Back to Journals » Clinical Ophthalmology » Volume 14
Preclinical Investigation of Goniotomy Using Four Different Techniques
Authors Ammar DA , Seibold LK, Kahook MY
Received 17 September 2020
Accepted for publication 15 October 2020
Published 28 October 2020 Volume 2020:14 Pages 3519—3525
DOI https://doi.org/10.2147/OPTH.S281811
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
David A Ammar,1 Leonard K Seibold,2 Malik Y Kahook2
1Research Department, Lions Eye Institute for Transplant & Research, Tampa, FL, USA; 2Department of Ophthalmology, University of Colorado School of Medicine, Aurora, CO, USA
Correspondence: Malik Y Kahook
University of Colorado School of Medicine, 1675 Aurora Ct, Aurora, CO 80045, USA
Tel +1 720 848 2500
Fax +1 720 848 5014
Email [email protected]
Purpose: To evaluate the tissue-level effects of goniotomy techniques on human trabecular meshwork (TM).
Design: Laboratory investigation.
Methods: The TM from human cadaveric corneal rim tissue was treated using 4 techniques: (1) microvitreoretinal (MVR) blade; (2) 360° trabeculotomy with 5-0 prolene suture; (3) the Kahook Dual Blade (KDB) Glide® device; (4) TrabEx™ device; tissue samples underwent standard histologic processing with H&E stain followed by comparative analyses.
Results: The MVR blade exhibited incision of TM extending into the scleral wall. The TrabEx device removed a small portion of TM with large leaflet tissue remnants in all treated areas. 360° suture trabeculotomy resulted in incision of the TM proximate to Schwalbe’s line with no excised tissue evident in all treated areas. Areas treated with the KDB Glide device resulted in nearly complete excision of TM without injury to surrounding tissues.
Conclusion: The various methods used for performing goniotomy or trabeculotomy resulted in varying degrees of incision or excision of TM. Only the KDB Glide device resulted in reliable excision of TM with the other devices producing incision or variable excision of tissue. Clinical correlation is required to better understand the implications of the current findings when using these methods to lower intraocular pressure in eyes with glaucoma.
Keywords: glaucoma, goniotomy, trabecular meshwork
Introduction
Goniotomy—the surgical technique of incising trabecular meshwork (TM) to create a continuous opening from the anterior chamber to the Canal of Schlemm—was first described in 19361 and was soon relegated to the surgical management of childhood glaucomas2 until a popular resurgence over recent years in modified form for adult eyes with glaucoma.3–6 Numerous variations on the theme of goniotomy are currently used to lower intraocular pressure (IOP) in eyes with various forms of glaucoma; these include incisional goniotomy using a microvitreoretinal (MVR) blade or the Trabectome® device (MicroSurgical Technology, Redmond, WA); excisional goniotomy using the Kahook Dual Blade® device (KDB, New World Medical, Rancho Cucamonga, CA) or the TrabEx™/TrabEx+™ device (Microsurgical Technology); and 360° trabeculotomy (gonioscopy-assisted transluminal trabeculotomy [GATT] using a suture or the iTrack® device [Ellex, Adelaide, Australia]) or the TRAB®360/OMNI® system (Sight Sciences, Menlo Park, CA).
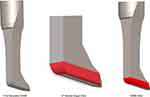
These various procedures have differential effects on the tissues of the anterior chamber angle. In a preclinical evaluation, the histologic effects of the KDB, MVR, and Trabectome—both on the TM and neighboring tissue structures—were previously described.7 The TrabEx device had not been commercialized at that time and was not included in the analysis. Also, since that report, a second-generation KDB instrument (KDB Glide, New World Medical) has been developed that incorporates features intended to enhance both its performance and its ease of use. The KDB Glide has incorporated beveled edges and rounded corners to the footplate, reducing its width for enhanced fit within Canal of Schlemm to facilitate smooth passage while excising TM (Figure 1). The second-generation instrument maintains the same ramp technology and distance between the parallel dual blades (230 microns) as the original KDB.
In this report, we compare the histologic appearance of the TM and neighboring angle structures after goniotomy using the KDB Glide, MVR blade, TrabEx, and 360° trabeculotomy using a 5-0 prolene suture (in a manner emulating gonioscopy-assisted transluminal trabeculotomy [GATT]8), in human eye bank eyes.
Methods
Exemption for this preclinical study was in place from the Colorado Multiple Institutional Review Board (COMIRB) for the use of human material prior to initiation of this study and the tenets of the Declaration of Helsinki were followed. COMIRB instructions state that IRB exemption applies to non-human subject studies where all information is de-identified and devoid of non-public information disclosures. A total of 4 corneoscleral rims were used for this study. All tissue samples were obtained from eyes lacking any history of glaucoma or surgical procedures in the anterior chamber other than cataract surgery. After removal from storage medium, each sample was mounted on a platform oriented with the TM facing up and secured with tissue pins. Each of the four techniques assessed consisted of >180° TM treatment under microscopic visualization. MVR blade: TM was incised with an MVR blade, with effort made to maintain incision depth into but not through the Canal of Schlemm. Suture trabeculotomy: A 360° ab interno trabeculotomy was performed by threading a 5-0 prolene suture into and fully around the Canal of Schlemm through a small goniotomy incision, and then applying tension to both ends to cheesewire through the complete TM circumference. This approach mimicked the GATT procedure,8 modified for direct visualization in this laboratory study. KDB Glide: As has been reported for the KDB,7 the Glide’s blade tip (Figure 1) was used to incise TM and the blade’s footplate positioned and then advanced along the Canal of Schlemm. At the end of the intended excision length, the device was redirected by inserting the tip just beyond the initial TM strip and moving the device in the direction of the original treatment to completely excise a whole strip of TM. TrabEx: In similar fashion to the KDB Glide, the instrument’s tip (Figure 2) was used to incise TM and positioned in Canal of Schlemm. The device was then moved forward to treat an arc of TM. At the end of the intended treatment length, the device was redirected by inserting the tip just beyond the initial treated TM and moving the device in the direction of the original treatment to attempt removal of a TM strip.
 |
Figure 2 TrabEx blade, featuring two serrated blades (arrow) without a non-cutting ramp. |
Following these procedures, each corneoscleral rim was preserved in 4% paraformaldehyde/phosphate-buffered saline overnight at 4° C and then cut into quadrants with radial incisions. Rim sections were embedded in paraffin with the cut edge of the tissue facing the front of the block. Tissue sections, each 6 μm thick, were then cut from randomly selected locations across the treated areas. Sections of untreated TM were also sectioned for comparison to treated areas. Each sample was stained using Mayer’s hematoxylin-eosin Y (Richard-Allan Scientific, Kalamazoo, Michigan, USA). A Nikon Eclipse 80i microscope (Nikon, Melville, New York, USA) fitted with a Nikon D5-Fi1 color camera and a Nikon CFI 10x/Plan Fluor objective lens was used to obtain bright-field imaging. Images were then evaluated in masked fashion to qualitatively characterize the tissue effects of each technique.
Results
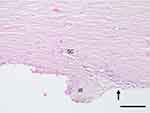
Systematic evaluation of tissue sections was undertaken. Findings were consistent across all sections within each eye for each procedure evaluated. The normal histology of the human anterior chamber angle is given in Figure 3, obtained from an unoperated portion of one of the four treatment samples. The iris is lost during recovery of the tissue from the donor leaving a remnant iris root; in contrast, the TM and Canal of Schlemm generally remain intact after recovery.
Figure 4 depicts the angle following MVR goniotomy. This technique created a complete incision of the full thickness of the TM (Figure 4A). TM tissue was not removed, and large tissue leaflets remained overlying Canal of Schlemm. The incision was noted to extend beyond the outer wall of the canal, producing scleral injury of variable depth along the incision (Figure 4A vs 4B).
Figure 5 depicts the angle following suture trabeculotomy. TM incision proximate to Schwalbe’s line was evident; no evidence of tissue excision or neighboring tissue injury was present.
Figure 6 depicts the angle following TrabEx goniotomy. Variable portions of TM removal were evident, and large leaflet tissue remnants ranging from 100 to 200 microns in total length were visible in all treated areas. No injury to neighboring tissue was present.
Figure 7 depicts the angle following goniotomy with the KDB Glide. Near complete excision of TM was noted in treatment areas, with intermittent areas of residual TM leaflet remnants of no more than 50 microns in width. No injury to neighboring tissue was present.
Discussion
The quest for a safe and effective alternative to trabeculectomy or tube-shunt implantation—specifically one that avoids the formation of a subconjunctival filtering bleb and its short- and long-term risks9,10 —has renewed interest in procedures that bypass the diseased TM in eyes with open-angle glaucoma. The bypass of TM facilitates egress of aqueous humor from the anterior chamber into the Canal of Schlemm and the distal outflow system. Methods by which the TM is bypassed in these various procedures include incisional and excisional procedures that cut TM from the anterior chamber into the Canal of Schlemm (goniotomy), incisional procedures that cut TM from the Canal of Schlemm into the anterior chamber (trabeculotomy), and various implantable trans-TM devices that shunt fluid across the TM through small lumens.3–6
Each of these surgical approaches produces an opening in the TM with unique characteristics, some of which have a significant impact on the aqueous flow capacity and thus potentially on IOP reduction. Incisional techniques disrupt but do not remove TM tissue, leaving remnants of tissue on either side of the incision that can reapproximate and close the ostomy.7,11 Excisional techniques remove or ablate a strip of TM, typically of 90°–180°. Ideally, the strip would be uniform in width and adequately wide to prevent reapproximation of remnant tissue leaflets; however, the strip can be variable in width over the course of the excision,7 producing ostomies of variable size. Devices such as the trabecular microbypass (iStent® and iStent Inject® stents, Glaukos, San Clemente, CA) produce small stented apertures in TM (80–120 microns in diameter12) without tissue removal; histological analysis has revealed a basement membrane like layer of fibrous tissue overlying the internal ostomy of this device in some eyes post-implantation.13
In the current study, of the 4 techniques evaluated, only two—the KDB Glide and TrabEx—perform excisional goniotomy. The KDB Glide produced an excisional opening in TM of consistent width along its entire length and did so without obvious damage to neighboring structures. This finding is consistent with a prior study of the original KDB instrument in cadaver eyes.7 The TrabEx device produced an excisional opening of variable width, leaving large (up to 200 microns) tissue leaflet remnants which can reapproximate to close the opening.7,11 While these devices have two blades, other key design features likely account for these histological differences. The KDB Glide features a non-cutting ramp that elevates, stretches, and guides TM to two parallel blades before any parallel incisions are created in the TM. The ramp’s role in putting TM on stretch before excision maximizes the width of the excised TM strip and minimizes residual TM tissue leaflets that could reapproximate and close the excisional ostomy between the anterior chamber and the lumen of Canal of Schlemm. The footplate of the KDB devices is designed to allow for proper fit and stability during the procedure which also likely leads to a more uniform treatment of tissue. In contrast, the TrabEx features two parallel serrated blades that extend to the tip of the device. The lack of a non-cutting ramp, as is found in KDB, leads to TM tissue cutting being initiated upon insertion of the device tip into the canal without the tissue being elevated and stretched. The TrabEx device also does not have a distinct footplate that would allow for evenly positioning the device on the anterior wall of the canal of Schlemm while advancing forward for treatment. These differences appear to lead to inconsistent excision of TM tissue along its path. The KDB Glide also improves on the original KDB by incorporating smoothed and rounded contours and a narrower footplate (203 microns compared to the TrabEx base width of 330 microns) to facilitate intra-canal passage (Figure 1). The diameter of Canal of Schlemm has been estimated to be approximately 240 microns or less;14 instruments less wide than this dimension should thus engage and move within the canal more easily than oversized instruments.
In summary, the various methods used for performing goniotomy or trabeculotomy resulted in varying degrees of incision or excision of TM. Only the KDB Glide device resulted in reliable excision of TM, with the other devices producing incision or variable excision of tissue. Clinical correlation is required to better understand the implications of the current findings when using these methods to lower IOP in eyes with glaucoma.
Acknowledgments
Assistance with manuscript preparation was provided by Tony Realini, MD, MPH, with support from New World Medical. Content from this paper was presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting in June of 2020 as an abstract presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Investigative Ophthalmology and Visual Science: https://iovs.arvojournals.org/article.aspx?articleid=2766810.
Disclosure
DA Ammar has received financial support from New World Medical for contract work through his employer. Malik Kahook is a consultant to New World Medical, SpyGlass Ophthalmics and Alcon. He receives patent royalties from Alcon, New World Medical, Johnson and Johnson Vision, Fluent Ophthalmics, SpyGlass Ophthalmics, ShapeTech and Aurea Medical. He also reports a patent US10327947B2 with royalties paid by New World Medical. Leonard K Seibold is a consultant to New World Medical and reports grants from Glaukos and Allergan, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Barkan O. A new operation for chronic glaucoma. Am J Ophthalmol. 1936;19:951–966. doi:10.1016/S0002-9394(36)93838-2
2. Barkan O. Present status of goniotomy. Am J Ophthalmol. 1953;36(4):445–453. doi:10.1016/0002-9394(53)90555-8
3. Chen DZ, Sng CCA. Safety and efficacy of microinvasive glaucoma surgery. J Ophthalmol. 2017;2017:3182935. doi:10.1155/2017/3182935
4. Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi:10.1371/journal.pone.0183142
5. Pillunat LE, Erb C, Junemann AG, Kimmich F. Micro-invasive glaucoma surgery (MIGS): a review of surgical procedures using stents. Clin Ophthalmol. 2017;11:1583–1600. doi:10.2147/OPTH.S135316
6. Richter GM, Coleman AL. Minimally invasive glaucoma surgery: current status and future prospects. Clin Ophthalmol. 2016;10:189–206.
7. Seibold LK, Soohoo JR, Ammar DA, Kahook MY. Preclinical investigation of ab interno trabeculectomy using a novel dual-blade device. Am J Ophthalmol. 2013;155(3):524–529 e522. doi:10.1016/j.ajo.2012.09.023
8. Grover DS, Godfrey DG, Smith O, Feuer WJ, Montes de Oca I, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855–861. doi:10.1016/j.ophtha.2013.11.001
9. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814. doi:10.1016/j.ajo.2011.10.024
10. Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC. Surgical complications in the tube versus trabeculectomy study during the first year of follow-up. Am J Ophthalmol. 2007;143(1):23–31. doi:10.1016/j.ajo.2006.07.022
11. Amari Y, Hamanaka T, Futa R. Pathologic investigation failure of trabeculotomy. J Glaucoma. 2015;24(4):316–322. doi:10.1097/IJG.0b013e31829e1d6e
12. Manning D. Real-world case series of iStent or iStent inject trabecular micro-bypass stents combined with cataract surgery. Ophthalmol Ther. 2019;8(4):549–561.
13. Capitena Young CE, Ammar DA, Seibold LK, Pantcheva MB, SooHoo JR, Kahook MY. Histopathologic examination of trabecular meshwork changes after trabecular bypass stent implantation. J Glaucoma. 2018;27(7):606–609. doi:10.1097/IJG.0000000000000968
14. Ten Hulzen RD, Johnson DH. Effect of fixation pressure on juxtacanalicular tissue and Schlemm’s canal. Invest Ophthalmol Vis Sci. 1996;37(1):114–124.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.