Back to Journals » International Journal of Nanomedicine » Volume 12
Polymeric mixed micelles loaded mitoxantrone for overcoming multidrug resistance in breast cancer via photodynamic therapy
Authors Li ZY, Cai Y, Zhao YQ, Yu H , Zhou HY, Chen MW
Received 29 March 2017
Accepted for publication 21 May 2017
Published 6 September 2017 Volume 2017:12 Pages 6595—6604
DOI https://doi.org/10.2147/IJN.S138235
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Thomas Webster
Zeyong Li,1,* Yuee Cai,2,* Yiqiao Zhao,1 Hua Yu,2 Haiyu Zhou,3 Meiwan Chen2
1Department of Laboratory Medicine, Guangdong No 2 Provincial People’s Hospital, Guangzhou, China; 2State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Macau, China; 3Department of Thoracic Surgery, Guangdong General Hospital, Guangdong Academy of Medical Sciences, Southern Medical University, South China University of Technology, Guangzhou, China
*These authors contributed equally to this work
Abstract: Mitoxantrone (MIT) is an anticancer agent with photosensitive properties that is commonly used in various cancers. Multidrug resistance (MDR) effect has been an obstacle to using MIT for cancer therapy. Photochemical internalization, on account of photodynamic therapy, has been applied to improve the therapeutic effect of cancers with MDR effect. In this study, an MIT-poly(ε-caprolactone)-pluronic F68-poly(ε-caprolactone)/poly(D,L-lactide-co-glycolide)–poly(ethylene glycol)–poly(D,L-lactide-co-glycolide) (MIT-PFP/PPP) mixed micelles system was applied to reverse the effect of MDR in MCF-7/ADR cells via photochemical reaction when exposed to near-infrared light. MIT-PFP/PPP mixed micelles showed effective interaction with near-infrared light at the wavelength of 660 nm and exerted great cytotoxicity in MCF-7/ADR cells with irradiation. Furthermore, MIT-PFP/PPP mixed micelles could improve reactive oxygen species (ROS) levels, decrease P-glycoprotein activity, and increase the cellular uptake of drugs with improved intracellular drug concentrations, which induced cell apoptosis in MCF-7/ADR cells under irradiation, despite MDR effect, as indicated by the increased level of cleaved poly ADP-ribose polymerase. These findings suggested that MIT-PFP/PPP mixed micelles may become a promising strategy to effectively reverse the MDR effect via photodynamic therapy in breast cancer.
Keywords: mitoxantrone, polymeric mixed micelles, multidrug resistance, photodynamic therapy, breast cancer
Introduction
Mitoxantrone (MIT, Figure 1A), a synthetic derivative of anthracenedione, is a topoisomerase II targeting drug with high efficacy in the treatment of various malignancies such as breast cancer and acute leukemia.1,2 Overexpression of adenosine triphosphate-binding cassette (ABC) transporters is associated with resistance to different anticancer agents, including MIT, which hinders the clinical application of MIT for cancer treatment.2 P-glycoprotein (P-gp) is one of the ABC transporters and is overexpressed in >40% of breast cancer cases. It participates in the active transport of anticancer drugs out of cancer cells, leading to decreased intracellular concentration of drugs and resulting in multidrug resistance (MDR) in clinical cases.3 Therefore, reversing the MDR effect on MIT in breast cancer has been a primary strategy to improve cancer therapy. Various methods have been applied to inhibit ABC transporters with the aim to improve the retention of anticancer drugs in cancer cells, including low-molecular weight P-gp inhibitors, polymer–drug conjugates, high-molecular weight polymer carriers, photodynamic therapy (PDT), and so on.4–6
PDT is a minimally invasive and clinically approved therapeutic modality with selective cytotoxicity against cancer. PDT requires three major components: photosensitizing agents, tissue oxygen, and laser irradiation at the wavelength of the photosensitizer.7 Photosensitizers kill cancer cells by generating reactive oxygen species (ROS) via transferring absorbed photon energy to oxygen molecules when the cells are exposed to irradiation with an appropriate wavelength. Photosensitizers are not toxic to cells without laser irradiation.8 PDT improves selectivity of anticancer agents and also decreases their side effects, compared to conventional radiotherapies and chemotherapies.9 Recently, photochemical internalization, an application of PDT, has been employed to improve the chemotherapeutic effect in cancers with MDR. Photochemical internalization destroys the cellular membrane with laser exposure by reactive oxygen-induced lipid perioxidation.6 MIT has two major absorption peaks at the wavelengths of 610 and 660 nm, and it is an efficient photosensitizer to mediate cell death with exposure to light at the wavelength of 660 nm.10 Studies of PDT using MIT to treat breast cancer and melanoma cancer have been reported.11,12 To conquer the MDR effect of MIT in breast cancer cells, using the photosensitizing property of MIT may exert unexpected anticancer effect due to the generation of ROS in cancer cells.
Polymeric micelles formed by high-molecular weight polymers are capable of encapsulating hydrophobic drugs through their core–shell structure with favorable size and enhanced permeability and retention effect, of which some function as a biologic modifier against MDR.13,14 In our previous study, a mixed micelle system, formed by poly(ε-caprolactone)-pluronic F68-poly(ε-caprolactone) (PFP) and poly(D,L-lactide-co-glycolide)–poly(ethylene glycol)–poly (D,L-lactide-co-glycolide) (PPP), has been designed to efficiently deliver the anticancer agent MIT, while reversing MDR in breast cancer.15 As mentioned above, the application of PDT could reverse the MDR effect in cancer cells.6 It is meaningful to investigate the photosensitizing property of a clinically used anticancer agent (MIT) as PDT against the cancer cells with MDR. In this study, the previously designed PFP/PPP mixed micelles were applied to deliver MIT (MIT-PFP/PPP mixed micelles) to evaluate the anticancer effect of MIT with exposure to near-infrared light on the MDR breast cancer cells, the MCF-7/ADR cells.
Materials and methods
Materials
MIT (98% purity) was obtained from Meilun Biology Technology Company (Dalian, China). Phosphate buffer saline (PBS), penicillin–streptomycin, fetal bovine serum (FBS), 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (w/v) and propidium iodide (PI) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Hoechst 33342, wortmannin, genistein, methyl-β-cyclodextrin, 2-deoxyglucose and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma Aldrich (St Louis, MO, USA). ROS Assay Kit was obtained from Beyotime (Shanghai, China). The primary antibodies against GAPDH and poly ADP-ribose polymerase (PARP) were obtained from Cell Signaling Technology (Boston, MA, USA). Multi-Drug Resistance Assay Kit was purchased from Cayman Chemical (Ann Arbor, MI, USA). The chemicals were all of analytical grade, and ultra-filtered water was obtained with a Milli-Q apparatus (EMD Millipore, Billerica, MA, USA).
Cell lines and cell culture
Doxorubicin-resistant MCF-7 cells, named MCF-7/ADR, were obtained by stepwise exposure of MCF-7 cells to doxorubicin with increasing concentrations as previously reported.16 MCF-7 cells were obtained from American Tissue Culture Collection. MCF-7/ADR cells were incubated in Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% FBS (v/v), 100 U/mL streptomycin and 100 μg/mL penicillin with 5% CO2 at 37°C.
Absorption properties of MIT
To obtain the light absorbance band of MIT, the absorption spectra of blank PFP/PPP mixed micelles, MIT and MIT-PFP/PPP mixed micelles were measured by DR6000 UV-Visible Spectrophotometer (HACH, Loveland, CO, USA). Various concentrations of MIT-PFP/PPP mixed micelles (12.5, 25, 50, 100, 200 μM) were detected by UV–Visible Spectrophotometer to obtain the MIT light absorption spectra.12 The experiments were carried out in triplicate.
Preparation and characterization of MIT-PFP/PPP mixed micelles
MIT-PFP/PPP mixed micelles were prepared by solvent evaporation as reported previously.15 Fourteen milligrams copolymers of PFP and PPP with the ratio of 1:2 were mixed with 1 mg MIT. The mixtures were then dissolved in water miscible organic solvent (800 μL) of methanol and acetonitrile (1:1, v/v) under ultrasonication. When the copolymers were completely dissolved, the mixed solution was added drop-wise into pure water and stirred for 5 h until the methanol and acetonitrile were evaporated. The final solution was filtered using a 0.45 μM Millipore filter to remove any large aggregate. Filtration isolated MIT-PFP/PPP micelles with particle size <450 nm. The whole procedure was performed in a dark room. The experiments were carried out in triplicate. The particle size of MIT-PFP/PPP mixed micelles was detected by dynamic light scattering with a Zetasizer Nano ZSP system. The drug concentration of MIT-PFP/PPP mixed micelles was detected by Waters e2695 HPLC with a C18 reverse-phase liquid chromatography column (250×4.6 mm) at a flow rate of 1 mL/min and maximum absorption wavelength of 609 nm. The mobile phase was methanol/0.25% acetic acid (50/50, v/v).
In vitro cytotoxicity by photodynamic treatment
MTT assay was used to determine the cytotoxicity of MIT-PFP/PPP mixed micelles with or without irradiation at 660 nm in MCF-7/ADR cells.17 MCF-7/ADR cells were seeded in 96-well plates with 100 μL medium at a density of 5×103 cells/well and then incubated for 24 h. Cells were treated with MIT and MIT-PFP/PPP mixed micelles at various concentrations ranging from 2.5 to 20 μM. After incubation for 4 h, the original medium was discarded and fresh medium containing 0.5% FBS was added with laser irradiation at the power of 6, 12 and 24 mW for 0.5 h with a 660 nm fiber-coupled laser system (LOSBLD-0660-2W; Hi-Tech Optoelectronics Co., Ltd., Beijing, China). After incubation for 24 h at 37°C, 20 μL MTT dye at a concentration of 5 mg/mL was used to replace the medium and cells were incubated for another 4 h to form formazan crystals via mitochondrial dehydrogenases. Formazan crystals were dissolved with dimethyl sulfoxide. A microplate reader (SpectraMax M5; Molecular Devices, LLC, Sunnyvale, CA, USA) was used to record the spectrophotometric absorbance at 570 nm, which was analyzed to demonstrate relative cell viability. The experiments were carried out in triplicate.
Measurement of ROS
An ROS assay kit was used to determine the intracellular ROS levels of cells treated with MIT or MIT-PFP/PPP mixed micelles.18 Briefly, MCF-7/ADR cells were seeded in 12-well plates at a density of 8×104 cells/well and incubated for 24 h. MIT and MIT-PFP/PPP mixed micelles at a concentration of 20 μM were added to MCF-7/ADR cells. After incubation for 4 h, fresh medium with 0.5% FBS was used to replace the medium and cells were irradiated with 660 nm fiber-coupled laser at a power of 24 mW for 0.5 h. The cells were then stained with 10 μM 2,7-dichlorodi-hydrofluorescein diacetate and incubated at 37°C for 30 min. Cells were washed with PBS three times and collected. Stained cells were analyzed by flow cytometry (BD FACS Canto™). The experiments were carried out in triplicate.
P-gp activity assay
A multidrug resistance assay kit (Cayman Chemical) was utilized to determine the activity of P-gp.19 MCF-7/ADR cells at a density of 5×103 cells/well were seeded in 96-well flat clear-bottom black-wall microplates and then incubated for 24 h. Then, the cells were treated with MIT and MIT-PFP/PPP mixed micelles and irradiated with laser mentioned above. One hundred microliters MDR dye-loading solution was added to each well followed by incubation at 37°C for another 1 h in the dark. Intracellular fluorescence was measured by a microplate reader with excitation wavelength of 490 nm and emission wavelength of 525 nm. The experiments were carried out in triplicate.
Cellular uptake of MIT and MIT-PFP/PPP and its mechanisms
The cellular uptake of MIT and MIT-PFP/PPP mixed micelles was determined by flow cytometry (BD Accuri), which could measure the fluorescence of MIT associated with the cells using FL4 channel.17,20 MCF-7/ADR cells at a density of 1×105 cells/wells were seeded in 12-well plates and cultured for 24 h. Cells were treated with MIT and MIT-PFP/PPP mixed micelles for 2 h, followed by laser irradiation as mentioned above. After incubation for another 1.5 h, the cells were collected by trypsinization and washed twice with PBS. Cells were suspended with 0.5 mL PBS and measured with flow cytometry. The experiments were carried out in triplicate.
The mechanisms of cellular uptake were determined using various endocytic inhibitors.21 Briefly, MCF-7/ADR cells were seeded at a density of 1×105 cells/well in 12-well plates and cultured for 24 h. Then, the cells were preincubated in the presence of different endocytic inhibitors for 1 h, followed by treatment with MIT and MIT-PFP/PPP mixed micelles at a concentration of 20 μM for 2 h. The endocytic inhibitors were added at the following concentrations: 10 μM of wortmannin, 50 μM of genistein, 5 mM of methyl-β-cyclodextrin and 20 mM of 2-deoxyglucose. The cells were irradiated as mentioned above. After incubation for another 1.5 h, the cells were trypsinized and washed twice with PBS. The cells were suspended in 0.5 mL PBS and analyzed by flow cytometry. The experiments were carried out in triplicate.
Assessment of cell apoptosis
Hoechst 33342 staining assay was used to observe nuclear morphologic changes and chromosome condensation in MCF-7/ADR cells.22 MCF-7/ADR cells were cultured in 96-well plates at a density of 5×103 cells/well for 24 h. MIT and MIT-PFP/PPP mixed micelles were added to the cells and irradiated with laser as mentioned above. After incubation for 24 h at 37°C, 4% paraformaldehyde was utilized to fix the cells for 15 min and they were washed with PBS. The cells were then stained with 1 μg/mL of Hoechst 33342 at 25°C for 20 min in darkness. Lastly, the cells were observed and imaged by Incell Analyzer 2000 (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA), which was equipped with a 4′,6-diamidino-2-phenylindole filter (excitation of 350 nm and emission of 455 nm). The experiments were carried out in triplicate.
Apoptosis was evaluated using the Annexin V-fluorescein isothiocyanate/PI detection kit (BD Pharmingen).23 MCF-7/ADR cells were cultured at a density of 5×103 cells/well in six-well plates for 24 h. The cells were treated with MIT and MIT-PFP/PPP mixed micelles and irradiated with a laser as mentioned above. After incubation for 24 h at 37°C, the cells were collected by trypsinization, washed twice with ice-cold PBS and gently suspended in 100 μL binding buffer containing 20 μg/mL Annexin V-fluorescein isothiocyanate stain for 30 min, followed by staining with PI (10 μL) for 5 min. Cell apoptosis was measured by flow cytometry. The experiments were carried out in triplicate.
Western blot analysis
Western blot analysis was used to measure apoptosis of cells treated with MIT and MIT-PFP/PPP mixed micelles.24 Briefly, MIT and MIT-PFP/PPP mixed micelles were added to MCF-7/ADR cells and irradiated with laser as mentioned above. After the cells were incubated for 24 h, total cellular proteins were obtained using radioimmunoprecipitation assay lysis buffer with 1% phenylmethanesulfonylfluoride and 1% protease inhibitor cocktail (Thermo Fischer Scientific). The concentrations of total protein were determined by a bicinchoninic acid protein assay kit (Thermo Fischer Scientific). Then, 15% sodium dodecylsulfate polyacrylamide gel electrophoresis was used to separate proteins and the corresponding proteins were transferred to polyvinylidene fluoride membranes (0.22 μm). After being blocked for 1 h by 5% non-fat dried milk, the membranes were incubated with specific primary antibodies (1:1,000; Cell Signaling Technology) against PARP and GAPDH, followed by incubation with secondary rabbit antibodies (1:1,000; Cell Signaling Technology). An electrochemiluminescence Advanced Western Blotting Detection Kit from GE Healthcare Bio-Sciences Corp. was used to visualize the protein bands. The experiments were carried out in triplicate.
Statistical analysis
The data were analyzed by one-way analysis of variance followed by Tukey’s Multiple Comparison Test using GraphPad Prism 5 software (GraphPad Software, Inc., San Diego, CA, USA). The results were expressed as mean ± SD. P-value lower than 0.05 was considered statistically significant.
Results and discussion
Absorption properties of MIT
It was reported that MIT has two photo absorption peaks, one at 600 nm and the other at 660 nm. MIT acts as a photosensitizer with laser irradiation at the wavelength of 660 nm to induce cell death.12 As shown in Figure 1B, both MIT and MIT-PFP/PPP mixed micelles have two major absorption peaks at 600 and 660 nm, while PFP/PPP micelles have no specific absorption peak at the wavelength range of 400–1,000 nm. This result indicated that the encapsulation of MIT by PFP/PPP mixed micelles had no influence in the absorption properties of MIT, enabling its effective interaction with near-infrared light at the wavelength of 660 nm. Additionally, the photo absorption of MIT-PFP/PPP mixed micelles increases in a dose-dependent manner (Figure 1C).
In vitro cytotoxicity by photodynamic treatment
MTT assay was used to determine the cytotoxicity of MIT-PFP/PPP mixed micelles via PDT in MCF-7/ADR cells. As shown in Figure 2, cells treated with or without free MIT showed negligible cytotoxicity in MCF-7/ADR cells with or without irradiation (6 and 12 mW) for 24 h, indicating that free MIT has no obvious anticancer effect in MCF-7/ADR cells. Meanwhile, MCF-7/ADR cells irradiated with different laser intensities (6, 12 and 24 mW) had no cytotoxicity. Cell viability reduced to around 60% in the cells treated with MIT-PFP/PPP mixed micelles (20 μM) and irradiation at 24 mW, while the reduction rates of cell viability at 6 and 12 mW were about 46% and 28.61%, respectively. With irradiation, MCF-7/ADR cells treated with MIT (20 μM) exhibited 11% cytotoxicity in MCF-7/ADR cells, which was much lower than that of MIT-PFP/PPP mixed micelles (20 μM) with irradiation (60%). Without irradiation, MCF-7/ADR cells treated with MIT-PFP/PPP mixed micelles (20 μM) exerted 43% cytotoxicity, which was also lower than that of MIT-PFP/PPP mixed micelles (20 μM) with irradiation (60%). These results indicate that PDT using MIT as a photosensitizer exerted strong anticancer effects under irradiation and MIT-PFP/PPP mixed micelles showed the highest cytotoxicity in MCF-7/ADR cells with irradiation among these treatments.
Measurement of ROS
PDT inhibited cell growth in cancer cells upon exposure to near-infrared irradiation through the generation of ROS.25 Whether the MIT-PFP/PPP mixed micelles generated ROS was measured in MCF-7/ADR cells under irradiation (24 mW). As shown in Figure 3A, MCF-7/ADR cells treated with MIT and MIT-PFP/PPP mixed micelles without irradiation exhibited negligible changes in ROS levels, while increased ROS levels were observed in the cells treated with both MIT and MIT-PFP/PPP mixed micelles after irradiation. These results suggest that both free MIT and MIT-PFP/PPP mixed micelles may induce cytotoxicity in MCF-7/ADR cells via the production of ROS by photochemical reaction under irradiation.
P-gp activity
Cayman’s MDR assay kit was used to determine P-gp activity.19 The kit used a cell-permeable nonfluorescent dye (calcein AM), which is cleaved intracellularly into a fluorescent molecule (calcein) for the detection of anticancer agents inhibiting MDR proteins. As shown in Figure 3B, P-gp activity was suppressed in MCF-7/ADR cells treated with MIT-PFP/PPP mixed micelles with irradiation, while there were no obvious changes on treating with MIT and MIT-PFP/PPP mixed micelles without irradiation. Meanwhile, there was also no obvious change in the P-gp activity of cells treated with MIT and irradiation, compared to that of cells treated with MIT and MIT-PFP/PPP mixed micelles without irradiation. This result demonstrated that MIT-PFP/PPP mixed micelles with irradiation were able to suppress P-gp activity in MCF-7/ADR cells.
Cellular uptake of MIT-PFP/PPP mixed micelles and its mechanisms
There might be different ways that cells can uptake MIT-PFP/PPP mixed micelles. We analyzed the internalization of MIT-PFP/PPP mixed micelles by using different endocytic inhibitors.21 As shown in Figure 3C, there was a significantly lower cellular uptake with 2-deoxyglucose exposure, indicating that cellular uptake with MIT-PFP/PPP mixed micelles may be associated with an energy-dependent endocytosis. Methyl-β-cyclodextrin and wortmannin exhibited a significant inhibitory effect, indicating that the internalization pathways were caveolae- and macropinocytosis-mediated pathways. Therefore, MIT-PFP/PPP mixed micelles could enter and stay in cancer cells while maintaining favorable drug concentration.
To investigate the ability of MIT-PFP/PPP mixed micelles to efficiently deliver anticancer drug to the cytosol in MCF-7/ADR cells under laser irradiation, the cellular internalization of MIT-PFP/PPP mixed micelles was examined by flow cytometry. Figure 3D shows an increasing trend in the cellular uptake of MIT from groups (MIT, MIT+660 nm, MIT-PFP/PPP and MIT-PFP/PPP+660 nm) after incubation for 2 h. This result suggested that MIT-PFP/PPP mixed micelles may improve intracellular concentration of anticancer drug under irradiation by photochemical reaction.
Assessment of cell apoptosis
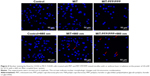
Nuclear staining and Annexin V/PI staining were used to measure the apoptosis of MCF-7/ADR cells treated with MIT-PFP/PPP mixed micelles. As shown in Figure 4, MCF-7/ADR cells treated with MIT and MIT-PFP/PPP mixed micelles without irradiation were rounded and showed no nuclear condensation, indicating that there were no apoptotic cells. Interestingly, the number of MIT-PFP/PPP mixed micelles-treated MCF-7/ADR cells was less than the cells treated with free MIT, indicating the antiproliferative ability of MIT-PFP/PPP mixed micelles. After irradiation with laser, MCF-7/ADR cells treated with free MIT showed less cell apoptosis than the cells treated with MIT-PFP/PPP mixed micelles. In addition, MCF-7/ADR cells treated with MIT-PFP/PPP mixed micelles had less proliferating cells. These results indicated that MIT-PFP/PPP mixed micelles exerted antiproliferative effect and induced cell apoptosis in MCF-7/ADR cells under irradiation.
To confirm the cell apoptosis of MIT-PFP/PPP mixed micelles under irradiation, Annexin V/PI staining was used to analyze apoptotic cells. As shown in Figure 5, about 7% and 9% apoptotic cells were observed in the cells treated with MIT and MIT-PFP/PPP mixed micelles, respectively, while the cells treated with MIT and MIT-PFP/PPP mixed micelles with irradiation showed 16% and 29% apoptosis, respectively. MIT-PFP/PPP mixed micelles with irradiation exhibited the highest cell apoptotic rate (29%). These results demonstrated that MIT-PFP/PPP mixed micelles could induce cell apoptosis in MCF-7/ADR cells upon irradiation.
Analysis of apoptosis-related biomarker by Western blot
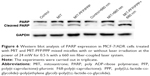
Western blot analysis was used to examine the expression of biomarker related to cell apoptosis in MIT-PFP/PPP mixed micelles-treated MCF-7/ADR cells. PARP was chosen because it is related to cell death and is specifically cleaved in apoptotic cells. As shown in Figure 6, the level of cleaved PARP in cells treated with MIT-PFP/PPP mixed micelles was increased under irradiation, indicating that MIT-PFP/PPP mixed micelles with irradiation induced apoptosis in MCF-7/ADR cells by increasing cleaved PARP.
Conclusion
In this study, MIT-PFP/PPP mixed micelles system was used to reverse MDR in MCF-7/ADR breast cancer cells by PDT. After irradiation, MIT-PFP/PPP micelles were able to increase ROS levels, decrease P-gp activity and increase cellular uptake of MIT-PFP/PPP mixed micelles, which induced cell apoptosis and reversed the effect of MDR. Meanwhile, the level of cleaved PARP was increased by treatment with MIT-PFP/PPP mixed micelles, indicating cell apoptosis under irradiation by PDT in MCF-7/ADR cells. Furthermore, it is necessary to perform further study in vivo. Collectively, MIT-PFP/PPP mixed micelles may provide a promising strategy to efficiently reverse MDR by PDT in breast cancer.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81403120), the Guangdong Province Medical Scientific Research Foundation (A2015333 and B2010004), and the Guangdong Province Science and Technology Planning Project (2014A020212406 and 2016ZC0158).
Disclosure
The authors report no conflicts of interest in this work.
References
Komeili-Movahhed T, Fouladdel S, Barzegar E, et al. PI3K/Akt inhibition and down-regulation of BCRP re-sensitize MCF7 breast cancer cell line to mitoxantrone chemotherapy. Iran J Basic Med Sci. 2015;18(5):472–477. | ||
Hou L, Feng Q, Wang Y, et al. Multifunctional hyaluronic acid modified graphene oxide loaded with mitoxantrone for overcoming drug resistance in cancer. Nanotechnology. 2016;27(1):015701. | ||
Yan CS, Wong IL, Chan KF, et al. A new class of safe, potent, and specific P-gp modulator: flavonoid dimer FD18 reverses P-gp-mediated multidrug resistance in human breast xenograft in vivo. Mol Pharm. 2015;12(10):3507–3517. | ||
Braunova A, Kostka L, Sivak L, et al. Tumor-targeted micelle-forming block copolymers for overcoming of multidrug resistance. J Control Release. 2017;245:41–51. | ||
Zhang J, Zhao X, Chen Q, et al. Systematic evaluation of multifunctional paclitaxel-loaded polymeric mixed micelles as a potential anticancer remedy to overcome multidrug resistance. Acta Biomater. 2017;50:381–395. | ||
Park H, Park W, Na K. Doxorubicin loaded singlet-oxygen producible polymeric micelle based on chlorine e6 conjugated pluronic F127 for overcoming drug resistance in cancer. Biomaterials. 2014;35(27):7963–7969. | ||
Agostinis P, Berg K, Cengel KA, et al. Photodynamic therapy of cancer: an update. CA Cancer J Clin. 2011;61(4):250–281. | ||
Wang S, Huang P, Nie L, et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer-functionalized gold nanostars. Adv Mater. 2013;25(22):3055–3061. | ||
Huang P, Xu C, Lin J, et al. Folic acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2011;1:240–250. | ||
Sazgarnia A, Montazerabadi AR, Bahreyni-Toosi MH, Ahmadi A, Aledavood A. In vitro survival of MCF-7 breast cancer cells following combined treatment with ionizing radiation and mitoxantrone-mediated photodynamic therapy. Photodiagnosis Photodyn Ther. 2013;10(1):72–78. | ||
Sazgarnia A, Montazerabadi AR, Bahreyni-Toosi MH, Ahmadi A. Photosensitizing and radiosensitizing effects of mitoxantrone: combined chemo-, photo-, and radiotherapy of DFW human melanoma cells. Lasers Med Sci. 2013;28(6):1533–1539. | ||
Montazerabadi AR, Sazgarnia A, Bahreyni-Toosi MH, Ahmadi A, Shakeri-Zadeh A, Aledavood A. Mitoxantrone as a prospective photosensitizer for photodynamic therapy of breast cancer. Photodiagnosis Photodyn Ther. 2012;9(1):46–51. | ||
Alvarez-Lorenzo C, Sosnik A, Concheiro A. PEO-PPO block copolymers for passive micellar targeting and overcoming multidrug resistance in cancer therapy. Curr Drug Targets. 2011;12(8):1112–1130. | ||
Kabanov AV, Batrakova EV, Miller DW. Pluronic block copolymers as modulators of drug efflux transporter activity in the blood-brain barrier. Adv Drug Deliv Rev. 2003;55(1):151–164. | ||
Cai Y, Wang S, Wu M, et al. PCL-F68-PCL/PLGA-PEG-PLGA mixed micelles mediated delivery of mitoxantrone for reversing multidrug resistant in breast cancer. RSC Adances. 2016;6:35318–35327. | ||
Wang S, Wang L, Shi Z, Zhong Z, Chen M, Wang Y. Evodiamine synergizes with doxorubicin in the treatment of chemoresistant human breast cancer without inhibiting P-glycoprotein. PLoS One. 2014;9(5):e97512. | ||
Ding F, Li HJ, Wang JX, et al. Chlorin e6-encapsulated polyphosphoester based nanocarriers with viscous flow core for effective treatment of pancreatic cancer. ACS Appl Mater Interfaces. 2015;7(33):18856–18865. | ||
Hao Y, Zhang B, Zheng C, et al. Multifunctional nanoplatform for enhanced photodynamic cancer therapy and magnetic resonance imaging. Colloids Surf B Biointerfaces. 2017;151:384–393. | ||
Wang S, Wang L, Chen M, Wang Y. Gambogic acid sensitizes resistant breast cancer cells to doxorubicin through inhibiting P-glycoprotein and suppressing survivin expression. Chem Biol Interact. 2015;235:76–84. | ||
Perillo E, Allard-Vannier E, Falanga A, et al. Quantitative and qualitative effect of gH625 on the nanoliposome-mediated delivery of mitoxantrone anticancer drug to HeLa cells. Int J Pharm. 2015;488(1–2):59–66. | ||
Chen F, Zhang J, He Y, Fang X, Wang Y, Chen M. Glycyrrhetinic acid-decorated and reduction-sensitive micelles to enhance the bioavailability and anti-hepatocellular carcinoma efficacy of tanshinone IIA. Biomater Sci. 2016;4(1):167–182. | ||
Wang S, Chen R, Morott J, Repka MA, Wang Y, Chen M. mPEG-b-PCL/TPGS mixed micelles for delivery of resveratrol in overcoming resistant breast cancer. Expert Opin Drug Deliv. 2015;12(3):361–373. | ||
Zhang J, Zhang M, Ji J, et al. Glycyrrhetinic acid-mediated polymeric drug delivery targeting the acidic microenvironment of hepatocellular carcinoma. Pharm Res. 2015;32(10):3376–3390. | ||
Wang S, Yang Y, Wang Y, Chen M. Gambogic acid-loaded pH-sensitive mixed micelles for overcoming breast cancer resistance. Int J Pharm. 2015;495(2):840–848. | ||
Pais-Silva C, de Melo-Diogo D, Correia IJ. IR780-loaded TPGS-TOS micelles for breast cancer photodynamic therapy. Eur J Pharm Biopharm. 2017;113:108–117. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.