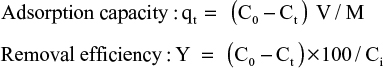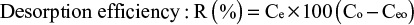Back to Journals » Nanotechnology, Science and Applications » Volume 10
Preparation and biosorption evaluation of Bacillus subtilis/alginate-chitosan microcapsule
Authors Tong K
Received 22 January 2016
Accepted for publication 10 March 2016
Published 3 February 2017 Volume 2017:10 Pages 35—43
DOI https://doi.org/10.2147/NSA.S104808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Ke Tong
College of Life Science and Engineering, Southwest University of Science and Technology, Mianyang, Sichuan, People’s Republic of China
Abstract: The aim of this study was to assess the effect of alginate–chitosan microcapsule on viability characteristics of Bacillus subtilis and the ability of B. subtilis/alginate–chitosan microcapsule to remove uranium ion from aqueous solution. The effects of particle size, chitosan molecular weight and inoculum density on viability characteristics were studied using alginate–chitosan microcapsule-immobilized B. subtilis experiments. In addition, the effects of pH, immobilized spherule dosage, temperature, initial uranium ion concentration and contact time on removal of uranium ion were studied using batch adsorption experiments. The results showed that alginate–chitosan microcapsule significantly improved the viability characteristics of B. subtilis and that B. subtilis/alginate–chitosan microcapsule strongly promoted uranium ion absorption. Moreover, the optimum values of pH was 6; immobilized spherule dosage was 3.5; temperature was 20°C; initial uranium ion concentration was 150 mg/L; contact time was 3 h of uranium ion absorption and the maximum adsorption capacity of uranium ion was 376.64 mg/g.
Keywords: alginate–chitosan microcapsule, Bacillus subtilis, viability characteristics, uranium ion, adsorption
Introduction
Pollutions caused due to heavy metals has become a serious environmental problem over the past few decades. Final industrial wastewater contains numerous heavy metal ions, which are extremely dangerous even at low concentrations due to their toxicity.1 For example, cadmium concentrations in the range of 0.1–100 mg × dm-3 are typical in wastewater from several industries, and an intake of excessively large doses by humans could lead to serious kidney failure.2,3 Uranium is an essential radioactive element and a nuclear fuel, but at a higher level it is toxic to plants, animals and humans.4 Uranium consumption in high doses could produce serious toxicological concerns such as cancer, kidney and liver injuries.4 Uranium is widely used in important nuke industrial applications; hence, its removal from wastewater is important for environmental protection and human health.
Biosorption course has been discovered to be better than other technologies because of its high efficiency, convenient operation, low cost, regeneration of biosorbents and recovery of metals, compared to the traditional effluent treatments.5,6 It was evident from a literature survey of >100 recent papers that low-cost adsorbents had demonstrated outstanding removal capabilities for various pollutants.7 Biosorption utilizes all kinds of microorganisms, including yeast, bacteria, algae, fungi and protozoa, which could be discovered everywhere.8 The mechanisms for removal of heavy metals include adsorption, uptake, reduction, methylation and oxidation.9 Living, dead and immobilized bacteria could be used in the process. Immobilized bacteria are usually easier to handle, require less complex separation systems, allow a high biomass density to be maintained and provide a greater opportunity for reuse and recovery.10
A previous study showed that Bacillus subtilis possessed high physiological activity in an industry waste treatment.6 The structure of B. subtilis cell wall is well known and consists primarily of peptidoglycan and teichoic acid.8 Peptidoglycan is a polymer of acetylmuramic and acetylglucosamine acids that display mainly carboxylic and hydroxyl functional groups.8 Teichoic acid is a polymer of copyranosyl glycerol phosphate that comprises mainly phosphate and hydroxyl groups.11,12
In this study, we reported alginate–chitosan as a B. subtilis microcapsule. The B. subtilis/alginate–chitosan microcapsule was composed of B. subtilis, sodium alginate, chitosan and calcium chloride. Therefore, in sterile conditions, B. subtilis was mixed with sodium alginate solution, and then the mixed solution was dropped into calcium chloride solution to immobilize using microcapsule preparation instrument. B. subtilis-loaded calcium alginate gel beads were obtained after immobilizing, and B. subtilis-loaded calcium alginate gel beads were mixed with chitosan solution to obtain the B. subtilis/alginate–chitosan microcapsule. The microcapsule system had good mechanical strength, flexibility and biocompatibility between B. subtilis and microcapsule. In addition, internal three-dimensional network structure of the microcapsule provided a sufficient space for B. subtilis growth and good encapsulating stability. Biosorption studies were conducted by the administration of B. subtilis/alginate–chitosan microcapsule to uranium-polluted wastewater, and the significant biosorption ability was evaluated in order to remove uranium ion from aqueous solution using free B. subtilis as the control.
Materials and methods
Strains and materials
B. subtilis was provided by College of Life Science and Engineering, Southwest University of Science and Technology. Alginate, chitosan, U3O8 and other reagents were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
B. subtilis cultivation
Cultivation of B. subtilis was carried out in 250 mL conical flasks with 100 mL culture medium on a rotary shaker at 200 rpm at a constant temperature of 37°C. The culture medium contained 3 g/L beef extract, 10 g/L peptone, 20 g/L glucose, 5 g/L NaCl and 0.005 g/L MnSO4. The pH of the medium was adjusted to 7.0.
Preparation of B. subtilis/alginate–chitosan microcapsule
A total of 15 mL of B. subtilis suspension of logarithmic phase was centrifuged for 10 min at 6,000 rpm. After centrifugation, B. subtilis was mixed with sodium alginate (5 mL, 15 g/L), and the concentration of the thallus was adjusted to 2.0 × 106/mL in miscible liquids. Then, CaCl2 was added to the solution (15 g/L) and immobilized for 20 min to obtain B. subtilis/calcium alginate gel beads using microcapsule preparation instrument. Chitosan solutions were chosen (4 g/L) to mix with B. subtilis/calcium alginate gel beads, and B. subtilis/alginate–chitosan microcapsule was obtained after 20 min.
B. subtilis/alginate–chitosan microcapsule cultivation
Cultivation of B. subtilis/alginate–chitosan microcapsule was carried out in 250 mL conical flasks with 100 mL culture medium on a rotary shaker at 200 rpm and a constant temperature of 37°C.
Morphologic observation of B. subtilis/alginate–chitosan microcapsule
The microcapsule sample was collected at regular intervals during B. subtilis/alginate–chitosan microcapsule cultivation, and the viability characteristics of B. subtilis were observed in microcapsules using an inverted microscope.
Measurement of cell biomass
The cultivation solution was collected in the free sample of cell culture at regular intervals, and the optical density was measured using a spectrophotometer at a wavelength of 650 nm to calculate the cell biomass. The microcapsule was opened after collecting the sample to release B. subtilis, and the optical density was measured using a spectrophotometer at a wavelength of 650 nm to calculate the cell biomass. The methods had been described previously.13
Configuration of the uranium solution and standard curve measurement
U3O8 (1.1790 g) was taken in a 1,000 mL volumetric flask to prepare 1 g/L uranium solution. The concentration of uranium ion in the experiment was measured by atomic absorption spectrophotometry. Standard stock solution of uranium ion was collected to prepare 0.02, 0.04, 0.06, 0.08 and 0.1 mg/L uranium solution using distilled water, and then, the absorbency was measured and the standard curve was drawn.
Characterization
The overall shape and surface characteristics of particles were observed using a scanning electron microscope (SEM; Quanta 200FEG; FEI Company, USA).
Adsorption experiment
A certain amount of immobilized B. subtilis adsorbent was taken in a 100 mL metallic solution at appropriate pH, oscillated at 100 rpm and centrifuged for 15 min at 10,000 rpm, then the supernatant was collected and the residual heavy metal ion concentration was measured.
|
where qt is the adsorption capacity of heavy metal ion under “t” time that the heavy metal ion was absorbed by per gram of immobilized adsorbent and was a index (mg/g) of measuring the adsorption capacity; C0 is the initial concentration (mg/L) of heavy metal ion, Ct is the concentration (mg/L) of heavy metal ion from solution after reactions, M is the absorbent dosage (g), V is the solution volume (L) and Y is the removal efficiency (%) of heavy metal ion. The methods had been described previously.8
pH experiment
The effect of adsorption process was observed under different pH values (1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 5.0, 5.5, 6.0, 6.5 and 6.8) by reacting for 24 h at 37°C and 100 rpm in an EZ thermoshake. In this process, the dosing quantity of immobilized pellets was 3.5% (w/v), and the initial concentration of heavy metal ion was 100 mg/L.
Adsorbent additive amount experiment
The effect of adsorption process was observed under different dosing quantities of immobilized pellets (1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, 4.5 and 5% [w/v]) by reacting for 24 h at 37°C and 100 rpm in an EZ thermoshake. In this process, the pH of the solution was 6.0, and the initial concentration of heavy metal ion was 100 mg/L.
Temperature experiment
The effect of adsorption process was observed under different temperatures (10, 15, 20, 25, 30, 35, 40, 45 and 50°C) by reacting for 24 h at 100 rpm in an EZ thermoshake. In this process, the pH of solution was 6.0, the dosing quantity of immobilized pellets was 3.5% (w/v) and the initial concentration of heavy metal ion was 100 mg/L.
Initial concentration of heavy metal ion experiment
The effect of adsorption process was observed under different initial concentrations of heavy metal ion (1, 5, 10, 20, 30, 50, 80, 100 and 150 mg/L) by reacting for 24 h at 37°C and 100 rpm in an EZ thermoshake. In this process, the pH of the solution was 6.0 and the dosing quantity of immobilized pellets was 3.5% (w/v).
Time experiment
The effect of adsorption process was observed under different initial concentrations of heavy metal ion (1, 50 and 100 mg/L) at 5 min, 10 min, 30 min, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h and 24 h. In this process, the pH of the solution was 6.0, the dosing quantity of immobilized pellets was 3.5% (w/v) and the reacted temperature was 37°C.
Desorption experiment
The desorption studies were performed using 3.5% (w/v) immobilized pellets with 100 mL of 100 mg/L uranium solution. After adsorption experiments (24 h), the adsorbed metal ions were eluted with 0.01 mol/L Na2CO3. The eluted biosorbent was then washed thoroughly with distilled water until the pH of the elute solution reached 7.0 and placed into metal solution for the subsequent adsorption–desorption cycle.
|
where Ce is the concentration of the metal ion in the solution after desorption. Co and Ceo are the concentrations of the heavy metal ion in the solution before and after adsorption.
Results
Growth state of B. subtilis in microcapsules
Very few B. subtilis were found in B. subtilis-loaded microcapsules, which were transparent gel microspheres. After cultivation for 8 h, B. subtilis grew and formed mycelial morphology in microcapsules, which further increased and the density of B. subtilis enlarged in the microcapsules after cultivation for 24 h (Figure 1A). The primary culture of microcapsules was transferred to fresh culture medium, where B. subtilis adapted to the new environment to achieve growth stability, and the density of B. subtilis further increased (Figure 1B).
  | Figure 1 The morphology of Bacillus subtilis/alginate–chitosan microcapsule in primary culture (A) and in batch culture (B). |
Effect of chitosan molecular weight on the growth characteristics of microencapsulated B. subtilis
The chitosans of 20,000 u, 50,000 u and 100,000 u molecular weight were chosen as microcapsule membrane. It was observed that B. subtilis reached the maximum quantity after 10 h in the sample of 100,000 u molecular weight chitosan, after 15 h in the sample of 50,000 u molecular weight chitosan and after 35 h in the sample of 20,000 u molecular weight chitosan. The maximum quantity of B. subtilis was similar in all 3 samples (Figure 2).
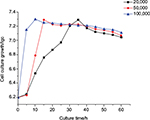  | Figure 2 Effect of chitosan molecular weight on the growth characteristics of Bacillus subtilis/alginate–chitosan microcapsule. Abbreviation: lgc, logarithmic expression of cell number. |
Effect of microcapsule particle size on the growth characteristics of microencapsulated B. subtilis
The microcapsule carriers of 100 and 1,000 µm particle size were chosen. It was observed that the culture growth of B. subtilis in the microcapsule carrier with a particle size of 100 µm was better than that in the microcapsule carrier with a particle size of 1,000 µm during primary culture and batch culture (Figure 3A and B).
Effect of inoculative density on the growth characteristics of microencapsulated B. subtilis
B. subtilis of 2.0×106, 2.6×106 and 5.2×106/mL inoculative density was chosen. It was observed that excess inoculative density led to decline of microcapsule mechanical strength, and the microcapsule ruptured. In addition, too low inoculative density caused the growth rate of B. subtilis to reach balance in the subsequent culture. The appropriate initial inoculative density was 2.6×106/mL (Figure 4).
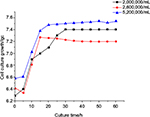  | Figure 4 Effect of inoculative density on the growth characteristics of Bacillus subtilis/alginate–chitosan microcapsule. Abbreviation: lgc, logarithmic expression of cell number. |
Standard curve measurement
As shown in Figure 5, the abscissa is uranium concentration and the ordinate is absorbency. It was observed that the uranium concentration showed a linear relation with the absorbency at 0.02–0.1 mg/L.
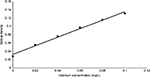  | Figure 5 The standard curve of uranium ions. |
SEM analysis
The surface areas of B. subtilis/alginate–chitosan microcapsule before and after uranium biosorption were observed by SEM. The SEM images are shown in Figure 6. Many tiny interspace structures distributed on the surface of the biosorbent could be clearly observed in Figure 6A, which thus provided the maximum surface area for the biosorption of uranium. Figure 6B reveals that the surface of the biosorbent became rough and had more protrusions.
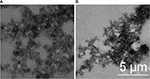  | Figure 6 SEM images of Bacillus subtilis/alginate–chitosan microcapsule before (A) and after (B) biosorption of uranium ions. Abbreviation: SEM, scanning electron microscope. |
Effect of initial pH on uranium adsorption
Figure 7 presents the effect of initial pH on the removal efficiency of uranium onto B. subtilis/alginate–chitosan microcapsule, with pH from 1.5 to 6.8. The removal efficiencies of uranium onto both adsorbents had the same trend, which increased with increasing pH. When the initial pH was adjusted to 1.5, a little uranium was removed by B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule. The removal efficiency of uranium increased sharply at the pH range of 1.5–5 while it increased slightly at pH >5. When the initial pH was 6, the removal efficiency of uranium onto B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule reached the maximum. When the initial pH was >6.5, the removal efficiency of uranium onto B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule significantly decreased, and the removal efficiency of uranium on B. subtilis/alginate–chitosan microcapsule was higher than that on alginate–chitosan microcapsule at all pH ranges.
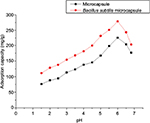  | Figure 7 Effect of initial pH on uranium ion adsorption by Bacillus subtilis/alginate–chitosan microcapsule. |
Effect of dosing quantity of immobilized pellets on uranium adsorption
The effect of dosing quantity of initial immobilized pellets on the removal efficiency of uranium onto B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule was assessed by varying the dosing quantity of immobilized pellets from 1.0 to 5 (w/v). The removal efficiencies of uranium onto both adsorbents had the same trend, which increased with increasing dosing quantity of immobilized pellets. When the initial dosing quantity of immobilized pellets was adjusted to 1.0 (w/v), a little uranium was removed by B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule. The removal efficiency of uranium increased sharply at the dosing quantity range of immobilized pellets of 1.0–3 (w/v), whereas it increased slightly when the dosing quantity of immobilized pellets was >3 (w/v). When the initial dosing quantity of immobilized pellets was 3.5 (w/v), the removal efficiency of uranium onto B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule reached the maximum. When the initial dosing quantity of immobilized pellets was >4 (w/v), the removal efficiency of uranium onto B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule significantly decreased. The removal efficiency of uranium onto B. subtilis/alginate–chitosan microcapsule was higher than that onto alginate–chitosan microcapsule at all dosing quantity ranges of immobilized pellets (Figure 8).
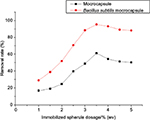  | Figure 8 Effect of dosing quantity of immobilized pellets on uranium adsorption by Bacillus subtilis/alginate–chitosan microcapsule. |
Effect of temperature on uranium adsorption
The effects of temperature on adsorption capacity of B. subtilis/alginate–chitosan microcapsule and alginate–chitosan microcapsule for uranium are shown in Figure 9. It could be observed from the figure that the adsorption capacity of B. subtilis/alginate–chitosan microcapsule for uranium increased with the increase in temperature from 10 to 20°C. The adsorption capacity reached a maximum at 20°C and then decreased with the increase in temperature to 50°C. This small change at 10−20°C led to a great change in adsorption capacity. These results indicated that B. subtilis was very sensitive to temperature. The adsorption capacity of alginate–chitosan microcapsule increased with increasing temperature, which indicated the endothermic nature of the adsorption process.
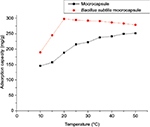  | Figure 9 Effect of temperature on uranium adsorption by Bacillus subtilis/alginate–chitosan microcapsule. |
Effect of initial uranium concentration on uranium adsorption
The removal efficiency and adsorption capacity at different uranium concentrations are presented in Figure 10. At a low uranium concentration of 1 mg/L, the removal efficiency of B. subtilis/alginate–chitosan microcapsule reached a maximum of 98.7% and then reduced with the increase in initial uranium concentration. The high biosorption efficiency (close to 99%) at low concentrations indicated the potential application of B. subtilis/alginate–chitosan microcapsule for removal of a trace amount of uranium from wastewater. The removal efficiency of uranium dropped sharply with an increase in initial uranium concentration from 5 to 150 mg/L. The adsorption capacity of B. subtilis/alginate–chitosan microcapsule increased with increasing initial uranium concentration and reached 376.64 mg/g at the initial uranium concentration of 150 mg/L.
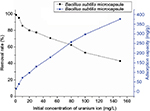  | Figure 10 Effect of initial uranium concentration on uranium adsorption by Bacillus subtilis/alginate–chitosan microcapsule. |
Effect of contact time on uranium adsorption
The effects of contact time for B. subtilis/alginate–chitosan microcapsule on the adsorption capacity at different initial concentrations (1, 50 and 100 mg/L) are shown in Figure 11. It was observed that a rapid uptake occurs within the first 3 h and then followed by a slow increase until the equilibrium state was attained after 8 h at all the initial uranium concentrations. After this equilibrium period, the amount of uranium adsorbed did not change significantly with time.
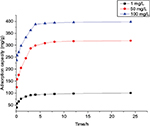  | Figure 11 Effect of contact time on uranium adsorption by Bacillus subtilis/alginate–chitosan microcapsule. |
Effect of desorption study
Table 1 lists the adsorption capacity and desorption ratio of uranium on the B. subtilis/alginate–chitosan microcapsule in 5 adsorption–desorption cycles. The adsorption capacity and desorption ratio of B. subtilis/alginate–chitosan microcapsule decreased by only 5.2 mg/g and 2.2% after the first cycle, which could still be maintained at 82.1 mg/g and 79.4%, respectively, at the 5th cycle.
  | Table 1 Desorption ratio of uranium ions adsorbed by Bacillus subtilis/alginate–chitosan microcapsule |
Discussion
Uranium is an environmentally important element because of its toxicity and to a lesser extent because of its radioactivity.14 With the rapid development of uranium mining industry, a large amount of wastewater containing uranium has been discharged into the environment. In addition, malfunctions of nuclear reactors and leaks of cooling water have also endangered the environment.15,16 Therefore, it is important to remove uranium from wastewater from uranium mining, reactor operations, fuel reprocessing and other anthropogenic activities.17 Conventional methods for removing uranium (VI) from wastewaters include chemical precipitation, coagulation and ion-exchange process. However, these conventional methods are expensive and ineffective, particularly at low metal concentrations.18,19
Many studies have shown that different groups of microorganisms, such as algae, bacteria, yeasts and fungi, could be used as natural adsorbents for uranium (VI) from aqueous solutions, and, among these, fungi and algae showed the greatest potential due to their high adsorption capacity and low cost.20–22 However, the physical and mechanical characteristics of the microbial biomass, such as the small particle size, difficult separation of solids and liquids and poor mechanical strength, had impeded their commercial application as a biosorbent.23,24 In order to overcome these difficulties, some researchers developed immobilization methods for biomass, and the immobilized biomass showed advantages over the free biomass in that it had higher mechanical strength and could more easily be separated from the aqueous solutions and the adsorbed metal ions could more easily be recovered.25,26
In our study, we report the synthesis of alginate–chitosan microcapsule with immobilized B. subtilis and showed that alginate–chitosan microcapsule had good mechanical strength, elasticity and biocompatibility with B. subtilis. Its internal three-dimensional network structure provided ample space for B. subtilis growth and good encapsulating stability. And B. subtilis was mycelial growth and did not leak in the culture process. In this study, microcapsule of small particle size had a high mass transfer efficiency, large specific surface area, easy to be the characteristics of a stable suspension and was advantageous to the pouch B. subtilis growth metabolism. Chitosan molecular weight had a significant effect on the microcapsule membrane performance and the microcapsule cell growth. It was observed that 100,000 u molecular weight chitosan could be used to prepare thin microcapsule membrane, which had good mechanical strength and mass transfer performance. Although B. subtilis inoculation density showed a positive correlation with the increase in growth of microcapsule B. subtilis, excess inoculation density led to microcapsule rupture. Therefore, in our study, it is demonstrated that 2.1×106/mL inoculation quantity was advisable.
On the other hand, in the biosorption evaluation of uranium, it was found that B. subtilis/alginate–chitosan microcapsule significantly improved biosorption of uranium. In the SEM analysis, many adsorbent particles were clearly observed on the surface of the biosorbent, which was attributed to the reactions occurring on the surface of the biosorbent that afterward changed the structure of alginate–chitosan microcapsule. Assessment of the effect of pH, dosing quantity of immobilized pellets, temperature, initial uranium concentration, contact time and desorption study on uranium adsorption showed that uranium ions could be effectively adsorbed by B. subtilis immobilized into alginate–chitosan microcapsule compared with alginate–chitosan microcapsule and that B. subtilis/alginate–chitosan microcapsule has great potential as a low-cost heavy metal biosorbent.
Although the removal of radionuclide, uranium, had been reported earlier, to our knowledge, this study is the first comprehensive report on its accumulation by alginate–chitosan microcapsule, which shows the feasibility of possible application of biosorbents in consecutive biosorption cycles. These results showed that immobilization methods could further promote the biosorption of uranium and increase the biomass of B. subtilis. This result was consistent with the results of previous studies;25,27,28 therefore, the immobilized B. subtilis alginate–chitosan microcapsule has great potential for removing uranium (VI) from aqueous solutions.
Conclusion
The following points can be concluded from this study: 1) B. subtilis was effectively encapsulated by alginate–chitosan; 2) Uranium ions could be effectively adsorbed by B. subtilis/alginate–chitosan microcapsule; 3) The pH, immobilized spherule dosage, temperature, initial uranium ion concentration and contact time highly affect the uranium biosorption; 4) Our results showed that the maximum adsorption capacity of uranium ion was 376.64 mg/g; and 5) The desorption rate was maintained at 79.4% after 5 cycles. This suggested that the B. subtilis/alginate–chitosan microcapsule has great potential as a low-cost uranium ion biosorbent.
Acknowledgment
This work was supported by College of Life Science and Engineering, Southwest University of Science and Technology, Mianyang, Sichuan, People’s Republic of China.
Author contributions
Ke Tong designed, carried out the experiment and wrote the manuscript. The author also revised the manuscript, gave useful suggestions on the early version and read and approved the final manuscript.
Disclosure
The author reports no conflicts of interest in this work.
References
Vijayaraghavan K, Yun YS. Bacterial biosorbents and biosorption. Biotechnol Adv. 2008;26(3):266–291. | ||
Vullo DL, Ceretti HM, Daniel MA, Ramírez SA, Zalts A. Cadmium, zinc and copper biosorption mediated by Pseudomonas veronii 2E. Bioresour Technol. 2008;99(13):5574–5581. | ||
Zheng W, Li XM, Yang Q, et al. Adsorption of Cd(II) and Cu(II) from aqueous solution by carbonate hydroxylapatite derived from eggshell waste. J Hazard Mater. 2007;147(1–2):534–539. | ||
Li ZJ, Wang L, Yuan LY, et al. Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite. J Hazard Mater. 2015;290:26–33. | ||
Yang CP, Wang JQ, Lei M, Xie GX, Zeng GM, Luo SL. Biosorption of zinc(II) from aqueous solution by dried activated sludge. J Environ Sci. 2010;22(5):675–680. | ||
Feng NC, Guo XY, Liang S. Kinetic and thermodynamic studies on biosorption of Cu(II) by chemically modified orange peel. Trans Nonferrous Metals Soc China. 2009;19:1365–1370. | ||
Ahmad T, Rafatullah M, Ghazali A, Sulaiman O, Hashim R. Oil palm biomass-based adsorbents for the removal of water pollutants – a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29(3):177–222. | ||
Liu YG, Liao T, He ZB, et al. Biosorption of copper(II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. Trans Nonferrous Met Soc China. 2013;23:1804–1814. | ||
Zahoor A, Rehman A. Isolation of Cr(VI) reducing bacteria from industrial effluents and their potential use in bioremediation of chromium containing wastewater. J Environ Sci. 2009;21(6):814–820. | ||
Tsekova K, Todorova D, Ganeva S. Removal of heavy metals from industrial wastewater by free and immobilized cells of Aspergillus niger. Int Biodeterior Biodegradation. 2010;64:447–451. | ||
Ji YL, Gao H, Sun JS, Fang C. Experimental probation on the binding kinetics and thermodynamics of Au(III) onto Bacillus subtilis. Chem Eng J. 2011;172:122–128. | ||
Frost PC, Maurice PA, Fein JB. The effect of cadmium on fulvic acid adsorption to Bacillus subtilis. Chem Geol. 2003;200:217–224. | ||
Liu X, Yu W, Ma X, Wang W, Xiong Y, Yuan Q. Polyelectrolyte microcapsules prepared by alginate and chitosan for biomedical application. Prog Chem. 2008;20:126–139. | ||
Benedict M, Levi H, Pigford T. Nuclear chemical engineering. AIChE J. 1982;28:702. | ||
Kazy SK, D’Souza SF, Sar P. Uranium and thorium sequestration by a Pseudomonas sp.: mechanism and chemical characterization. J Hazard Mater. 2009;163(1):65–72. | ||
Preetha CR, Gladis JM, Rao TP, Venkateswaran G. Removal of toxic uranium from synthetic nuclear power reactor effluents using uranyl ion imprinted polymer particles. Environ Sci Technol. 2006;40:3070–3074. | ||
Singh H, Mishra S, Vijayalakshmi R. Uranium recovery from phosphoric acid by solvent extraction using a synergistic mixture of di-nonyl phenyl phosphoric acid and tri-n-butyl phosphate. Hydrometallurgy. 2004;73:63–70. | ||
Martins M, Faleiro ML, Rose da Costa AM, et al. Mechanism of uranium (VI) removal by two anaerobic bacterial communities. J Hazard Mater. 2010;184(1–3):89–96. | ||
Lloyd JR, Macaskie LE. In: Lovley DR, editor. Environmental Metal Microbe Interaction. Washington, DC: American Society of Microbiology;2000:277–327. | ||
Akhtar K, Akhtar MW, Khalid AM. Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Res. 2007;41(6):1366–1378. | ||
Ghasemi M, Keshtkar AR, Dabbagh R, Safdari SJ. Biosorption of uranium (VI) from aqueous solutions by Ca-pretreated Cystoseira indica alga: breakthrough curves studies and modeling. J Hazard Mater. 2011;189(1–2):141–149. | ||
Tsuruta T. Removal and recovery of uranyl ion using various microorganisms. J Biosci Bioeng. 2002;94(1):23–28. | ||
Kumar R, Singh R, Kumar N, Bishnoi K, Bishnoi NR. Response surface methodology approach for optimization of biosorption process for removal of Cr(VI), Ni (II) and Zn (II) ions by immobilized bacterial biomass sp. Bacillus brevis. Chem Eng J. 2009;146:401–407. | ||
McHale AP, McHale S. Microbial biosorption of metals: potential in the treatment of metal pollution. Biotechnol Adv. 2000;12(4):647–652. | ||
Akhtar K, Khalid AM, Akhtar MW, Ghauri MA. Removal and recovery of uranium from aqueous solutions by Ca-alginate immobilized Trichoderma harzianum. Bioresour Technol. 2009;100(20):4551–4558. | ||
Arica MY, Kaçar Y, Genc O. Entrapment of white-rot fungus Trametes versicolor in Ca-alginate beads: preparation and biosorption kinetic analysis for cadmium removal from an aqueous solution. Bioresour Technol. 2001;80(2):121–129. | ||
Ding DX, Tan X, Hu N, Li G-Y, Wang Y-D, Tan Y. Removal and recovery of uranium (VI) from aqueous solutions by immobilized Aspergillus niger powder beads. Bioprocess Biosyst Eng. 2012;35(9):1567–1576. | ||
Akhtar K, Waheed Akhtar M, Khalid AM. Removal and recovery of uranium from aqueous solutions by Trichoderma harzianum. Water Res. 2007;41:1366–1378. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.