Back to Journals » International Journal of Women's Health » Volume 12
Placenta Accreta Spectrum Disorders: Challenges, Risks, and Management Strategies
Authors Morlando M , Collins S
Received 8 July 2020
Accepted for publication 26 September 2020
Published 10 November 2020 Volume 2020:12 Pages 1033—1045
DOI https://doi.org/10.2147/IJWH.S224191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Everett Magann
Maddalena Morlando,1 Sally Collins2
1Department of Woman, Child and General and Specialized Surgery, Obstetrics and Gynecology Unit, University of Campania “Luigi Vanvitelli”, Naples, Italy; 2Nuffield Department of Women’s and Reproductive Health, University of Oxford, Fetal Medicine Unit, John Radcliffe Hospital, Oxford, UK
Correspondence: Sally Collins
Nuffield Department of Women’s and Reproductive Health, University of Oxford, Fetal Medicine Unit, John Radcliffe Hospital, Oxford, UK
Tel +44 (0)1865 851165
Fax +44 (0) 7958 656 885
Email [email protected]
Abstract: The worldwide incidence of placenta accreta spectrum (PAS) is rapidly increasing, following the trend of rising cesarean delivery. PAS is an heterogeneous condition associated with a high maternal morbidity and mortality rate, presenting unique challenges in its diagnosis and management. So far, the rarity of this condition, together with the absence of high quality evidence and the lack of a standardized approach in reporting PAS cases for the ultrasound, clinical, and pathologic diagnosis, represented the main challenges for a deep understanding of this condition. The study of the available management strategies of PAS has been hampered by the heterogeneity of the available epidemiological data on this condition. The aim of this review is to provide a critical view of the current available evidence on the screening, the diagnosis, and the management options for PAS disorders, with a special focus on the challenges we foresee for the near future.
Keywords: placenta accreta spectrum, placenta accreta, abnormal placentation, abnormal invasive placenta, morbidly adherent placenta
Introduction
Placenta accreta spectrum disorder (PAS), also called abnormally invasive placenta (AIP), describes a clinical situation where the placenta does not detach spontaneously after delivery and cannot be forcibly removed without causing massive and potentially life-threatening bleeding.1,2 The incidence of PAS is rising worldwide.3,4 This is most likely due to the increasing rates of cesarean delivery, which is the major risk factor for PAS in subsequent pregnancies. PAS is one of the most dangerous conditions of the pregnancy as it is significantly associated with maternal morbidity and mortality.5 Maternal and neonatal outcomes are generally improved when diagnosis is made before delivery, and the woman is managed by a multidisciplinary team with expertise in the condition.6,7
Pathophysiology of PAS
Several theories have been proposed to explain why and how PAS occurs. The prevailing hypothesis is that an iatrogenic defect of the endometrium–myometrial interface leads to a failure of normal decidualization at the site of a uterine scar, enabling abnormally deep trophoblast infiltration.8 The decidua potentially regulates trophoblast invasion, as demonstrated by the aggressive invasion of the muscular and serosal layers seen when ectopic implantation occurs in areas where the decidua is physiologically absent, such as the fallopian tube or the abdominal cavity.9,10 Disruption of the decidua, for example by a previous cesarean delivery incision, may result in loss of the inherent regulation and uncontrolled invasion of extravillous trophoblast through the entire depth of the myometrium. The extent of penetration of the villous tissue within the myometrium is likely to be related to the degree of the deciduo-myometrial damage. Conditions like manual removal of the placenta, uterine curettage, and endometritis11,12 are more likely to result in abnormally adherent placentation (accreta). On the other hand, a full thickness surgical scar is associated with both the absence of endometrial re-epithelialization and vascular remodelling around the scar area, and this may lead to abnormally invasive placentation (increta/percreta).13
One additional mechanism has been recently suggested in studies investigating the role of in vitro fertilization as a risk factor for PAS A characteristic hormonal milieu at the time of implantation and placentation resulting from IVF may enhance trophoblast invasion and cause PAS.14 Aberrant placentation may be the effect of elevated serum estrogens at the time of embryo implantation, which may lead to excessive trophoblastic invasion through the endometrium. Alternatively, lower serum estradiol levels together with the presence of a thinner decidualized endometrium may result in abnormal trophoblastic growth leading to PAS.15
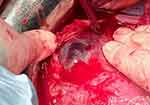
In normal placentation, extravillous trophoblast cells undertake a remodeling process of uterine arteries leading to the progressive loss of myocytes and their internal elastic lamina, which are replaced by fibrinoid material. Consequently, the terminal coils of the spiral arteries are dilated by an approximately 4-fold increase in their diameter at the myometrial–endometrial interface and within the distal myometrium. Conversely, the segment just below the myometrial-endometrial interface represents the limit of physiological trophoblast invasion and the arteries below this point remain highly vaso-reactive throughout pregnancy.16 One additional finding observed in cases of abnormally invasive placentation is an unusual uteroplacental vasculature in which physiological changes are present in large arteries deeper in the myometrium in comparison with normal pregnancies.17 Ultrasound imaging and macroscopic observation at delivery of the hyper-vascularity of the placental bed in cases of invasive placentation suggest a phenomenon of neovascularization in the area of uterine scar in addition to the vasodilatation of the uterine vessels13 (Figure 1).
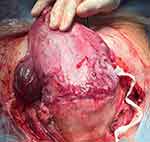 |
Figure 1 Placenta percreta, showing aberrant neovascularization of the lower uterine segment. This extends down behind the urinary bladder (this can just be seen at the top of the bladder). |
A recent commentary on PAS highlighted the importance to abandon the old terminology focusing on PAS as an invasive trophoblastic disease and to start to consider PAS as a disease resulting from a combination of many factors such as a defective decidua, abnormal trophoblastic attachment, abnormal angiogenesis and vascular remodelling, and progressive uterine scar dehiscence.18
Definition
According to the depth of trophoblast invasion into the myometrium, three known variants of PAS can be differentiated by pathologists:1 placenta accreta (also called placenta creta, vera, or adherenta), where the chorionic villi attach directly to the surface of the myometrium in the absence of the decidual layer;2 placenta increta, where the chorionic villi penetrate deeply into the myometrium reaching the external layer;3 and placenta percreta, where the invasive chorionic villi reach and penetrate through the uterine serosa.1,8
The first challenge when dealing with PAS is the heterogeneous definition of this condition used in the available literature. In the majority of the studies published in the last few years there is no correlation between ultrasound signs, clinical presentation, and histopathologic findings.19 In addition to this, in many series the inclusion of both adherent and invasive forms of PAS in the same category has made the interpretation of clinical data even more difficult. Abnormal placentation includes both abnormally adherent placenta (placenta accreta) and abnormally invasive placenta (AIP – including placenta increta and placenta percreta); the term PAS encompasses the whole spectrum of the disorder (Figure 2). In abnormally adherent placenta the implantation of the villi is in direct contact with the myometrium in the absence of an obvious plane of cleavage. In abnormally invasive placentation, the villi invade deeply the myometrium, and cannot be easily removed either manually or by curettage. Another issue in the diagnostic conundrum is the potential confusion between a retained placenta and an adherent one, especially when the placenta is only partially adherent.20,21 Placental retention occurs when the placenta separates from the uterine wall, but it remains entrapped within the uterus due to constriction of the cervix. This condition should not be regarded as PAS, as inclusion of placental retention among PAS cases might be responsible for overestimation of PAS prevalence. The result of different, sometimes incorrect, diagnostic criteria is a wide variability in the reported predictive value of antenatal imaging strategies, and the outcomes associated with different management strategies22 (Table 1).
 |
Table 1 A Clinical and Histologic Grading System to Assess and Categorize Placental Adherence or Invasion at Delivery According to FIGO Guidelines22 |
Epidemiology and Risk Factors
Another challenge is the accurate estimation of the prevalence of this condition. The prevalence of PAS will vary among different populations according to the prevalence of the risk factors associated with this condition. However, more importantly, differences in prevalence estimates will also arise from the variety of definitions used to diagnose PAS. On top of the lack of differentiation in the degree of invasion and the problem of the inclusion of retained placenta among PAS cases, there is one additional issue. In many medical conditions, the histopathologic findings represent the gold standard for the diagnosis of the condition. However, in the case of PAS this may also lead to some inaccuracies. Historically, the main histopathological criterion used to confirm the diagnosis of PAS was the absence of a decidual layer between the tip of anchoring villi and superficial myometrium. However, myometrial fibers can be found also in the basal plate of normal placentas, and in the same placentas the decidua can be undetectable, as it usually becomes thinner towards the end of gestation. On the other hand, in many cases of placenta percreta the depth of the invasion to the uterine wall is such that no decidual and myometrial tissue are left at the site of placental implantation, making histopathologic diagnosis impossible.1 Moreover, different degrees of villous invasion have been described throughout the same placenta, with areas of accreta and percreta coexisting on the same specimen, further limiting the accuracy of microscopic diagnosis as it becomes dependent on the site of sampling. One more scenario that makes histopathologic diagnosis of PAS impossible is conservative management where the placenta is left in situ, with no histopathologic specimen available for the diagnosis.
The literature is controversial on the sensitivity and specificity of the clinical criteria compared with histopathologic diagnosis. The exclusion of the cases with negative or unavailable histopathologic examination may underestimate the real incidence,23–26 as the absence of indicative histological features in cases of clinically suspected PAS does not exclude the diagnosis.26 Therefore, the clinical definition has to be the most important criteria for definition of PAS disorders3 and should always be taken into account. This is particularly true when the placenta is found under a uterine dehiscence. Lower segment scar dehiscence becomes likely in the third trimester due to the pressure of the fetus and to uterine contractions, both of which increase the disruption of the scar tissue. In such cases the placental tissue can be seen under the serosa at the time of cesarean section (see Figure 3). This is a “uterine window” and can be incorrectly diagnosed as PAS (Figure 3). This error can then be compounded by histology if the pathologist only looks for placental villi directly adjacent to the serosa. However, in the case of a uterine window the myometrium surrounding the defect is completely normal.1 In order to overcome all the limitations in the study of PAS, a standardized clinical classification has been proposed by FIGO to describe and categorize the different aspects of PAS at the time of delivery22 (Table 1). The use of a standardized classification for both the clinical and histopathological diagnosis of PAS disorders is crucial to obtaining accurate and reliable data from future studies.
PAS may occur after any kind of procedure that causes damage to the endometrium, including curettage, manual removal of the placenta, uterine-artery embolization, or myomectomy.27,28 Additional risk factors are advanced maternal age, high parity, IVF, and a diagnoses of PAS in a previous pregnancy.14,15,29,30
However, the major risk factor for PAS is a prior cesarean delivery in combination with placenta previa, namely, a placenta implanting over the cervical os. Moreover, the risk of PAS increases progressively with increasing number of previous cesarean deliveries. A large multicenter US study found that the risk of PAS in women with a placenta previa and previous cesarean deliveries was 3%, 11%, 40%, 61%, and 67% for the first, second, third, fourth, and fifth or more cesareans, respectively.31 Placenta previa is reported in around half of all cases of PAS,32 and, again, the risk of previa increases with higher numbers of previous cesarean sections.33 Over the last 40 years, cesarean section rates have risen globally from less than 10% to over 30%, and at the same time a 10-fold increase in the incidence of PAS has been reported.34 Strong epidemiologic data support a direct link between the increase in prevalence of PAS disorders and the increase in cesarean delivery rates in most middle- and high-income countries.3,–4,–29,–31–33,35 In the beginning of the 20th century, the first studies on placenta accreta reported the estimated incidence to be one in 30,000 deliveries in the US.11 By contrast, recent publications from all over the world reported a notable increase in the prevalence of this condition, with an incidence of one in 533 births33 and even one in 321 births in populations with higher rates of cesarean section.4
One emerging problem related with the development of PAS is the presence of a cesarean scar pregnancy, which is the implantation of the blastocyst into the hysterotomy scar. In this condition the pregnancy can invade the myometrium and give rise to the clinical expression of PAS conditions. Most but not all scar pregnancies will develop into clinically significant PAS, therefore, the real challenge is to identify the cases that will potentially pose serious adverse effects on maternal health, from the cases that will proceed as relatively normal pregnancies.36,37 The ability to discriminate between these two scenarios is of utmost importance, as in cesarean scar pregnancy, termination of pregnancy should be discussed with the woman. The relationships between the ectopic gestational sac, previous cesarean scar, and anterior uterine wall thickness can predict both the evolution of the scar during pregnancy towards the most severe types of PAS, and the clinical outcome of these women.38 Recently, a new ultrasound marker, the cross-over sign (COS), has been suggested to have the potential to stratify the risk of women with cesarean scar pregnancy evolving towards PAS and to predict the surgical outcome.38 A recent systematic review39 suggests that expectant management is a reasonable option for scar pregnancies with no detectable fetal heart activity, as the majority of women did not experience any major complication. On the contrary, the presence of fetal heart activity might be associated with a high burden of first- and second-trimester maternal complications and for the pregnancies progressing to the third trimester, with the occurrence of PAS conditions in about three-quarters of the women. There is still a need for adequately powered studies to ascertain whether and how prenatal imaging can identify women with a cesarean scar pregnancy at higher risk of PAS, and what is the optimal treatment of this condition. These represent some of the important challenges of the future research.
Diagnosis
Ultrasound Diagnosis
Accurate antenatal diagnosis of PAS has been demonstrated to improve maternal outcomes, allowing appropriate risk assessment and planned delivery in a tertiary referral center with an experienced multidisciplinary team.6,40 While antenatal diagnostic accuracy reaches 95% in a series from experienced centers, several population studies found that PAS remains undetected before delivery in half of the cases in the overall population.13,41 Therefore, women with relevant clinical risk factors for PAS (eg, placenta previa and a cesarean scar) should undergo ultrasound evaluation in a center with expertise in this condition.
Several studies have investigated the predictive value of different ultrasound signs of PAS. However, the performance of these signs has shown considerable variability.42 These differences have been mainly attributed to the principal limitations of the available studies: the limited sample size, the retrospective design, and the variability of the diagnosis at delivery. In addition to this, a huge heterogeneity and complexity is reported in the terminology applied to describe the ultrasound characteristics of PAS, with the same sign being described using different names, and the same term being used for different findings. One additional problem is the fact that all the diagnostic techniques rely strongly on the subjective opinion of the operator which will vary according to their experience.43 As PAS is still a rare condition, many clinicians will not have much experience with the ultrasound appearance. Other factors which may lead to over- or underestimation of the disease are the scanning conditions (eg, a too full or too empty bladder), the ultrasound equipment and settings used, and gestational age. In particular, the color Doppler signs are more susceptible to operator error as the color Doppler appearance of the placenta is strongly dependant on the US machine settings. Three-dimensional power Doppler ultrasound is currently under investigation and its use might improve the accuracy of PAS antenatal diagnosis.44
One additional problem is the lack of an ultrasound sign or combination of signs for the effective definition of the degree of invasion in PAS.1,13,19 This is mainly due to the absence of correlation between ultrasound signs, macroscopic aspects at delivery, and histopathology findings in all the available studies. Providing good quality evidence by applying a standardized approach in reporting PAS cases for the ultrasound, clinical, and pathologic diagnosis, represents the first challenge to obtain effective screening, management, and to optimize the outcome of women with PAS disorders.
In 2016, the “European Working Group on Abnormally Invasive Placenta (AIP)” (now the International Society for PAS) proposed a standardized definition of the PAS ultrasound descriptors, in order to improve the comparability among studies, to increase the diagnostic accuracy, and to facilitate the international collaboration on the study of PAS.43 A reporting proforma based on these standardized definitions has also been suggested.45 This standardized terminology has been subsequently incorporated into the 2019 FIGO guidelines on the prenatal diagnosis and screening of PAS20 (Table 2).
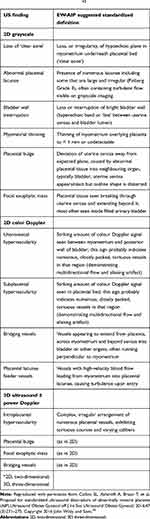 |
Table 2 Unified Descriptors, as Suggested by the European Working Group on Abnormally Invasive Placenta (EW-AIP), for Ultrasound (US) Diagnosis of AIP. l43 |
MRI Diagnosis
Ultrasound is the first-line imaging tool for the screening and diagnosis of PAS. However, it is now well-established that magnetic resonance imaging (MRI) has a role in the diagnosis of PAS, with high sensitivity and specificity.46 Despite this, MRI has not demonstrated superiority over ultrasound in the diagnosis of PAS so far. One confounding factor to take into account when comparing the diagnostic accuracy of US and MRI for the diagnosis of PAS is that MRI is not usually employed as a screening tool in women with higher risk of PAS. Indeed, the first screening is performed by US, and only women in which a suspicion of PAS has been raised at the US are subjected to MRI examination. This might lead to an overestimation of the diagnostic capability of the MRI. In a recent study, MRI resulted in a change in diagnosis that could alter clinical management of PAS in more than one third of cases, but, when changed, the diagnosis was often incorrect.47
MRI has been recommended as a second-line imaging tool for the diagnosis of PAS to assess the depth of invasion and the lateral extension of myometrial invasion, especially with posterior placentation and in women with US suspicion of parametrial invasion.48 In fact, in certain circumstances MRI can overcome the technical limitations related with the US diagnosis of PAS. An unfavorable placental location or a high maternal body mass index do not present a problem for the MRI. The entire pelvis can be studied easily, and it also allows offline revaluation by different physicians. The counterpart is that MRI is more expensive and less available than US. As with US, the terminology in the literature for MRI is not consistent. Therefore, to facilitate international collaboration and comparison among studies, a standardized definition of the MRI descriptors of PAS has been recently proposed by the International Society for Placenta Accreta Spectrum (www.is-pas.org; formerly the EW-AIP).49
Clinical Diagnosis
In all cases of antenatal suspicion of PAS, ultimate confirmation of this condition should always be undertaken intrapartum, before surgical treatment is commenced. There is no robust evidence demonstrating the best clinical diagnostic method for the intrapartum diagnosis of PAS. However, a stepwise process for the diagnosis of PAS after laparotomy has been recently proposed.50 This will include:
- Step 1: Thorough inspection of the external surface of the uterus and the pelvis for frank signs of placental invasion including: a) abnormal appearance of the uterus over the placental bed (bluish/purple appearance) with evident distension (placental bulge); and b) obvious invasion of the placental tissue through the surface of the uterus with or without invasion of the serosa. If these aspects are clearly seen, the diagnosis of PAS disease can be confirmed.
- Step 2: If there is no clinical evidence of the most invasive forms of PAS, with no placental tissue seen to be invading through the uterine serosa, the uterine incision should be made leaving the placenta undisturbed, and gentle cord traction can be attempted. If traction on the umbilical cord causes the uterine wall to be visibly pulled inward in the direction of traction with no separation of the placenta (the “dimple” sign) and there is apparent contraction of the uterus separate from the placental bed, then PAS can be diagnosed.
- Step 3: When PAS has not been diagnosed by the previous two steps, then gentle digital exploration can be attempted to assess the presence of a plane of cleavage between the uterus and the placenta.
A clinical and histologic grading system to assess and categorize placental adherence or invasion at delivery has been recently recommended (Table 1).
Biomarkers of PAS Disorders
Several potential biomarkers have been studied in PAS, showing variability of measurement depending on gestational age at sampling.51 In the serum of women with PAS disorders, the human chorionic gonadotropin (hCG) and its free beta-subunit (β-hCG) have been shown to be lower and pregnancy-associated plasma protein A (PAPP-A) higher, at 11–12 weeks of gestation. At the same time, at 14–22 weeks, serum β-hCG and alpha-fetoprotein (AFP) were found to be higher in cases of PAS. In a recent study, women with PAS were found to have a unique and distinct plasma protein profile compared with control subjects, characterized by dysregulation of about 50 proteins involved in the inflammatory response, in the regulation of vascular remodelling, and extracellular matrix proteins regulating invasion.52 Currently, there is no evidence of an effective biomarker for a serological screening of PAS.20 However, we can imagine that in the near future the combination of ultrasound details with serological analytes will give us the opportunity to offer screening for PAS disorders, as we currently do for aneuploidies or preeclampsia.
Management Strategies
The depth of placental invasiveness is one of the main factors affecting maternal outcome.7 Therefore, in order to identify the best strategies for the management of PAS, a correct assessment of the degree of the invasion at the time of delivery, stratification of women according to this, and a precise correlation between prenatal imaging, intra-operative and pathological aspects are of utmost importance when comparing data from different studies. However, due to the relative rarity of this condition, and given the ethical issues that randomized trials would face, high quality studies dealing with the management of PAS disorders are still lacking. Most of the information to guide the management are taken from retrospective cohort studies, case series, and opinion papers. As a result, different strategies for the management of PAS have been described, with some clinicians opting for the traditional radical approach, and some others proposing conservative techniques.53,54
One of the cornerstones of the management of PAS is to avoid any attempt to remove the placenta, either in the conservative or in the radical approach. In fact, in abnormally invasive placentation, any attempt to forcibly remove the placenta will leave placental fragments within a very deficient myometrium, resulting in uncontrolled major obstetric hemorrhage. Making no attempt to remove any of the placenta, either during conservative management or prior to cesarean hysterectomy, is associated with decreased levels of hemorrhage and a reduced need for blood transfusion.5
One more challenge when dealing with PAS is to define the best time of delivery in order to optimize maternal and neonatal outcome. Earlier elective cesarean delivery may reduce the risk of bleeding or labor, leading to an emergency delivery, which has been associated with higher maternal complications;55 however, earlier delivery will also increase the risks to the neonate related with prematurity. Several management strategies have been proposed, suggesting planned elective delivery ranging from 34–38 weeks,48,–50,–53,–56–58 further demonstrating that there is still insufficient evidence to recommend one gestational age over another. One reasonable approach could be to tailor the timing of delivery based on the individual woman’s risk of emergent delivery. Expectant management until after 36+0 weeks can be considered a safe option for women with no previous history of preterm delivery and who are stable with no vaginal bleeding, preterm premature rupture of the membranes (PPROM), or uterine contractions suggestive of preterm labour. On the contrary, planned delivery at around 34+0 weeks’ gestation should be arranged for women with a history of previous preterm birth, multiple episodes of small amounts of vaginal bleeding, a single episode of a significant amount of vaginal bleeding, or PPROM. Antenatal steroids prophylaxis should be administered in accordance based to the current local guidelines for the specific gestation at delivery.50
Maternal morbidity has been proven to be significantly reduced when care is provided in a center of excellence for the management of PAS conditions.6,7,55,59 The hallmark features of a center of excellence for the management of PAS have been recently defined by many international societies. A center of excellence is usually a tertiary referral hospital, which can provide a multidisciplinary team (MDT) with significant experience in managing the most invasive forms of PAS providing both antenatal diagnosis and preoperative planning. The MDT should be available 24 hours a day, 7 days a week, to ensure that expertise is available for emergency situations, including prompt availability of the interventional radiologist, colorectal surgeon, vascular surgeon, hematologist, and massive transfusion facilities. Communication and collaboration with the blood bank is crucial in view of the planned delivery given the probable need for large-volume blood transfusion. A high volume of cases per year is necessary to maintain the experience of the MDT.50,53,57,60
Traditional Surgical Management
Cesarean hysterectomy is considered the gold standard for the treatment of invasive placentation. However, also this radical approach is associated with high rates (40–50%) of severe maternal morbidity, mostly related to hemorrhage and insult to surrounding organs during surgery, and mortality rates as high as 7% due to massive untreatable hemorrhage.61,62 However, a recent meta-analysis suggested that when prenatal diagnosis and multidisciplinary expert management are available, rates in the range of 0.05% are achievable.63 In a recent systematic review and meta-analysis almost 90% of antenatally suspected cases of PAS underwent cesarean hysterectomy.13
A vertical skin incision is the preferred option for many clinicians, as it allows adequate access to the uterus and pelvic walls. However, large transverse incisions, such as a modified Maylard, have been reported and might be preferred due to a faster healing as well as for cosmetic reasons. There is no strong evidence to recommend one type of skin incision over another. Therefore, the decision should be made in accordance to the preference of the operating team taking into consideration the location of the placenta, the degree of invasion suspected, the likelihood of intraoperative complications, the maternal body habitus, and the gestational age.50,53 The uterine incision should be performed avoiding placental transection in order to reduce maternal morbidity related to blood loss from the placental bed. This is a fundal incision in many cases. Intraoperative ultrasound of the exposed uterus, undertaken in a sterile manner, can be considered to identify the upper placental edge and guide the decision regarding the site of hysterotomy.64 After delivery of the infant the uterine incision will be rapidly closed and the hysterectomy will be carried out. The type of hysterectomy performed should be individualized on a case-by-case basis. In the majority of cases, a total hysterectomy is needed because of the cervical invasion involved in a complete previa.
Cesarean hysterectomy in women with PAS is technically challenging, and the reported risk of adjacent organ injury is relevant (adjusted OR=8.2; 95% CI=5.2–13.1).65 Urinary tract injuries are described in 29% of the procedures performed in women with PAS, with a reported rate of 76% for bladder lacerations, 17% for ureteral injuries, and 5% for genitourinary fistulas.66 The main risk factors for urinary tract injury are reported to be the depth and extension of placental invasion, the intraoperative blood loss, and the number of previous cesarean deliveries.67 The occurrence of injury to other abdominal organs, such as the bowel and the pelvic vessels and nerves, has also been reported, but these complications are less common.68
Delayed hysterectomy is an alternative radical surgical management strategy for PAS. This involves the delivery of the baby, then closure of the uterus with the placenta left in situ, and closure of the maternal abdomen. A planned hysterectomy can then be scheduled 3–12 weeks postpartum.48 The rationale of this approach is that the uterine perfusion reduces after delivery, even with the placenta in situ, and involution of the uterus and reduction of the vascularity will make later surgery less risky for the woman.
One more scenario where delayed hysterectomy should be applied is the case of unsuspected highly invasive PAS diagnosed at the opening of the abdomen for an elective repeat cesarean section. A high degree of invasion of surrounding structures would mean an extremely difficult cesarean hysterectomy. If the surgeon has limited experience in performing complex surgical procedures and both mother and baby are stable, the cesarean section should be delayed to wait for trained staff and adequate resources or to arrange maternal transfer to a center of excellence.48 If the baby needs delivering urgently and the placenta is not bleeding, a delayed hysterectomy can be undertaken with the woman being transferred to a center of excellence for a hysterectomy at a later date.
Conservative Management
Conservative management of PAS consists of any approach whereby hysterectomy is avoided. The conservative approach might be considered in two circumstances: 1) when the intraoperative findings suggest that hysterectomy will be likely complicated and associated with a high risk of massive hemorrhage or adjacent tissue injury that may be reduced by leaving the placenta in situ; and 2) for women who desire future childbearing, or whose fertility is inextricably linked with social status and self-esteem.69
In the conservative approach, the umbilical cord is ligated close to its placental insertion after delivery, and without any attempt of removal, the placenta is left in-situ adherent to the myometrium. The use of adjunctive measures to reduce blood loss and to speed up the process of placental resorption has been reported. Among them: methotrexate, compression sutures, balloon tamponade, uterine artery embolization and/or uterine artery ligation. No efficacy for any adjunct has been proven, in fact they may be correlated to adverse outcomes. Several case reports exist of uterine necrosis in conservative management with uterine artery embolization.50 The use of methotrexate was linked to a maternal death in the largest case series70 and therefore the use of methotrexate is not recommended by any international consensus guideline and should be actively discouraged. Postoperative antibiotic therapy is usually prescribed to minimize the risk of infection. Placental expulsion or resorption usually takes from 4 weeks (expulsion) to 9–12 months (reabsorption), with a median of 13.5 weeks. The success rate is reported to be 78%. Severe maternal complications have been reported in as many as 6% of the women, including sepsis, uterine necrosis, postpartum uterine rupture, fistula, acute pulmonary edema, renal failure, venous-thromboembolism, and maternal death.70
Subsequent pregnancies have been reported in the 89% of women attempting to become pregnant, with a risk of recurrence of PAS of the 29%.71 Overall, these data suggest that leaving the placenta in situ may be a promising option for women who desire to preserve their fertility. However, when opting for conservative management, adjuvant therapy should be avoided and women must be appropriately counseled about the risks, and the need for potentially lengthy follow-up in centers with expertise.
Local surgical resection, namely the removal of the areas of the myometrium where the placenta is abnormally attached, has been proposed as a conservative technique for the management of PAS. Many different surgical techniques have been described by many authors, making interpretation of the available evidence difficult.54,–72–74 However, in appropriately selected cases with no placental invasion into the uterine cervix and/or parametrium, local resection is a reasonable option and may reduce blood loss and improve maternal morbidity compared to hysterectomy. The IS-AIP expert consensus defined an “appropriate case” for local resection a case with focal disease, with an adherent/invasive area which is <50% of the anterior surface of the uterus. Further studies are needed to identify the subgroup of women which will most benefit from this management strategy.
Intra-Partum Adjuvant Measures to Improve Maternal and Fetal Outcome
Ureteric Stents and Cystoscopy
Ureteric stents may be beneficial in preventing ureteric injury and early morbidity, however, the evidence is not strong enough to recommend routine placement of ureteric stents for all suspected cases of PAS.50,66 Therefore, placement of ureteric stents should be limited to cases where hysterectomy is anticipated to be highly complex. Routine preoperative cystoscopy is not recommended, as it was not demonstrated to improve maternal outcomes. If preoperative cystoscopy is performed for insertion of ureteric stents, the appearance of the bladder should not change the planned management based on the prenatal imaging.50
Prophylactic Endovascular Balloon Catheters
Endovascular balloon occlusion of the pelvic vessels has been proposed as a method to reduce intraoperative blood loss, in order to improve maternal outcome related to hemorrhage and to allow the surgeon to operate in a cleaner field with improved visibility.
However, PAS is associated with extensive aberrant neovascularization, and in such cases, occlusion of some of the pelvic vessels might lead to increased blood loss from the collateral vessels. In addition to this, endovascular balloon occlusion has been associated with significant maternal morbidity, mainly related to vessel rupture and thromboembolism. Two small randomized controlled trials found no differences in the number of packed RBC units transfused in women with antenatally suspected PAS who underwent placement of balloon catheters into the iliac arteries compared to those who did not.75,76 Larger studies are needed to truly demonstrate both the safety and efficacy of prophylactic balloon occlusion. Therefore, the routine use of prophylactic pelvic arterial balloon catheters for women with PAS suspected antenatally is not currently recommended from many international societies.48,50,53,57
Intraoperative Measures to Treat Life-Threatening Hemorrhage
Several operators recommend different strategies for the management of massive intraoperative bleeding in women with PAS. The surgical treatments proposed include internal iliac artery ligation, uterine devascularization, uterine compression sutures, uterine balloon tamponade, and pelvic tamponade. There are no randomized controlled trials comparing the effectiveness of different strategies in controlling maternal blood loss at delivery. Therefore, the procedure of choice will have to be chosen according to operator experience and resources available. A reasonable approach suggests that the simplest techniques with the lowest rate of complications should be performed first.50 In the case of massive bleeding occurring after placenta removal, the first-line measure should be the intrauterine tamponade (eg, balloon tamponade). If this measure is not effective, or the placenta remains in situ, an additional useful measure is uterine devascularization, with or without uterine compressive sutures. The last measure to be tried is the ligation of the internal iliac artery, as this procedure is associated with the highest risk of post-operative complications. When the woman is unstable or the bleeding is life threatening, the surgical team should focus on the source of the blood loss. In most cases this will be found in the placental bed. In such cases an emergency hysterectomy should be performed rapidly. Compression of the common iliac arteries or aorta has been reported as a temporary measure to gain time to temporarily stop the bleeding and to quickly complete the definitive treatment.50
Conclusion
PAS is a potentially life-threatening condition. Given the increasing rates of cesarean section worldwide, the incidence of PAS will be likely to increase further over time. Therefore, clinicians should be aware of the difficulties related with the diagnosis and the challenges associated with the management of this condition. Future research should focus on the collection of data for prospective studies on the diagnosis and management of PAS providing correlation between prenatal imaging, clinical grading of PAS at the time of delivery, and histopathology. This is of paramount importance to provide the best screening, diagnosis, and management options to women affected by PAS disorders.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Jauniaux E, Collins S, Burton GJ. Placenta accreta spectrum: pathophysiology and evidence-based anatomy for prenatal ultrasound imaging. Am J Obstet Gynecol. 2018;218(1):75–87. doi:10.1016/j.ajog.2017.05.067
2. Chantraine F, Braun T, Gonser M, Henrich W, Tutschek B. Prenatal diagnosis of abnormally invasive placenta reduces maternal peripartum hemorrhage and morbidity. Acta Obstetricia et Gynecologica Scandinavica. 2013;92(4):439–444. doi:10.1111/aogs.12081
3. Jauniaux E, Chantraine F, Silver RM, Langhoff-Roos J. FIGO consensus guidelines on placenta accreta spectrum disorders: epidemiology. Int J Gynecol Obstet. 2018;140(3):265–273. doi:10.1002/ijgo.12407
4. Morlando M, Sarno L, Napolitano R, et al. Placenta accreta: incidence and risk factors in an area with a particularly high rate of cesarean section. Acta Obstet Gynecol Scand. 2013;92(4):457–460.
5. Fitzpatrick K, Sellers S, Spark P, Kurinczuk J, Brocklehurst P, Knight M. The management and outcomes of placenta accreta, increta, and percreta in the UK: a population-based descriptive study. BJOG Int J Obstet Gynaecol. 2014;121(1):62–71.
6. Eller AG, Bennett MA, Sharshiner M, et al. Maternal Morbidity in Cases of Placenta Accreta Managed by a Multidisciplinary Care Team Compared With Standard Obstetric Care. Obstet Gynecol. 2011;117(2, Part 1):331–337.
7. Shamshirsaz AA, Fox KA, Salmanian B, et al. Maternal morbidity in patients with morbidly adherent placenta treated with and without a standardized multidisciplinary approach. Am J Obstet Gynecol. 2015;212(2):
8. Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(4):244–251.
9. Randall S, Buckley CH, Fox H. Placentation in the Fallopian Tube. Int J Gynecol Pathol. 1987;3(2):132–139.
10. Godyn JJ, Hazra A, Gulli VM. Subperitoneal placenta accreta succenturiate in the case of a successful near-term extrauterine abdominal pregnancy. Hum Pathol. 2005;36(8):922–926. doi:10.1016/j.humpath.2005.05.020
11. Irving C, Hervig AT. A Study of Placenta Accreta. Surg Gynecol Obstet.
12. Badr DA, Al Hassan J, Salem Wehbe G, Ramadan MK. Uterine body placenta accreta spectrum: A detailed literature review. Placenta. 2020;95:44–52. doi:10.1016/j.placenta.2020.04.005
13. Jauniaux E, Bhide A. Prenatal ultrasound diagnosis and outcome of placenta previa accreta after cesarean delivery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;217(1):27–36. doi:10.1016/j.ajog.2017.02.050
14. Modest AM, Toth TL, Johnson KM, Shainker SA. Placenta Accreta Spectrum: in Vitro Fertilization and Non-In Vitro Fertilization and Placenta Accreta Spectrum in a Massachusetts Cohort. Am J Perinatol. 2020;5:
15. Salmanian B, Fox KA, Arian SE, et al. In vitro fertilization as an independent risk factor for placenta accreta spectrum. Am J Obstet Gynecol. 2020;S0002937820305111.
16. Burton GJ, Woods AW, Jauniaux E, Kingdom JCP. Rheological and Physiological Consequences of Conversion of the Maternal Spiral Arteries for Uteroplacental Blood Flow during Human Pregnancy. Placenta. 2009;30(6):473–482. doi:10.1016/j.placenta.2009.02.009
17. Khong TY, Robertson WB. Placenta creta and placenta praevia creta. Placenta. 1987;8(4):399–409. doi:10.1016/0143-4004(87)90067-1
18. Einerson BD, Comstock J, Silver RM, Branch DW, Woodward PJ, Kennedy A. Placenta Accreta Spectrum Disorder: uterine Dehiscence, Not Placental Invasion. Obstet Gynecol. 2020;135(5):1104–1111. doi:10.1097/AOG.0000000000003793
19. Jauniaux E, Collins SL, Jurkovic D, Burton GJ. Accreta placentation: a systematic review of prenatal ultrasound imaging and grading of villous invasiveness. Am J Obstet Gynecol. 2016;215(6):712–721. doi:10.1016/j.ajog.2016.07.044
20. Jauniaux E, Bhide A, Kennedy A, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: prenatal diagnosis and screening. Int J Gynecol Obstet. 2018;140(3):274–280. doi:10.1002/ijgo.12408
21. Jauniaux E, Hussein AM, Fox KA, Collins SL. New evidence-based diagnostic and management strategies for placenta accreta spectrum disorders. Best Pract Res Clin Obstet Gynaecol. 2019;61:75–88. doi:10.1016/j.bpobgyn.2019.04.006
22. Jauniaux E, Ayres‐de‐Campos D, Langhoff‐Roos J, Fox KA, Collins S. FIGO Duncombe FIGO classification for the clinical diagnosis of placenta accreta spectrum disorders. Int J Gynaecol Obstet off Organ Int Fed Gynaecol Obstet. 2019;146(1):20–24. doi:10.1002/ijgo.12761
23. Miller DA, Chollet JA, Goodwin TM. Clinical risk factors for placenta previa–placenta accreta. Am J Obstet Gynecol. 1997;177(1):210–214. doi:10.1016/S0002-9378(97)70463-0
24. Gielchinsky Y, Rojansky N, Fasouliotis SJ, Ezra Y. Placenta Accreta—Summary of 10 Years: A Survey of 310 Cases. Placenta. 2002;23(2–3):210–214. doi:10.1053/plac.2001.0764
25. Hung TH, Shau WY, Hsieh CC, Chiu TH, Hsu JJ, Hsieh TT. Risk factors for placenta accreta. Obstet Gynecol. 1999;93(4):545–550.
26. Khong TY, Werger AC. Myometrial fibers in the placental basal plate can confirm but do not necessarily indicate clinical placenta accreta. Am J Clin Pathol. 2001;116(5):703–708. doi:10.1309/M9BF-6JHH-VF2U-2B8T
27. Silver RM, Branch DW, Solomon CG. Placenta Accreta Spectrum. N Engl J Med. 2018;378(16):1529–1536. doi:10.1056/NEJMcp1709324
28. Baldwin HJ, Patterson JA, Nippita TA, et al. Antecedents of Abnormally Invasive Placenta in Primiparous Women: risk Associated With Gynecologic Procedures. Obstet Gynecol. 2018;131(2):227–233. doi:10.1097/AOG.0000000000002434
29. Fitzpatrick KE, Sellers S, Spark P, Kurinczuk JJ, Brocklehurst P, Knight M. Incidence and risk factors for placenta accreta/increta/percreta in the UK: a national case-control study. PLoS One. 2012;7(12):e52893. doi:10.1371/journal.pone.0052893
30. Bowman ZS, Eller AG, Bardsley TR, Greene T, Varner MW, Silver RM. Risk factors for placenta accreta: a large prospective cohort. Am J Perinatol. 2014;31(9):799–804.
31. Silver RM, Landon MB, Rouse DJ, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226–1232. doi:10.1097/01.AOG.0000219750.79480.84
32. Thurn L, Lindqvist PG, Jakobsson M, et al. Abnormally invasive placenta-prevalence, risk factors and antenatal suspicion: results from a large population-based pregnancy cohort study in the Nordic countries. BJOG: An International Journal of Obstetrics & Gynaecology. 2016;123(8):1348–1355. doi:10.1111/1471-0528.13547
33. Wu S, Kocherginsky M, Hibbard JU. Abnormal placentation: twenty-year analysis. Am J Obstet Gynecol. 2005;192(5):1458–1461.
34. Jauniaux E, Ayres-de-Campos D, Placenta Accreta FIGO. Diagnosis and Management Expert Consensus Panel. FIGO Consensus Guidelines on Placenta Accreta Spectrum Disorders: Introduction Int J Gynaecol Obstet off Organ Int Fed Gynaecol Obstet. 2018;140(3):261–264.
35. Cheng KKN, Lee MMH. Rising incidence of morbidly adherent placenta and its association with previous caesarean section: a 15-year analysis in a tertiary hospital in Hong Kong. Hong Kong Med J Xianggang Yi Xue Za Zhi. 2015;21(6):511–517.
36. Jurkovic D. Cesarean scar pregnancy and placenta accreta. Ultrasound in Obstetrics & Gynecology. 2014;43(4):361–362. doi:10.1002/uog.13346
37. Timor-Tritsch IE, Monteagudo A, Cali G, et al. Cesarean scar pregnancy and early placenta accreta share common histology. Ultrasound in Obstetrics & Gynecology. 2016;215(6):712–721. doi:10.1002/uog.13282
38. Calì G, Forlani F, Minneci G, et al. First-trimester prediction of surgical outcome in abnormally invasive placenta using the cross-over sign. Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2018;51(2):184–188.
39. Calì G, Timor-Tritsch IE, Palacios-Jaraquemada J, et al. Outcome of Cesarean scar pregnancy managed expectantly: systematic review and meta-analysis. Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2018;51(2):169–175.
40. Chantraine F, Langhoff-Roos J. Abnormally invasive placenta–AIP. Awareness and pro-active management is necessary. Acta Obstet Gynecol Scand. 2013;92(4):369–371.
41. Bailit JL, Grobman WA, Rice MM, et al. Morbidly adherent placenta treatments and outcomes. Obstet Gynecol. 2015;125(3):683–689.
42. D’Antonio F, Iacovella C, Bhide A. Prenatal identification of invasive placentation using ultrasound: systematic review and meta-analysis. Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2013;42(5):509–517.
43. Collins SL, Ashcroft A, Braun T, et al. Proposal for standardized ultrasound descriptors of abnormally invasive placenta (AIP). Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2016;47(3):271–275.
44. Collins SL, Stevenson GN, Al-Khan A, et al. Three-Dimensional Power Doppler Ultrasonography for Diagnosing Abnormally Invasive Placenta and Quantifying the Risk. Obstet Gynecol. 2015;126(3):645–653.
45. Alfirevic Z, Tang A-W, Collins SL, Robson SC, Palacios-Jaraquemada J. Ad-hoc International AIP Expert Group. Pro Forma Ultrasound Rep Suspected Abnormally Invasive Placenta. 2016;47(3):276–278.
46. Familiari A, Liberati M, Lim P, et al. Diagnostic accuracy of magnetic resonance imaging in detecting the severity of abnormal invasive placenta: a systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2018;97(5):507–520.
47. Einerson BD, Rodriguez CE, Kennedy AM, Woodward PJ, Donnelly MA, Silver RM. Magnetic resonance imaging is often misleading when used as an adjunct to ultrasound in the management of placenta accreta spectrum disorders. Am J Obstet Gynecol. 2018;218(6):
48. Jauniaux E, Alfirevic Z, Bhide AG, et al. Placenta Praevia and Placenta Accreta: diagnosis and Management: green-top Guideline No. 27a BJOG Int J Obstet Gynaecol. 2019;126(1):e1–48.
49. Morel O, Collins SL, Uzan-Augui J, et al. A proposal for standardized magnetic resonance imaging (MRI) descriptors of abnormally invasive placenta (AIP) - From the International Society for AIP. Diagn Interv Imaging. 2019;100(6):319–325.
50. Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. Am J Obstet Gynecol. 2019;220(6):511–526.
51. Bartels HC, Postle JD, Downey P, Brennan DJ. Placenta Accreta Spectrum: A Review of Pathology, Molecular Biology, and Biomarkers. Dis Markers. 2018;2018:1507674.
52. Shainker SA, Silver RM, Modest AM, et al. Placenta accreta spectrum: biomarker discovery using plasma proteomics. Am J Obstet Gynecol. 2020;223(3):
53. Allen L, Jauniaux E, Hobson S, Papillon-Smith J, Belfort MA, Placenta Accreta FIGO. Diagnosis and Management Expert Consensus Panel. FIGO Consensus Guidelines on Placenta Accreta Spectrum Disorders. 2018;140(3):281–290.
54. Sentilhes L, Kayem G, Chandraharan E, Palacios-Jaraquemada J, Jauniaux E, Placenta Accreta FIGO. Diagnosis and Management Expert Consensus Panel. FIGO Consensus Guidelines Placenta Accreta Spectrum Dis. 2018;140(3):291–298.
55. Eller AG, Porter TF, Soisson P, Silver RM. Optimal management strategies for placenta accreta. BJOG Int J Obstet Gynaecol. 2009;116(5):648–654.
56. Rac MWF, Wells CE, Twickler DM, Moschos E, McIntire DD, Dashe JS. Placenta accreta and vaginal bleeding according to gestational age at delivery. Obstet Gynecol. 2015;125(4):808–813.
57. American College of Obstetricians and Gynecologists, Society for Maternal-Fetal Medicine. Obstetric Care Consensus No. 7: placenta Accreta Spectrum. Obstet Gynecol. 2018;132(6):e259–75.
58. Robinson BK, Grobman WA. Effectiveness of timing strategies for delivery of individuals with placenta previa and accreta. Obstet Gynecol. 2010;116(4):835–842.
59. Shamshirsaz AA, Fox KA, Erfani H, et al. Multidisciplinary team learning in the management of the morbidly adherent placenta: outcome improvements over time. Am J Obstet Gynecol. 2017;216(6):
60. Silver RM, Fox KA, Barton JR, et al. Center of excellence for placenta accreta. Am J Obstet Gynecol. 2015;212(5):561–568.
61. Sentilhes L, Goffinet F, Kayem G. Management of placenta accreta. Acta Obstet Gynecol Scand. 2013;92(10):1125–1134.
62. Hoffman MS, Karlnoski RA, Mangar D, et al. Morbidity associated with nonemergent hysterectomy for placenta accreta. Am J Obstet Gynecol. 2010;202(6):
63. Jauniaux E, Bunce C, Grønbeck L, Langhoff-Roos J. Prevalence and main outcomes of placenta accreta spectrum: a systematic review and meta-analysis. Am J Obstet Gynecol. 2019;221(3):208–218.
64. Al-Khan A, Gupta V, Illsley NP, et al. Maternal and fetal outcomes in placenta accreta after institution of team-managed care. Reprod Sci Thousand Oaks Calif. 2014;21(6):761–771.
65. Upson K, Silver RM, Greene R, Lutomski J, Holt VL. Placenta accreta and maternal morbidity in the Republic of Ireland, 2005-2010. J Matern Fetal Neonatal Med off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2014;27(1):24–29.
66. Tam Tam KB, Dozier J, Martin JN. Approaches to reduce urinary tract injury during management of placenta accreta, increta, and percreta: a systematic review. J Matern Fetal Neonatal Med off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2012;25(4):329–334.
67. Woldu SL, Ordonez MA, Devine PC, Wright JD. Urologic considerations of placenta accreta: a contemporary tertiary care institutional experience. Urol Int. 2014;93(1):74–79.
68. Silver RM. Abnormal Placentation: placenta Previa, Vasa Previa, and Placenta Accreta. Obstet Gynecol. 2015;126(3):654–668.
69. Fox KA, Shamshirsaz AA, Carusi D, et al. Conservative management of morbidly adherent placenta: expert review. Am J Obstet Gynecol. 2015;213(6):755–760.
70. Sentilhes L, Ambroselli C, Kayem G, et al. Maternal outcome after conservative treatment of placenta accreta. Obstet Gynecol. 2010;115(3):526–534.
71. Sentilhes L, Kayem G, Ambroselli C, et al. Fertility and pregnancy outcomes following conservative treatment for placenta accreta. Hum Reprod Oxf Engl. 2010;25(11):2803–2810.
72. Teixidor Viñas M, Belli AM, Arulkumaran S, Chandraharan E. Prevention of postpartum hemorrhage and hysterectomy in patients with morbidly adherent placenta: a cohort study comparing outcomes before and after introduction of the Triple-P procedure. Ultrasound Obstet Gynecol off J Int Soc Ultrasound Obstet Gynecol. 2015;46(3):350–355.
73. Shabana A, Fawzy M, Refaie W. Conservative management of placenta percreta: a stepwise approach. Arch Gynecol Obstet. 2015;291(5):993–998.
74. Barinov S, Tirskaya Y, Medyannikova I, Shamina I, Shavkun I. A new approach to fertility-preserving surgery in patients with placenta accreta. J Matern Fetal Neonatal Med off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2019;32(9):1449–1453.
75. Salim R, Chulski A, Romano S, Garmi G, Rudin M, Shalev E. Precesarean Prophylactic Balloon Catheters for Suspected Placenta Accreta: A Randomized Controlled Trial. Obstet Gynecol. 2015;126(5):1022–1028.
76. Chen M, Liu X, You Y, et al. Internal Iliac Artery Balloon Occlusion for Placenta Previa and Suspected Placenta Accreta: A Randomized Controlled Trial. Obstet Gynecol. 2020;135(5):1112–1119.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.