Back to Journals » Journal of Blood Medicine » Volume 9
Prevalence, morphological characterization, and associated factors of anemia among children below 5 years of age attending St. Mary’s Hospital Lacor, Gulu District, Northern Uganda
Authors Ocan A, Oyet C , Webbo F , Mwambi B, Taremwa IM
Received 15 August 2018
Accepted for publication 28 September 2018
Published 30 October 2018 Volume 2018:9 Pages 195—201
DOI https://doi.org/10.2147/JBM.S184126
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin Bluth
Apollo Ocan,1 Caesar Oyet,1 Fred Webbo,1,2 Bashir Mwambi,1 Ivan Mugisha Taremwa1
1Institute of Allied Health Sciences, Clarke International University, Kampala, Uganda; 2Lancet Laboratories, Kampala, Uganda
Aim/objective: The aim of this study was to determine the prevalence, severity, morphological characterization, and the associated factors of anemia among children under the age of 5 years at St. Mary’s Hospital Lacor, Gulu District, Northern Uganda.
Materials and methods: A structured questionnaire was administered to each participant’s parent/caregiver to collect data on sociodemographic factors, feeding pattern, and history of chronic illness. Hemoglobin (Hb) estimation was performed using a HemoCue 201+ analyzer. Peripheral thin and thick blood films were made from venous blood and stained with Giemsa to morphologically characterize red blood cells (RBCs) and investigate hemoparasites, respectively. We collected and examined stool specimens from each participant using wet preparations and formol–ether concentration technique for intestinal parasites. Descriptive statistics was used to describe study participants and to determine the prevalence of anemia. Logistic regression analysis was done to determine the factors associated with acquiring anemia at a P-value≤ 0.05.
Results: The study enrolled 343 children below the age of 5 years. Of these, 62.7% (N=215) were females. The IQR, median, and mean Hb levels were 5.1±3.2 g/dL, 8.2 g/dL, and 7.9 g/dL, respectively. Overall, 160 (46.6%, 95% CI: 42.1–51.46) children had anemia. The magnitude of severe, moderate, and mild anemia was 11.9%, 58.8%, and 29.4%, respectively. Morphologic characterization of anemia revealed hypochromic-microcytic (65.4%, N=106), hypochromic-macrocytic (15.4%, N=25), and normochromic-microcytic (19.1%, N=31) anemia. Factors associated with anemia were parasitic infestation, history of chronic disease, lack of complementary foods, complementary feeding for not more than twice a month, and households’ with annual income less than 200,000 Ugandan Shillings.
Conclusion: We report the high prevalence of anemia among children below 5 years of age in Gulu District, Northern Uganda. Thus, strategies geared at addressing the etiologic causes (such as, nutrient deficiency and parasitic infections) are key to reduce it in the region.
Keywords: anemia, associated factors, children below 5 years, Uganda
Introduction
The global epidemiologic burden of anemia has been widely recognized, as one-third of the global population remain anemic.1 According to WHO, childhood anemia is diagnosed when hemoglobin (Hb) levels are below 11.0 g/dL.2 The burden is more among pregnant women and children below the age of 5 years, and with peak during the first 2 years of life.3 Though complex, its causes are ascribed to micronutrient deficiencies like iron deficiency, vitamin B12 deficiency, and folate deficiency and infections like malaria, worm infestations, and a complex interplay of these factors.3
Childhood anemia is of concern as it leads to poor health, physical and mental growth retardations with eventual cognitive difficulties, and increased risk of morbidity and mortality.1,4,5 This phenomenological attribute makes anemia a public health concern, mainly in sub-Saharan Africa, where malaria and nutrient deficiencies are common.1,6,7 This has inexorably necessitated concerted interventions like deworming, malaria presumptive treatment, and provision of fortified foods.4,5,8 Despite the lifesaving maneuvers, numerous research studies conducted in Africa indicate that the prevalence of anemia among children below 5 years remains unacceptably very high. For example, in Northeast Ethiopia, anemia prevalence was found to be 47.4%;9 in Cape Verde, it was reported to be 51.8%;10 28.8% in Kenya;11 43% in Democratic Republic of Congo,6 and 45% in southern Cameroon.12 The burden has been reported even higher at 78.4% in Ghana13 and 77.2% in Tanzania.14 Relatedly, the burden of anemia among under 5-year old children in Uganda was reported to be 67.5%;15 however, this varies with different districts, that is 58.8% in Namutumba District16 and 26.2% in Bushenyi District.7 At the same time, clinical diagnosis remains wanting as children with mild-to-moderate degrees of anemia may go undiagnosed and therefore untreated. This factor in addition to limited data on the burden and associated factors of anemia among children below the age of 5 years in Gulu District, Northern Uganda may complicate both clinical intervention and policy formulation. Thus, the aim of this study was to determine the prevalence and factors associated with anemia among preschool children at St. Mary’s Hospital, Lacor, Gulu District in Northern Uganda.
Materials and methods
Study design and study setting
This was a cross-sectional study, conducted from July to November of 2016 at St. Mary’s Hospital Lacor Pediatric Outpatients’ Department. Founded by Comboni missionaries in 1959, St. Mary’s Hospital Lacor is located about 338 km from Kampala city, 6 km off Gulu town along Juba road in Obiya Village, Bardege subcounty, Gulu Municipality, Northern Uganda. The hospital runs under the management of the Roman Catholic Archdiocese of Gulu. It has a bed capacity of 554, of which 157 are in pediatric ward. It receives approximately 200 cases daily in the outpatient department, of which about 40 are children below 5 years of age.
Sample size estimation, study participants, and sampling
We calculated the sample size of our study using a two-sided 95% binomial CI with a 5% margin of error assuming the national prevalence of anemia as 67.5%.15 Our study participants comprised a total of 343 children under 5 years of age, whose parents or caregivers consented for the study. We excluded participants with obvious clinical anemia, hemorrhage, suspected or confirmed bleeding disorders, known sickle cell disease children, HIV-infected children, those with a blood transfusion history, and/or were operated within the past 3 months. We also excluded children who were too ill and in critical condition. Our participants were enrolled using a consecutive sampling technique. As this study lasted for a duration of 5 months, we ensured that a participant was not repeated.
Data collection and processing
Sociodemographic, socioeconomic, and other related data were obtained using questionnaire-based interview. Our questionnaire comprised the child’s gender, age category in months, history of chronic illness, caregiver’s knowledge of anemia, mothers’ parity, education level attained, average household income, frequency of feeding, and complementary foods. To ensure quality and reliable data, the questionnaire was pilot-tested using 15 caregivers of children under 5 years of age attending Benedict Medical Centre Luzira in Nakawa division, and changes were effected.
Laboratory investigation to determine Hb levels was done using HemoCue HB 201+ analyzer (HemoCue, Angelholm, Sweden). Blood samples were collected by finger pricking with a sterile disposable lancet after careful disinfection with cotton immersed in 70% ethanol. A drop of blood was allowed to enter the optical window of the microcuvette by capillary action. The microcuvette was placed into the cuvette holder for photometric determination of Hb level, and anemia was considered for children with Hb levels lower than 11 g/dL.3 Mild, moderate, and severe anemia were considered for Hb levels of 10.0–10.9 g/dL, 7.0–9.9 g/dL, and <7.0 g/dL, respectively.3 Furthermore, about 1.8 mL of venous blood was collected in a 2.0 mL EDTA vacutainer and used to make thin and thick blood films that were stained using Giemsa.16 The blood films were air-dried and examined for morphological characterization of anemia based on a comprehensive blood film comment. Anemia was reported based on: 1) red blood cells (RBCs) size that is, normocytic, microcytic, and macrocytic, and 2) RBCs staining, that is, normochromic and hypochromic.17 The prepared thick and thin peripheral blood films were examined to detect the type of hemoparasite. The microcytic RBC picture is characteristic of iron-deficiency anemia, the macrocytic RBC picture is seen in megaloblastic anemia, while the normocytic RBCs are found in different conditions as in a chronic disease, aplastic anemia, blood loss, and suppressed production of RBCs or hemolysis.3 Additionally, about 5 g of fresh stool was collected from each participant and analyzed using wet preparations and formol–ether concentration technique for intestinal parasites. Blood and stool samples were analyzed using standard operating procedure. All blood and stool-positive slides were confirmed by a proficient laboratory technologist (FW) attached to Lancet laboratories. Additionally, blood film comments were made by two independent readers and discussed before a conclusive report was made. The final reports on comprehensive blood film comment were cross-checked and validated by a proficient person (IMT) of the Hematology and Blood Transfusion Unit of Clarke International University (formerly, International Health Sciences University). The obtained data were cross-checked to ensure their completeness and kept under lock and key, only available to the investigation team.
Data analysis
Data were entered using Microsoft Excel 2013, cleaned, and analyzed using SPSS, version 17.0. Descriptive statistics (mean, frequencies, cross tabulation) were used to describe study participants and to determine the prevalence of anemia. Univariate and multivariate logistic regression analyses were done to look for statistical significance with associated factors of anemia. This was based on those variables with P-value of 0.05 or less in the univariate logistic regression and was considered for the multivariable logistic regression analysis. Variables with a P-value of <0.05 were considered statistically significant.
Ethics considerations
Ethical approval was sought and obtained from research and ethics committees of Clarke International University and St. Mary’s Hospital, Lacor. All caregivers gave written informed consent. Laboratory results were given to the attending doctor for prompt patient care. Anonymity of the participants was ensured at all stages of data analysis.
Results
Sociodemographic characteristics of study participants
This study included 343 children, whose characteristics are indicated in Table 1. They comprised 62.7% (N=215) females. The predominant age group was between 12 and 24 months (30.3%, N=104). The IQR, median, and mean Hb levels were 5.1±3.2 g/dL, 8.2 g/dL, and 7.9 g/dL, respectively. Six (3.7%) of the participants were infested with Plasmodium falciparum, while 2 (1.2%) had intestinal parasites.
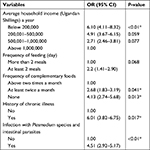
  | Table 1 Sociodemographic factors associated with anemia among children under 5 years of age Note: 3,700 Ugandan Shilling is an equivalent of 1 USD. |
Prevalence and severity of anemia
Overall, 160 (46.6%, 95% CI: 42.1–51.46) participants had anemia. Of these, 19 (11.9%) participants had severe anemia, 94 (58.8%) had moderate anemia, while 47 (29.4%) had mild anemia as presented in Figure 1.
  | Figure 1 A bar graph showing the severity of anemia among children under 5 years of age. Abbreviation: Hb, hemoglobin. |
Morphological characterization of anemia
Examination of the peripheral thin blood film indicated that majority of the anemic children (N=106, 65.4%) had a hypochromic-microcytic blood picture, while 15.4% (N=25) had a hypochromic-macrocytic picture. Thirty-one (19.1%) participants presented with normochromic-microcytic RBCs.
Associated factors of anemia
Logistic regression was used to elucidate the effect of sociodemographic, socioeconomic, feeding pattern, history of chronic illnesses, and risk of parasite infestation to the acquisition of anemia as indicated in Table 2. From the analysis, the variables (OR, 95% CI) that had statistically significant association were infestation with parasites (4.51, 2.92–5.17), history of chronic disease (6.01, 3.82–6.75), lack of complementary feeding (4.13, 2.74–5.68), insufficient (at least twice a month) complementary feeding (2.68, 1.83–3.19), households whose annual income was below 200,000 Ugandan Shillings (an equivalent of 55 USD; 6.10, 4.11–8.32) as indicated in Table 3.
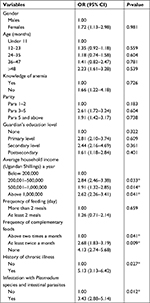  | Table 2 Univariate logistic analysis of risk factors toward causation of anemia among children under 5 years of age Note: *Statistically significant values; 1.00 denotes a reference population. |
Discussion
This study found that 160 children below the age of 5 years had Hb levels less than 11.0 g/dL, indicative of a 46.6% (95% CI: 42.1–51.46) prevalence of anemia. This is slightly lower than the global prevalence of 47.7%,18 and 47.4% reported in Ethiopia.9 The prevalence is however higher than 26.2% reported in Bushenyi,7 28.8% in Kenya,11 and 45% in Cameroon.12 While the causes of this markedly higher variance are multifactorial, it is probably attributed to a number of factors: first, our study population comprised children who were sick and had sought for formal health care, which may imply that they had already developed anemia considering the poor health care seeking behaviors in our communities. Second, this population is characterized by low standards of living due to post conflict environment, and this may warrant high parasitic infection burdens which are known risk factors of anemia. Contrary to our study findings, a number of studies have reported a higher prevalence, for example, 78.4% in Ghana,13 77.2% in Tanzania,14 51.8% in Cape Verde,10 and 58.8% in Namutumba District in Uganda.19
The majority of our participants had mild-to-moderate forms of anemia (29.4% and 58.8%, respectively), while 11.9% had severe anemia. This finding is in agreement with other studies.20–23 The moderate form of anemia may require instant clinical intervention since it can progress to more severe and life-threatening outcome, mainly in limited resource setups, where apt diagnosis may not be done.18,20 As defined by WHO,3 identification of the severity of anemia could help to overcome morbidity and associated mortality. The high percentage of severe anemia may be ascribed to the endemic parasitic infection, as evidenced by the positive indices in this study. This observation is consistent with what was reported in Tanzania24 and Kenya.25
Our study has further revealed the predominant morphological forms of anemia as hypochromic-microcytic and normochromic-microcytic anemia. This feature collaborates with results from a study done in Serbia.26 Based on the RBC size, the microcytic picture is indicative of iron-deficiency anemia, while macrocytic anemia is a feature of megaloblastic anemia due to either vitamin B12 or folate deficiency.27 Finding of parasitic infections (3.7% P. falciparum and 1.2% intestinal parasites) is suggestive of nutrient-deficiency anemia as the underlying mechanism, as reported earlier.6
In this study, we have explored that children infested with parasites (P=0.00), history of chronic disease (P=0.02), lack of complementary feeding (P=0.01), insufficient (at least twice a month) complementary feeding (P=0.04), and households whose annual income was below 200,000 Ugandan Shillings (an equivalent of 55 USD; P=0.00) were predictor variables of anemia. This is consistent with what has been reported.7,9,12 The presence of P. falciparum is a known phenomenon that causes iron deficiency through destruction of RBCs. On the other hand, chronic diseases exert their effect through interference with hematologic balance between RBC productions via erythropoietin release, consequently, inducing anemia.8 The low socioeconomic status of less than 55 USD annually indicates a low standard of living that may not permit the special feeding demands of children under 5 years of age. These findings have been reported in other regions.7,11,12
A limitation of our study is that confirmatory tests including iron studies, bone marrow biopsy, and Hb electrophoresis were not performed. This may limit the estimation of prevalence and severity of anemia in these children. The results of our hospital-based study and consecutively enrolling participants make it rather difficult to generalize the findings to the entire Gulu District for children under 5 years of age. Furthermore, the observed macrocytosis of the RBC morphology may be pseudo due to exaggerated flattening, while making the peripheral blood thin film. Additionally, the use of a questionnaire is dependent on the caregivers’ assessment, and finally, our laboratory assays are not regarded as the gold standard to establish a causal relationship, making it difficult to eliminate anemia in the study area.
Conclusion
This study has demonstrated evidence of a high prevalence of childhood anemia at St. Mary’s Hospital Lacor, Gulu District in Northern Uganda. Based on the WHO benchmarks, a population prevalence rate of greater than 40% is a severe public health concern, demanding immediate actions and measures to help diminish the burden of the disease. Considering a high prevalence of anemia, prevention may necessitate to be revised: 1) regular screening of Hb level must target all children under 5 years of age irrespective of their clinical status, 2) interventions aimed at lowering the anemia rates are key in this region, and 3) community education programs would assist lower the risk of childhood anemia.
Acknowledgments
Our deep gratitude goes to our study subjects who were volunteers and took their time to give us all the relevant laboratory samples and questionnaire responses to the study. We are grateful to laboratory staff of St. Mary’s Hospital, Lacor for the assistance accorded during the time of data collection. We wholeheartedly thank the Emmaus Foundation and Benedict Medical Centre, Luzira for the logistical support toward this study. We did not receive funding for this study; however, we obtained laboratory consumables (vacutainers, HemoCue 201+ analyzer and cuvettes, gloves, syringes, and cotton) as a generous donation from Emmaus Foundation and Benedict Medical Centre, Luzira. This did not in any way interfere with our study design, results obtained, and interpretation as well as the decision to publish.
Author contributions
AO, CO, FW, BM, and IMT conceived the study idea, participated in study design, data acquisition, analysis, and interpretation. AO, CO, FW, and BM participated in drafting data collection tools and manuscript drafting. IMT oversaw the entire research process, scheduling for internal responsibilities and critically reviewed the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. | ||
Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–e25. | ||
WHO. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/MNM/11.1). Geneva: World Health Organization; 2011. Available from: http://www.who.int/vmnis/indicators/haemoglobin. Accessed October 15, 2018. | ||
Bain LE, Awah PK, Geraldine N, et al. Malnutrition in sub-Saharan Africa: burden, causes and prospects. Pan Afr Med J. 2013;15:120. | ||
Scott SP, Chen-Edinboro LP, Caulfield LE, Murray-Kolb LE. The impact of anemia on child mortality: an updated review. Nutrients. 2014;6(12):5915–5932. | ||
Hedberg K, Shaffer N, Davachi F, et al. Plasmodium falciparum-associated anemia in children at a large urban hospital in Zaire. Am J Trop Med Hyg. 1993;48(3):365–371. | ||
Kikafunda JK, Lukwago FB, Turyashemererwa F. Anaemia and associated factors among under-fives and their mothers in Bushenyi District, Western Uganda. Public Health Nutr. 2009;12(12):2302–2308. | ||
Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–624. | ||
Woldie H, Kebede Y, Tariku A. Factors associated with anemia among children aged 6–23 months attending growth monitoring at Tsitsika Health Center, Wag-Himra Zone, Northeast Ethiopia. J Nutr Metab. 2015;2015:928632–928639. | ||
Semedo RML, Santos M, Baião MR, Luiz RR, da Veiga GV. Prevalence of anaemia and associated factors among children below five years of age in Cape Verde, West Africa. J Health Popul Nutr. 2014;32(4):646–657. | ||
Ngesa O, Mwambi H. Prevalence and risk factors of anaemia among children aged between 6 months and 14 years in Kenya. PLoS One. 2014;9(11):e113756. | ||
Cornet M, Le Hesran JY, Fievet N, et al. Prevalence of and risk factors for anemia in young children in southern Cameroon. Am J Trop Med Hyg. 1998;58(5):606–611. | ||
Ewusie JE, Ahiadeke C, Beyene J, Hamid JS. Prevalence of anemia among under-5 children in the Ghanaian population: estimates from the Ghana demographic and health survey. BMC Public Health. 2014;14(1):626. | ||
Simbauranga RH, Kamugisha E, Hokororo A, Kidenya BR, Makani J. Prevalence and factors associated with severe anaemia amongst under-five children hospitalized at Bugando Medical Centre, Mwanza, Tanzania. BMC Hematol. 2015;15(1):13. | ||
Uganda Bureau of Statistics. Uganda Demographic and Health Survey. Vol. 2012. Calverton, MD: Uganda Bureau of Statistics (UBOS) and ICF International Inc; 2011. | ||
Tropical Laboratory Medicine. Department of Clinical Sciences. Preparation thin blood films and Giemsa staining. TLM_PRO_026 Version No.001; 2018. Available from: http://www.labquality.be/Documents/TLM_PRO_026_V01_Thin_film_preparation.pdf. Accessed September 21, 2018. | ||
Lynch EC. Peripheral blood smear. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Boston: Butterworths; Chapter 155. Available from: https://www.ncbi.nlm.nih.gov/books/NBK263/ Accessed September 21, 2018. | ||
World Health Organization [webpage on the Internet]. Special subjects: causes of death. Anaemias. World Health Stat Q. 2015;15:594. Available from: https://www.who.int/healthinfo/global_burden_disease/estimates/en/index1.html. Accessed October 23, 2018. | ||
Kuziga F, Adoke Y, Wanyenze RK. Prevalence and factors associated with anaemia among children aged 6 to 59 months in Namutumba District, Uganda: a cross-sectional study. BMC Pediatr. 2017;17(1):25. | ||
Mclean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12(4):444–454. | ||
Mamabolo RL, Alberts M. Prevalence of anaemia and its associated factors in African children at one and three years residing in the Capricorn District of Limpopo Province, South Africa. Curationis. 2014;37(1):1160. | ||
Madoori S, Ramya C, Valugula S, Sandeep G, Kotla S. Clinico hematological profile and outcome of anemia in children at tertiary care hospital, Karimnagar, Telangana, India. Int J Res Med Sci. 2015;3(12):3567–3571. | ||
Magesa AS, Magesa PM. Association between anaemia and infections (HIV, malaria and hookworm) among children admitted at Muhimbili National Hospital. East Afr J Public Health. 2012;9(3):96–100. | ||
Kahigwa E, Schellenberg D, Sanz S, et al. Risk factors for presentation to hospital with severe anaemia in Tanzanian children: a case-control study. Trop Med Int Health. 2002;7(10):823–830. | ||
Foote EM, Sullivan KM, Ruth LJ, et al. Determinants of anemia among preschool children in rural, western Kenya. Am J Trop Med Hyg. 2013;88(4):757–764. | ||
Djokic D, Drakulovic MB, Radojicic Z, et al. Risk factors associated with anemia among Serbian school-age children 7–14 years old: results of the first national health survey. Hippokratia. 2010;14(4):252–260. | ||
Monica Cheesbrough. District Laboratory Practice in Tropical Countries. 2nd ed. New York: United States of America by Cambridge University Press; 2006. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.