Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 9
Preliminary study on the development of an antistretch marks water-in-oil cream: ultrasound assessment, texture analysis, and sensory analysis
Authors Bogdan C, Moldovan ML, Man I, CRISAN M
Received 26 February 2016
Accepted for publication 3 June 2016
Published 6 September 2016 Volume 2016:9 Pages 249—255
DOI https://doi.org/10.2147/CCID.S107298
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Jeffrey Weinberg
Cătălina Bogdan,1 Mirela L Moldovan,1 Ioana Manuela Man,2 Maria Crișan,2
1Department of Dermopharmacy and Cosmetics, Faculty of Pharmacy, 2Department of Histology, Faculty of Medicine, University of Medicine and Pharmacy “Iuliu Hațieganu”, Cluj-Napoca, Romania
Purpose: Striae distensae represent the result of the failure of the dermis to sustain intrinsic mechanical forces. Intensive moisturization of the lesions and use of emollient oils have been recommended for the prevention and treatment of striae distensae rubra. The aim of this research was to formulate an emollient water-in-oil cosmetic cream containing argan oil, which may be helpful in the prevention or early treatment of striae distensae.
Patients and methods: Sensory evaluation of the consistency, firmness, adhesiveness, oiliness, spreadability, and rapidity of penetration into the skin was evaluated by 22 volunteers using 10-point scales for each descriptor. The instrumental characterization of the cream was performed using Brookfield® CT3 Texture Analyzer. The cutaneous changes induced by the topical use of the cream were evaluated by assessing the thickness of the epidermis, hydration, and elasticity of the skin using DermaLab® Combo scanner.
Results: Ultrasound measurements showed an improvement in the elasticity of the epidermis following the application of cream. The product was well tolerated and appreciated by the consumers in terms of its spreadability, penetration ability, and lack of stickiness. The values recorded for texture analysis were firmness 10.16±0.15 mJ, adhesiveness 30.94±6.87 g, consistency 1229.50±119.78 g, spreadability 481.50±39 g, and stringiness 0.56±0.09 mJ.
Conclusion: A water-in-oil cream containing argan oil and emollient ingredients with appropriate physical characteristics was obtained. In vivo study of clinical efficacy revealed a positive effect on increasing the skin elasticity, suggesting that the cream may be helpful in the prevention or early treatment of striae distensae.
Keywords: striae distensae, cosmetic cream, sensory properties, cream evaluation
Introduction
Striae distensae, also known as stretch marks, are the result of the failure of the dermis to sustain intrinsic mechanical forces. They are depressed lines or bands of thin, reddened skin, which later become white, smooth, and shiny, affecting almost half of adolescents and young adults, especially pregnant women. They occur most commonly on the abdomen during and after pregnancy (striae gravidarum) and on the breasts after lactation.1 They also occur on the buttocks and thighs, the inguinal areas, the posterior zone of the arm, and over the knees and elbows in children during the growth spurt of puberty. The shape of the lesions is linear or fusiform, with variable length. Their surface is often smooth and tense when the striae are recent. Older lesions tend to become crumpled and atrophic, giving the sensation of vacuity at palpation. They are hairless and no sweat or sebum excretion seems to be present. Striae distensae are a reflection of “breaks” in the connective tissue. Skin distension may lead to excessive mast cell degranulation and subsequent damage of collagen and elastin fibers.2
In striae distensae, both the mechanical functions and the structure of the dermal fibrous networks are modified and the epidermis also becomes thinner. Striae result from ruptures in the direction where the tissue is the weakest to sustain the mechanical stress. As a result, the axis of striae is oriented along the extension lines and the alterations in the matrix structure occur in the direction of the long axis of the striae. The causes responsible for this process are represented by factors distending the skin, such as fat expansion when gaining weight, visceral pressure during pregnancy, muscular hypertrophy, and movement-related skin extension. Compared to normal skin, striae distensae have diverse colors. Recent lesions look reddish at first and finally become white, but sometimes the color palette is broader. Blue striae are characteristic in case of patients with endogenous or exogenous hypercorticism.1
During the early stage of striae distensae (striae rubra), there are a few options to improve the appearance of stretch marks, such as pharmacologic and nonpharmacologic treatment and also procedures such as peeling, microdermabrasion, laser therapy, and surgical methods. For the treatment of striae distensae rubra, intensive moisturization of the lesions and use of vitamin C, fruit acids, and retinoids show a positive effect. When stretch marks become white (striae alba), they become difficult to treat and only few treatment modalities exist.3 Overall, in the management of striae distensae, prevention is a priority, with emphasis on topical formulations that maintain the elasticity and hydration of the skin.
In order to improve the skin condition predisposed to stretch marks, an emollient water-in-oil (W/O) cream containing argan oil was formulated and tested. Formulation of a cosmetic product represents a challenging work, especially in terms of stability and textural and sensorial properties of the final product.4 Rheological properties of the emulsions offer useful information about the colloidal structure of the systems and long-term stability. Besides, sensory characteristics of the emulsions are related to rheological properties. A combined approach using texture analysis for evaluation of the mechanical characteristics of emulsions associated with the sensory profile could be helpful in the cosmetic emulsion formulation process.5
Evaluation of efficacy of a cosmetic formulation requires an objective measurement tool. For this purpose, high-frequency ultrasound can be a useful tool to evaluate the skin improvements during the clinical trial. Ultrasonography is widely used in clinical medicine as a noninvasive method of diagnosis. During the past decade, diagnostic ultrasonography has been used in clinical dermatology as well because of the multiple benefits. High-resolution ultrasound systems with probes of at least 20 MHz can provide useful details regarding skin lesions such as tumor extension and inflammatory infiltrate. High-frequency ultrasound is also an appropriate tool for monitoring the evolution and therapeutic efficacy of diseases associated with skin sclerosis (eg, morphea, systemic scleroderma, and scleroderma-like diseases).6–10
Ultrasonography represents a valuable tool that can be used to evaluate the normal skin in a rapid and noninvasive way through cross-sectional images, especially for the measurement of skin thickness. Skin thickness is considered an objective physiological parameter for monitoring the influence of endogenous or environmental factors on skin structure. It also reflects changes in the cutaneous structure during studies that assess the effects of topical and systemic drugs or studies on efficacy of cosmetic creams. Currently, many techniques are used for assessing skin thickness such as pulsed ultrasound, conventional ultrasound, and skin-fold measurements.8–14
The main objectives of the present study were
- Preparation of the emollient cream containing argan oil and the most suitable substances selected as ingredients for the cosmetic cream base.
- Assessment of sensory properties of the cosmetic cream using volunteers’ evaluation as an innovative tool for predicting skinfeel properties.
- Assessment of firmness, consistency, adhesiveness, and stringiness of the cream performed using texture analysis.
- Ultrasound assessment of the cutaneous changes induced by the topical use of the antistretch marks cream.
Patients and methods
Cream preparation
Argan oil (Transvital Cosmetics, Cluj-Napoca, Romania) was selected as active ingredient of the W/O cream. The ingredients used were glyceryl stearate (Elemental, Oradea, Romania), Rapithix A 60 (sodium polyacrylate [and] hydrogenated polydecene [and] trideceth-6; Ashland Inc., Covington, KY, USA), Montane 481 VG (sorbitan oleate [and] beeswax [and] hydrogenated castor oil [and] stearic acid; Seppic, Paris, France), Estasan GT8-60 (caprylic/capric triglycerides; Croda, Snaith, UK), Euxyl PE 9010 (phenoxyethanol [and] ethylhexylglycerin; Schülke & Mayr, Norderstedt, Germany), silicone oil (Elemental), and butylated hydroxyanisole (Sigma-Aldrich Co., St. Louis, MO, USA). The cocoa butter, carbopol 940, glycerol, and magnesium sulfate were supplied by Vitamar, Bucharest, Romania. The following other materials were used: paraffin oil (Remed Prodimpex, Bucharest, Romania), cetaceum (Cognis, Monheim, Germany), cera alba (Mosselman, Ghlin, Belgium), cetylstearyl alcohol (Sabo, Levate, Italy), and distillated water (European Pharmacopoeia). All substances and reactants used were of pharmaceutical purity.
The W/O cream was prepared by heating separately the aqueous phase and the oily phase containing the lipophilic surfactants and the active ingredient. The aqueous phase was added to the oily phase under agitation at high temperature at 50°C±2°C, for different time intervals.
Evaluation of sensory properties
Sensory analysis represents a valuable tool in qualifying consumer perception, regarding a cosmetic product.15 Sensory evaluation provides helpful input to formulators and supports them to improve the texture properties of the cosmetic product. The sensory panel consisted of 22 volunteers aged from 22 years to 34 years. The volunteers were asked to fill in a questionnaire evaluating the sensory properties of the tested product from their point of view. They received the cosmetic formulation and the analysis form containing instructions of the sensory evaluation for the firmness, stickiness, consistency, spreadability, oiliness, and penetration degree into the skin, following a 1 to 10 scale for each descriptor (1 minimum and 10 maximum of a characteristic). In order to facilitate the evaluation process, reference standards of the scale (values 1 and 10) were made available to the volunteers.
Sensory attributes were evaluated between two fingers or when rubbed into the skin on the dorsal side of the hand. Thus, firmness was evaluated as the force necessary to press the surface of the sample (0.2 g) between two fingers, while the adhesiveness of the cosmetic product was appreciated as the work necessary to overcome the attractive forces between the surfaces of the two fingers joined together by a layer of cosmetic cream. The assessors evaluated consistency as the perceived amount of sample (0.2 g) between the two fingers. Each sample was characterized also regarding spreadability (the ease of spreading the cosmetic emulsion over a given distance), oiliness (the greasy feel perceived after cosmetic product application), and the penetration degree (the moment when the product is no longer felt at the skin surface).
Texture analysis
Texture profile analysis was performed using CT3 Texture Analyzer (Brookfield Engineering Laboratories, Middleboro, MA, USA). The following parameters were calculated: firmness, consistency, adhesiveness, stringiness, and spreadability.
The assessment of firmness, consistency, adhesiveness, and stringiness of the cream was performed using fixture base table (TA-BT-KIT) and back extrusion cell (TA-DEC), while the probe penetrated into the sample containers, to a depth of 25 mm at a rate of 2 mm/s. The force exerted to the probe was recorded using Texture ProCT Software 1.5 (Brookfield Engineering Laboratories, Middleboro MA, USA). The spreadability test was performed using fixture base table (TA-BT-KIT) and spreadability accessory (TA-SF). A conical shape sample holder was filled evenly with the sample while the cone analytical probe was forced down into each sample at a defined test speed (2 mm/s) and to a defined depth (15 mm). The samples were analyzed in triplicate, and the average values were calculated.
In vivo study design
A pilot study was performed on 12 female volunteers with age between 22 years and 60 years, who used the tested cream during 7 days. Every subject was informed about the nature and the purpose of the study and signed an informed consent before enrolling into the study. The study was carried out according to Declaration of Helsinki, regarding Ethical Principles for Medical Research Involving Human Subjects and has been approved by the Research Ethics Committee of the Iuliu Hat¸ieganu University of Medicine and Pharmacy (registration number: 534 and date of approval: December 23, 2015).
The cosmetic product was applied twice daily, by massage, at the upper arms level on the area predisposed to stretch marks occurrence. All measurements were made in the same area in controlled temperature and humidity conditions (T=22°C, relative humidity =50%±5%). The volunteers have been preconditioned in the test room for at least 15 minutes before the measurements.
The instrumental assessments were carried out at the beginning of the study (t0) and at the end of the study (t1). For every subject, the following parameters were measured: thickness of the epidermis, the hydration level of the stratum corneum, and the elasticity of the upper skin layer.
Volunteer selection
Inclusion criteria
The target population was composed by the patients predisposed to stretch marks occurrence due to important variation in weight, glucocorticoid treatment, genetic influence, or after giving birth.
The volunteers included in the study accomplished the following criteria: absence of cutaneous diseases and absence of any type of lesion on the interest area that could interfere with evaluation study.
Exclusion criteria
The study excluded patients with known allergies to one of the cream components, with known skin diseases and those who used topical treatment for increasing the elasticity of the skin in the last 2 months.
Ultrasonographic evaluation
The cutaneous changes induced by the topical use of the cream in the sites predisposed to stretch marks at the arms level were assessed with DermaLab® Combo (Cortex Technology, Hadsund, Denmark), a computer-supported skin diagnostics system equipped with a rotating high-resolution ultrasound sensor probe (20 MHz).
DermaLab® Combo represents a complex instrument for skin analysis, which combines ultrasound assessment with hydration and elasticity measurements and may be used in order to assess the efficacy of topical applied therapies or cosmetic creams. Previous studies have shown that the thickness of the epidermis and dermis, as well as the dermal density represents important parameters that assess the cutaneous regeneration process, hydration is important for all skin functions and also to improve skin appearance, and elasticity is important to prevent stretch marks.16
The thickness of the epidermis was obtained by calculating the mean of three measurements performed at three different sites of each image (the two extremities of the analyzed image and the center of the image). For the skin hydration status and elasticity, measurements data collection from the volunteers enrolled in the study consisted of quantitative assessment of hydration level (μS) and retraction time (ms) as an indicator for the elasticity of the skin.
Statistical analysis
The analysis was performed by relating the data of the cosmetic product-treated sites to the corresponding starting value. Data were collected in a Microsoft® Excel file and classified by study code, code of the volunteer subject, and day of the study.
The obtained data were analyzed, calculating the mean and standard deviation for the quantitative variables of every group and the proportions for the qualitative variables. The difference in mean values before and after treatment was tested using a Student’s t-test for paired samples. A P-value <0.05 was considered significant.
In order to evaluate the correlation between the parameters of the sensory analysis, the Pearson’s correlation coefficients were determined. The Pearson’s correlation coefficient is a measure of the linear correlation between two variables. The range of values for the coefficient is between -1 (total negative correlation) and +1 (total positive correlation), where zero values would mean no correlation at all. The dependent variable was considered to be the consistency of the cosmetic product. The variables were also tested for linearity by creating scatter plots. The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) Version 19.0 (IBM Corporation, Armonk, NY, USA).
Results
The product was very well tolerated by all the volunteers, without evoking any adverse effects (erythema and pruritus). Thus, this product may be considered as nonirritant, regarding its primary skin tolerance. Subjectively, an increase in skin firmness was noticed by the volunteers enrolled into the study.
Our study revealed significant reduction in the retraction time (time required for the raised skin to return to flat, in ms) from 720.42±108.08 to 569.33±146.30 (P=0.019). Shorter retraction time in elasticity measurements reveals an improvement in skin elasticity. Although the causes of striae distensae are not elucidated, the condition is known to relate to changes in the structures that provide skin elasticity.17–19
Regarding the hydration level of the stratum corneum, the statistic analysis showed no significant increase during the study. The mean thickness of the epidermis before the application of the cosmetic cream was 893.91 μm, while the thickness increased up to 955.25 μm (P>0.05).
The general variation pattern of the quantifiable ultrasonographic parameters after cream application is illustrated in Table 1 and Figure 1.
  | Table 1 Cutaneous parameters quantified by high-frequency ultrasound before and after cream application Abbreviation: SD, standard deviation. |
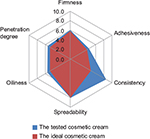
Figure 2 presents medium values of the assessors’ appreciation for each established characteristic of the tested product during the sensory analysis. As we can observe, all volunteers considered that the tested product has a good spreadability, despite the high consistency of the cosmetic cream formulation. The volunteers also appreciated that the product was not sticky and the degree of oiliness felt after application was acceptable. The tested product revealed good penetration ability into the skin.
The Pearson’s coefficient shows that there is a positive correlation between consistency and oiliness of the cream and also between adhesiveness and oiliness. A negative correlation is perceived between penetration degree and consistency values. The correlations between these variables are not very strong, but the results show that there is significance between the coefficients of the sensory analysis (Table 2).
  | Table 2 Pearson’s correlation coefficients between the parameters of the sensory analysis |
The values recorded for texture analysis were firmness 10.16±0.15 mJ, adhesiveness 30.94±6.87 g, consistency 1229.50±119.78 g, spreadability 481.50±39 g, and stringiness 0.56±0.09 mJ.
Discussion
So far, a great number of topical antistretch marks formulations containing herbal extracts have been claimed for their efficacy in the treatment of striae distensae. The present study investigated the potential effect of an emollient W/O formulation containing argan oil on preventing stretch marks. Argan oil is obtained from the fruits of argan trees (Argania spinosa) following a multistep process. The acylglycerols constitute 99% of the argan oil including 95% triacylglycerols and the remaining 4% are composed of monoacylglycerols (0.27%–0.65%), diacylglycerols (0.68%–1.53%) and free fatty acids (1.1%–2%). Unsaponifiable fraction constitutes 1% of argan oil and it is represented by carotenes (37%), tocopherols (8%), triterpene alcohols (20%), sterols (29%), and xanthophylls (5%). In extra virgin argan oil, the level of tocopherols varies between 600 mg/kg and 900 mg/kg. Other phenols that may act as antioxidants have been identified by mass spectroscopy. The chemical composition justifies the use of argan oil in antistretch marks creams for benefits in restoration of the skin water–lipid layer, neutralization of free radical agents, and protection of the conjunctive tissue.20,21
The other ingredients of the cosmetic cream were chosen for the most suitable properties in patients with striae, because in case of antistretch marks cosmetic formulations, the efficacy is given by the actives incorporated in the moisturizing formula and also by the emollient ingredients, whose role is to maintain skin elasticity.
In a previous study on early striae distensae, Sheu et al22 found that sequential changes in elastolysis accompanied by mast cell degeneration occur in the very early stage of striae distensae. Elastic fiber is the primary target of the pathological process and the abnormalities extend as far as 3 cm beyond the lesion into the normal skin.22,23 There are suggestions that deficiencies in elastic properties, endocrine imbalances, and toxins may be factors in the cause of striae distensae.24–27 Hence, an increase in the elasticity of the connective tissue presents an important aspect in the prevention of striae distensae.
Based on these observations, argan oil was incorporated in a W/O formula containing several emollients as mineral oil, silicone oil, cocoa butter, caprylic/capric triglycerides, cetaceum, glycerol stearate, beeswax, and cetylstearylic alcohol. In this formulation, triethanolamine was used to neutralize carbopol in order to achieve maximum viscosity. Glycerin was incorporated in the cream formula as moisturizer to increase stratum corneum hydration. Usually, when it is used as a humectant agent or moisturizer, it is associated with occlusive compounds, in our case glycerol stearate, beeswax, cocoa butter, and caprylic/capric triglycerides.28 Besides, occlusion and moisturizing agents are known to improve the signs and symptoms of scarring. The moisturizing agents reduce excessive scarring through a suppressive effect on collagen production in fibroblasts, while the occlusion regulates epidermal cytokine production and reduces the formation of hypertrophic scars.29 As striae have been suggested to be anatomically similar to scars, it is expected that the occlusive and moisturizing ingredients reduce the signs and symptoms of striae.30
Sensory analysis of the cosmetic cream revealed that the product was well tolerated and appreciated by the consumers regarding its spreadability, penetration ability, and lack of stickiness, mainly due to the emollients with textural qualities. Decreasing the consistency is recommended in order to increase the ease of pick up the cream from the container and to facilitate the cream application because antistretch marks products are indicated to be used for a long period on large body surfaces. Increasing the content of silicone oil and decreasing the cetylstearylic alcohol and beeswax would improve the consistency characteristics and also will ameliorate the oily feel after cream application and the adhesiveness of the cosmetic product.
Texture analysis and sensory analysis are complementary methods used in order to characterize the sensorial properties of the cream. Sensory evaluation provides useful information about perceptual attributes of the creams and consumer perception regarding cosmetic product, as these contribute to the improvements in the texture properties of the final product. Moreover, the different texturizing excipients reveal different texture properties and show the importance of the choice of excipients in order to obtain an appropriate cosmetic product.15,31
Our study revealed statistically significant changes in the skin elasticity, but no significant changes in the hydration and thickness of the epidermis, probably due to the short period of application of the cosmetic product. In the same time, the preliminary results offer an encouraging perspective for the continuation of the study for a longer time frame and reveal that the ultrasonographic assessment may be an appropriate tool in order to observe the variation in the skin parameters.
Conclusion
An emollient W/O cream containing argan oil was formulated. The study of clinical efficacy showed an increase in the skin elasticity; therefore, the formulation may be useful in the prevention and early treatment of striae distensae. For this purpose, the effect should be proven by follow-up, by observing the dynamics of the ultrasonographic parameters for a longer period of time.
Acknowledgments
This research was financially supported by the University of Medicine and Pharmacy, Cluj-Napoca, Romania, grant 1495/7/28.01.2014. The authors gratefully acknowledge Cortex Technology, Hadsund, Denmark, for allowing the use of the ultrasound equipment and Farmec Company, Cluj-Napoca, Romania, for the substances supplied.
Disclosure
The authors report no conflicts of interest in this work.
References
Hermanns JF, Piérard GE. High-resolution epiluminescence colorimetry of striae distensae. J Eur Acad Dermatol Venereol. 2006;20(3):282–287. | ||
Kim BJ, Lee DH, Kim MN, et al. Fractional photothermolysis for the treatment of striae distensae in Asian skin. Am J Clin Dermatol. 2008;9(1):33–37. | ||
Alaiti S [webpage on the Internet]. Striae Distensae Treatment & Management [cited May 20, 2015]. Available from: http://emedicine.medscape.com/article/1074868-treatment. Accessed July 26, 2016. | ||
Djuris J, Vasiljevic D, Jokic S, Ibric S. Application of D-optimal experimental design method to optimize the formulation of O/W cosmetic emulsions. Int J Cosmet Sci. 2014;36(1):79–87. | ||
Lukic M, Jaksic I, Krstonosic V, Cekic N, Savic S. A combined approach in characterization of an effective W/O hand cream: the influence of emollient on textural, sensorial and in vivo skin performance. Int J Cosmet Sci. 2012;34(2):140–149. | ||
Schmid-Wendtner MH, Burgdorf W. Ultrasound scanning in dermatology. Arch Dermatol. 2005;141(2):217–224. | ||
Szyman´ska E, Nowicki A, Mlosek K, et al. Skin imaging with high frequency ultrasound-preliminary results. Eur J Ultrasound. 2000;12(1):9–16. | ||
Stojanoviæ S, Poljacki M, Ros T. Diagnostic importance of ultrasound in dermatology. Med Pregl. 2002;55(9–10):392–396. | ||
Tan CY, Statham B, Marks R, Payne PA. Skin thickness measurement by pulsed ultrasound: its reproducibility, validation and variability. Br J Dermatol. 1982;106(6):657–667. | ||
Bleve M, Capra P, Pavanetto F, Perugini P [webpage on the Internet]. Ultrasound and 3D skin imaging: methods to evaluate efficacy of striae distensae treatment. Dermatol Res Prat. 2012 [cited May 12, 2014]. Available from: http://www.hindawi.com/journals/drp/2012/673706/#B30. Accessed July 26, 2016. | ||
Gammal S, Auer T, Hoffman K, Altmeyer P. High-resolution ultrasound of human epidermis. In: Serup J, Jemec G, editors. Handbook of Noninvasive Methods and the Skin. Vol. 1. Boca Raton, FL: CRC Press; 1995:125–131. | ||
Seidenari S, Pagnoni A, Di Nardo A, Giannetti A. Echographic evaluation with image analysis of normal skin: variations according to age and sex. Skin Pharmacol. 1994;7(4):201–209. | ||
Crisan D, Lupsor M, Boca A, Crisan M, Badea R. Ultrasonographic assessment of skin structure according to age. Indian J Dermatol Venereol Leprol. 2012;78(4):519. | ||
Crisan M, Badea R, Cattani C, Crisan D. Senescence: imagistic noninvasive assessment of skin aging and anti-aging therapies. In: Nagata T, editor. Senescence. Rijeka: Intech; 2012:773–796. | ||
Kulkamp-Guerreiro IC, Berlitz SJ, Contri RV, et al. Influence of nanoencapsulation on the sensory properties of cosmetic formulations containing lipoic acid. Int J Cosmet Sci. 2013;35(1):105–111. | ||
Crisan D, Crisan M, Moldovan M, Lupsor M, Badea R. Ultrasonographic assessment of the cutaneous changes induced by topical flavonoid therapy. Clin Cosmet Investig Dermatol. 2012;5:7–13. | ||
Kozel BA, Bayliss SJ, Berk DR, et al. Skin findings in Williams syndrome. Am J Med Genet A. 2014;164A(9):2217–2225. | ||
Hadi H, Awadh AI, Hanif NM, Md Sidik NF, Mohd Rani MR, Suhaimi MS. The investigation of the skin biophysical measurements focusing on daily activities, skin care habits, and gender differences. Skin Res Technol. 2016;22(2):247–254. | ||
Kelekci KH, Kelekci S, Destegul E, Aksoy A, Sut N, Yilmaz B. Prematurity: is it a risk factor for striae distensae? Int J Dermatol. 2011;50(10):1240–1245. | ||
Charrouf Z, Guillaume D. Argan oil: occurrence, composition and impact on human health. Eur J Lipid Sci Technol. 2008;110(7):632–636. | ||
El Monfaloutia H, Guillaume D, Denheza C, Charrouf Z. Therapeutic potential of argan oil: a review. J Pharm Pharmacol. 2010;62(12):1669–1675. | ||
Sheu HM, Yu HS, Chang CH. Mast cell degranulation and elastolysis in the early stage of striae distensae. J Cutan Pathol. 1991;18(6):410–416. | ||
Singh G, Kumar L. Striae distensae. Indian J Dermatol Venereol Leprol. 2005;71(5):370–372. | ||
Mitts TF, Jimez F, Hinek A. Skin biopsy reveals predisposition to stretch mark formation. Aesthet Surg J. 2005;25(6):593–600. | ||
Tsuji T, Sawabe M. Elastic fibres in striae distensae. J Cutan Pathol. 1988;15(4):215–222. | ||
Zheng P, Lavker RM, Kligman AM. Anatomy of striae. Br J Dermatol. 1985;112(2):185–193. | ||
Ud-Din S, McAnelly SL, Bowring A, et al. A double-blind controlled clinical trial assessing the effect of topical gels on striae distensae (stretch marks): a non-invasive imaging, morphological and immunohistochemical study. Arch Dermatol Res. 2013;305(7):603–617. | ||
Chularojanamontri L, Tuchinda P, Kulthanan K, Pongparit K. Moisturizers for acne: what are their constituents? J Clin Aesthet Dermatol. 2014;7(5):36–44. | ||
Gallant-Behm CL, Mustoe TA. Occlusion regulates epidermal cytokine production and inhibits scar formation. Wound Repair Regen. 2010;18(2):235–244. | ||
Rawlings AV, Bielfeldt S, Lombar KJ. A review of the effects of moisturizers on the appearance of scars and striae. Int J Cosmet Sci. 2012;34(6):519–524. | ||
Guest S, McGlone F, Hopkinson A, Schendel Z, Blot K, Essick G. Perceptual and sensory-functional consequences of skin care products. J Cosmet Dermatol Sci Appl. 2013;3(1A):66–78. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.