survival rate.48 This observation further buttresses the reported effect of racial factor on the outcome of FSGS.
Furthermore, this systematic review has shown that the calculated mean prevalence rates of MPGN, MCN, and MN among children with SRNS in the West African sub-region are 22.4%, 14.0%, and 6.73% respectively. However, the picture in South Africa appears different as the distribution of the histopathologic subtypes was reported in a single study as follows: FSGS (36.1%), MCN (36.1%), and MesPGN (8.2%).43 In contrast, children with SRNS in the developed world have the following histopathologic subtypes on light microscopy: FSGS (63–73%), MCN (0–15%), diffuse mesangial sclerosis (3–15%), and IgM nephropathy (3–15%).49,50 More importantly, these causes of SRNS account for 5–20% of all pediatric patients with ESRD.51 Again, this shows that SRNS in children, irrespective of the underlying histopathologic subtype, most likely ends up with ESKD. While there are obvious differences between SSA and the Western world with respect to the prevalence rates of the histopathologic subtypes like diffuse mesangial sclerosis and IgM nephropathy, the prevalence rate of steroid-resistant MCN appears similar in these two regions.
MCN generally responds well to steroids with over 90% response rate while steroid resistance occurs in 7–10% of cases.12 Its mean prevalence rate of about 14% among SRNS patients in West Africa agrees with this well-established figure. But in South Africa, MCN appears to have the same frequency and steroid-resistance rate with FSGS.43 Historically, the degenerative glomerular lesions of FSGS were those observed in the progression of MCN (hitherto known as “lipoid nephrosis”), and it was later noted that these patients with MCN had an accelerated clinical trajectory (with possible transformation to FSGS).52 This may explain the similarity in the frequencies of both histopathologic subtypes, although this conclusion remains conjectural. Nevertheless, the rising prevalence rate of steroid-resistant FSGS in South Africa and in the West African sub-region, could be attributed to the current pandemic of the human immunodeficiency virus (HIV). In virus-associated FSGS, HIV-1 is the most implicated virus which is strongly linked with this histopathologic subtype (especially the collapsing glomerular variant of FSGS) as it directly infects the podocytes.5,53 Interestingly, the effect of HIV on podocytes is strongest in individuals with two APOL1 risk alleles, with a high odds ratio reported in South Africa.53 Remarkably, subjects with HIV-associated nephropathy (HIVAN) have one or two APOL1 risk alleles.49 Although APOL1-associated FSGS constitutes a major type of FSGS in SSA, the effect of APOL1 is mainly recessive, needing two risk alleles. But in HIV-positive South Africans, a single copy of a risk allele has a significant link with HIVAN.53
Recently, genetic/familial FSGS is increasingly being documented as several genes have been described for this form of the disease.10,54 However, in SSA there appears to be paucity of genetic investigations for childhood nephrotic syndrome due to inadequate human and financial resources.35 In fact only one study has reported the genetic evaluation of nephrotic syndrome in Nigerian children.55 The authors genetically evaluated two siblings with familial FSGS. Although the sequencing analysis was conducted outside the country, sequencing of both exons of NPHS2, WT1, and APOL1 did not reveal the presence of common mutations in these genes: prompting the authors to conclude that undiscovered gene mutations may account for FSGS in SSA.55 Nevertheless, to bridge these diagnostic gaps, the Human Heredity and Health (H3) Africa Kidney Disease Research Network (H3A-KDRN) has initiated a project to provide data on a cohort of children with nephrotic syndrome from SSA countries such as Ghana, Nigeria, Tanzania, South Africa, and Cameroon.56 The H3A-KDRN will establish if clinical, infectious, and genetic factors are responsible for differences in first steroid response, steroid resistance, frequent relapse/steroid dependence, and a four-year chronic kidney disease-progression trajectory in childhood nephrotic syndrome.56
Another prominent histopathologic subtype associated with SRNS in SSA is MPGN (or mesangiocapillary glomerulonephritis). It was particularly reported in all the studies from Nigeria.18,22,23,26,27 MPGN has a documented steroid-response rate of only 7% and thus has a high rate of steroid resistance.12 In one of the studies in Western Nigeria, its frequency in cases of SRNS surpassed that of FSGS.26 In fact, both histopathologic subtypes (FSGS and MPGN) are frequently associated with steroid resistance.57 Idiopathic MPGN is regarded as one of the least common types of glomerulonephritis which account for about 4–7% of primary renal causes of nephrotic syndrome in childhood and adulthood respectively.58 Despite the decline in its incidence in developed countries,59 it has been reported as one of the most frequent histopathologic subtypes in biopsied children with nephrotic syndrome both in Nigeria,60 and Turkey.61 Like FSGS, it progresses to ESKD although it has a slowly progressive clinical course: with 2.8% of ESKD in children on dialysis and 3.3% of ESRD in pediatric kidney transplant recipients caused by MPGN.62
Finally, MN appears to be the least common of the histopathologic subtypes associated with idiopathic SRNS in SSA. While some authors from the West African sub-region failed to document any case of MN in their series,18,19,27 others reported the least prevalence rates in comparison with other histopathologic subtypes.22,26 This finding agrees with the report from a recent study in Eastern Europe which noted prevalence rates of 36.4%, 31.8%, 13.6% and 4.5% for MCN, FSGS, MPGN, and MN respectively in children with SRNS.63 However, it differs from the high prevalence rate reported among black children with nephrotic syndrome in a South African study.20 It should be noted that this study was a retrospective review of all cases of childhood nephrotic syndrome irrespective of treatment responses. Interestingly, a later study by the same South African authors which explored the treatment responses of the histopathologic subtypes associated with SRNS suggested a low prevalence rate.24
Conclusion
The pattern of the major histopathologic subtypes seen in childhood SRNS has changed over the years in SSA. The prevalence of FSGS is currently high in this region, which is similar to the picture in other parts of the world. In fact, it remains the commonest histopathologic lesion associated with SRNS in SSA, although other histopathologic subtypes like MPGN and MCN have also been reported with high prevalence figures. There is a need to improve facilities for diagnostic nephrology in this part of the world (such as genetic testing, electron microscopy and immunofluorescent microscopy) in order to promote ongoing research on this subject. With the discovery of genetic forms of FSGS and the current H3A-KDRN study, data on prevalence rates of these histopathologic subtypes in SSA are obviously still evolving and may thus warrant a follow-up systematic review
Limitations of the systematic review
The use of the Newcastle-Ottawa quality assessment scale, originally designed for case-control studies (but adapted for use in cross-sectional studies), may have compromised the quality rating of the included studies: which were all retrospective. Secondly, the few numbers of reviewed studies precluded a meta-analysis of the reported prevalence rates
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi:10.1016/S0140-6736(03)14184-0
2. Lane JC, Langman CB Pediatric nephrotic syndrome. Available from: https://emedicine.medscape.com/article/982920-overview.
3. Downie ML, Gallibois C, Parekh RS, Noone DG. Nephrotic syndrome in infants and children: pathophysiology and management. Paediatr Int Child Health. 2017;37(4):248–258. doi:10.1080/20469047.2017.1374003
4. Kiffel J, Rahimzada Y, Trachtman H. Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv Chronic Kidney Dis. 2011;18(5):332–338. doi:10.1053/j.ackd.2011.03.005
5. Marras D, Bruggeman LA, Gao F, et al. Replication and compartmentalization of HIV-1 in kidney epithelium of patients with HIV-associated nephropathy. Nat Med. 2002;8:522–526. doi:10.1038/nm0502-522
6. Chandra P, Kopp JB. Viruses and collapsing glomerulopathy: a brief critical review. Clin Kidney J. 2013;6:1–5. doi:10.1093/ckj/sft002
7. Mohamed N, Goldstein J, Schiff J, John R. Collapsing glomerulopathy following anthracycline therapy. Am J Kidney Dis. 2013;61:778–781. doi:10.1053/j.ajkd.2012.08.048
8. Wickman C, Kramer H. Obesity and kidney disease: potential mechanisms. Semin Nephrol. 2013;33:14–22. doi:10.1016/j.semnephrol.2012.12.006
9. Ikezumi Y, Suzuki T, Karasawa T, et al. Low birthweight and premature birth are risk factors for podocytopenia and focal segmental glomerulosclerosis. Am J Nephrol. 2013;38:149–157. doi:10.1159/000353898
10. Tae-Sun H. Genetics of hereditary nephrotic syndrome: a clinical review. Korean J Pediatr. 2017;60(3):55–63. doi:10.3345/kjp.2017.60.3.55
11. Churg J, Habib R, White RH. Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet. 1970;760:1299–1302.
12. International Study of Kidney Disease in Children. Nephrotic syndrome in children. Prediction of histopathology from clinical and laboratory characteristics at the time of diagnosis. Kidney Int. 1978;13:15–65.
13. Trumpeter RS, Lloyd BW, Hicks J, White RH, Cameron JS. Long-term outcome for children with minimal change nephrotic syndrome. Lancet. 1985;1:368–370.
14. International Study of Kidney Disease in Children. Primary nephrotic syndrome in children. Clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. Kidney Int. 1981;20:765–771.
15. Teeninga N, Kist-van Holthe JE, Nauta J. Extending prednisolone treatment does not reduce relapses in childhood nephrotic syndrome. J Am Soc Nephrol. 2012;24:149–159. doi:10.1681/ASN.2012070646
16. Uwaezuoke SN. Steroid-sensitive nephrotic syndrome in children: triggers of relapse and evolving hypotheses on pathogenesis. Italian J Pediatr. 2015;41:19. doi:10.1186/s13052-015-0123-9
17. Olowu WA, Ademola A, Ajite AB, Saad YM. Childhood nephrotic syndrome in tropical Africa: then and now. Paediatr Int Child Health. 2017;37:259–268. doi:10.1080/20469047.2017.1374002
18. Asinobi AO, Ademola AD, Okolo CA, Yaria JO. Trends in the histopathology of childhood nephrotic syndrome in Ibadan Nigeria: preponderance of idiopathic focal segmental glomerulosclerosis. BMC Nephrol. 2015;16:213. doi:10.1186/s12882-015-0208-0
19. Doe JY, Funk M, Mengel M, Doehring E, Ehrich JHH. Nephrotic syndrome in African children: lack of evidence for ‘tropical nephrotic syndrome’? Nephrol Dial Transplant. 2006;21:672–676. doi:10.1093/ndt/gfi297
20. Bhimma R, Coovadia HM, Adhikari M. Nephrotic syndrome in South African children: changing perspectives over 20 years. Pediatr Nephrol. 1997;11:429–434.
21. Abdurrahman MB, Babaoye FA, Aikhionbare HA. Childhood renal disorders in Nigeria. Pediatr Nephrol. 1990;4:88–93.
22. Obiagwu PN, Aliyu A, Atanda AT. Nephrotic syndrome among children in Kano: A clinicopathological study. Niger J Clin Pract. 2014;17:370–374. doi:10.4103/1119-3077.130247
23. Uwaezuoke SN, Okafor HU, Eneh CI, Odetunde OI. The triggers and patterns of relapse in childhood idiopathic nephrotic syndrome: a retrospective, descriptive study in a tertiary hospital, south-east Nigeria. J Clin Nephrol Res. 2016;3(1):1032.
24. Bhimma R, Adhikari M, Asharam K. Steroid-resistant nephrotic syndrome: the influence of race on cyclophosphamide sensitivity. Pediatr Nephrol. 2006;21(12):1847–1853. doi:10.1007/s00467-006-0276-2
25. Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomized intervention studies. Health Technol Assess. 2003;7:27. doi:10.3310/hta7270
26. Olowu WA, Adelusola KA, Adefehinti O. Childhood idiopathic steroid-resistant nephrotic syndrome in Southwestern Nigeria. Saudi J Kidney Dis Transpl. 2010;21(5):979–990.
27. Ladapo TA, Esezobor CI, Lesi FE. Cyclosporine in the treatment of childhood idiopathic steroid-resistant nephrotic syndrome: a single center experience in Nigeria. Pan Afr Med J. 2016;25:258. doi:10.11604/pamj.2016.25.261.2630
28. Aloni MN, Sysleyne LM, Ekulu PM, Babio FL, Ngiyulu RM, Gini-Ehungu JL. The challenges of caring for children with nephrotic syndrome in a tertiary institution in the Democratic Republic of Congo. Acta Paediatr. 2014;103(8):e365–e369. doi:10.1111/apa.12647
29. Gulati S, Sharma AP, Sharma RK, Gupta A. Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis. 1999;34(4):646–650. doi:10.1016/S0272-6386(99)70388-4
30. Srivastava T, Simon SD, Alon US. High incidence of focal segmental glomerulosclerosis in nephrotic syndrome of childhood. Pediatr Nephrol. 1999;13(1):13–18. doi:10.1007/s004670050555
31. Kari JA. Changing trends of histopathology in childhood nephrotic syndrome in Western Saudi Arabia. Saudi Med J. 2002;23(3):317–321.
32. Bonilla-Felix M, Parra C, Dajani T, et al. Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int. 1999;55(5):1885–1890. doi:10.1046/j.1523-1755.1999.00408.x
33. Filler G, Young E, Geier P, Carpenter B, Drukker A, Feber J. Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis. 2003;42(6):1107–1113.
34. Olowu WA, Adelusola KA, Adefehinti O, Oyetunji TG. Quartan malaria-associated childhood nephrotic syndrome: now a rare clinical entity in malaria-endemic Nigeria. Nephrol Dial Transplant. 2010;25(3):794–801. doi:10.1093/ndt/gfp536
35. Anigilaje EA, Olutola A. Prospects of genetic testing for steroid-resistant nephrotic syndrome in Nigerian children: a narrative review of challenges and opportunities. Int J Nephrol Renovasc Dis. 2019;12:119–136. doi:10.2147/IJNRD.S193874
36. Rosenberg AZ, Kopp JB. Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2017;12. doi:10.2215/CJN.05960616
37. Gulati S, Sharma AP, Sharma RK, Gupta A, Gupta RK. Do current recommendations for kidney biopsy in nephrotic syndrome need modifications? Pediatr Nephrol. 2002;17(6):404–408. doi:10.1007/s00467-002-0840-3
38. Corwin HL, Schwartz MM, Lewis EJ. The importance of sample size in the interpretation of the renal biopsy. Am J Nephrol. 1988;8(2):85–89. doi:10.1159/000167563
39. Schachter AD. Computational simulation of renal biopsy accuracy in focal segmental glomerulosclerosis. Pediatr Nephrol. 2006;21(7):953–957. doi:10.1007/s00467-006-0127-1
40. Kopp JB. Focal segmental glomerulosclerosis and collapsing glomerulopathy syndromes. In: Fadem SZ, editor. Essentials of Chronic Kidney Disease. Hauppauge: Nova Science Publishers; 2015;30–33.
41. Ingulli E, Tejani A. Racial differences in the incidence and renal outcome of idiopathic focal segmental glomerulosclerosis in children. Pediatr Nephrol. 1991;5:393–397. doi:10.1007/BF01453661
42. Boyer O, Moulder JK, Somers MJ. Focal and segmental glomerulosclerosis in children: a longitudinal assessment. Pediatr Nephrol. 2007;22(8):1159–1166. doi:10.1007/s00467-007-0493-3
43. Chanchlani R, Parekh RS. Ethnic differences in childhood nephrotic syndrome. Front Pediatr. 2016;4:39. doi:10.3389/fped.2016.00039
44. Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic APOL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi:10.1126/science.1193032
45. Andreoli SP. Racial and ethnic differences in the incidence and progression of focal segmental glomerulosclerosis in children. Adv Ren Replace Ther. 2004;11(1):105–109. doi:10.1053/j.arrt.2003.10.015
46. Sorof JM, Hawkins EP, Brewer ED, Boydstun II, Kale AS, Powell DR. Age, and ethnicity affect the risk and outcome of focal segmental glomerulosclerosis. Pediatr Nephrol. 1998;12(9):764–768. doi:10.1007/s004670050542
47. Gbadegesin R, Hinkes B, Vlangos C, et al. Mutational analysis of NPHS2 and WT1 in frequently relapsing and steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2007;22:509–513. doi:10.1007/s00467-006-0377-y
48. El-Refaey AM, Bakr A, Hammad A, et al. Primary focal segmental glomerulosclerosis in Egyptian children: a 10-year single-center experience. Pediatr Nephrol. 2010;25(7):1369–1373. doi:10.1007/s00467-010-1448-7
49. Ruf RG, Schultheiss M, Lichtenberger A, et al. Prevalence of WT1 mutations in a large cohort of patients with steroid-resistant and steroid-sensitive nephrotic syndrome. Kidney Int. 2004;66:564–570. doi:10.1111/j.1523-1755.2004.00775.x
50. Kitiyakara C, Eggers P, Kopp JB. The twenty-one-year trend in ESRD due to focal segmental glomerulosclerosis in the United States. Am J Kidney Dis. 2004;44:815–825.
51. Meehan SM, Kim L, Chang A. A spectrum of morphologic lesions of focal segmental glomerulosclerosis by Columbia criteria in human immunodeficiency virus infection. Virchows Arch. 2012;460:429–435. doi:10.1007/s00428-012-1213-3
52. Rich AR. A hitherto undescribed vulnerability of the juxtamedullary glomeruli in lipoid nephrosis. Bull Johns Hopkins Hosp. 1957;100:173–186.
53. Kasembeli AN, Duarte R, Ramsay M, et al. APOL1 risk variants are strongly associated with HIV-associated nephropathy in black South Africans. J Am Soc Nephrol. 2015;26:2882–2890. doi:10.1681/ASN.2014050469
54. Kang HG, Cheong HII. Nephrotic syndrome: what’s new, what’s hot? Korean J Pediatr. 2015;58:275–282. doi:10.3345/kjp.2015.58.8.275
55. Anochie IC, Eke FU, Okpere AN. Familial focal segmental glomerulosclerosis (FSGS) in a Nigerian family and exclusion of mutations in NPHS2, WT1 and APOL1. West Afr J Med. 2012;31:273–276.
56. Rheault MN, Gbadegesin RA. The genetics of nephrotic syndrome. J Ped Gen. 2016;5(1):15–24.
57. NIH awards by location and organization - NIH research Portfolio. Available from: https://report.nih.gov/award/index.cfm?ot/state/fm/orgid/distr/rfa/pid.
58. Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1988;338:1202–1211. doi:10.1056/NEJM199804233381707
59. Alchi B, Jayne D. Membrano-proliferative glomerulonephritis. Pediatr Nephrol. 2010;25:1409–1418. doi:10.1007/s00467-009-1322-7
60. Asinobi AO, Gbadegesin RA, Adeyemo AA, et al. The predominance of membrano-proliferative glomerulonephritis in childhood nephrotic syndrome in Ibadan, Nigeria. West Afr J Med. 1999;18:203–206.
61. Ozkaya N, Cakar N, Ekim M, Kara N, Akkök N, Yalçinkaya F. Primary nephrotic syndrome during childhood in Turkey. Pediatr Int. 2004;46:436–438. doi:10.1111/j.1442-200x.2004.01920.x
62. Warady BA, Hébert D, Sullivan EK, Alexander SR, Tejani A. Renal transplantation, chronic dialysis, and chronic renal insufficiency in children and adolescents. The 1995 annual report of the North American pediatric renal transplant cooperative study. Pediatr Nephrol. 1997;11:49–64.
63. Pokrajac D, Kamber AH, Karasalihovic Z. Children with steroid-resistant nephrotic syndrome: a single center experience. Mater Sociomed. 2018;30(2):84–88. doi:10.5455/msm.2018.30.84-88
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
