Back to Journals » Clinical Ophthalmology » Volume 12
Physiologic anisocoria under various lighting conditions
Authors Steck RP, Kong M, McCray KL, Quan V, Davey PG
Received 22 July 2017
Accepted for publication 25 November 2017
Published 4 January 2018 Volume 2018:12 Pages 85—89
DOI https://doi.org/10.2147/OPTH.S147019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Video abstract presented by Pinakin Davey
Views: 1316
Ryan P Steck,1 Min Kong,2 Kaydee L McCray,3 Valerie Quan,3 Pinakin Gunvant Davey3
1Western University of Health Sciences, College of Osteopathic Medicine of the Pacific, Pomona, CA, USA; 2Visual Science and Optometry Center, The People’s Hospital of Guangxi Nanning, Zhuang Autonomous Region, China; 3Western University of Health Sciences, College of Optometry, Pomona, CA, USA
Purpose: To evaluate the measurement of anisocoria in a group of ocular healthy subjects using a standardized protocol in scotopic, mesopic, and photopic lighting conditions, and determine the optimal threshold of difference in pupil diameter in determining physiologic anisocoria.
Methods: Right and left pupil diameters of 126 ocular healthy subjects with a mean age 30.5±7.8 years (40 males and 86 females) were measured sequentially under photopic conditions using a monocular infrared pupillometer. A sub-group of 51 individuals had right and left pupil measurements performed under three additional lighting conditions, allowing for a 2-minute recovery between measurements. A white light emitting diode (LED) in the eyecup of the pupillometer produced three controlled light settings: scotopic (0 lux), low mesopic (0.3 lux), and high mesopic (3 lux). The criterion for anisocoria was defined as ≥0.4 mm difference in pupil diameter between the eyes.
Results: In the 126 subjects tested, 23.8% (n=30) exhibited anisocoria in photopic conditions. In the sub-group measured under three additional light settings, 43.1% (n=22) exhibited anisocoria in scotopic conditions, 43.1% (n=22) in low mesopic conditions, and 47.1% (n=24) in high mesopic conditions. Approximately 73% of subjects exhibited anisocoria in at least one light setting, while only approximately 8% had anisocoria in every light setting. When the criterion for anisocoria was shifted to ≥0.2 mm or ≥0.6 mm, the prevalence of anisocoria shifted significantly. Using a higher cutoff of ≥0.6 mm effectively reduced the number of healthy individuals who exhibit anisocoria in every light setting to almost zero.
Conclusion: Based on our data, anisocoria is more prevalent under varied lighting conditions. To ensure the anisocoria is due to physiologic reasons, one should ensure that it is present under all lighting conditions to avoid excessive false positives.
Keywords: anisocoria, pupillometry, pupil diameter
Introduction
Anisocoria is defined as an inequality in pupil size, and its presence in the absence of ocular and neurologic pathology is known as physiologic anisocoria. Generally, a small difference in pupil diameter in both dim and bright illumination, with normal light response, no dilation lag, and no ptosis, does not warrant concern. The characteristics of normal physiologic anisocoria have been studied previously in attempts to assist eye care professionals in separating benign from pathologic findings.1–8 Historically, pupil gauges and flashlights were used to measure the pupil, but these devices were limited to static estimations of pupil size in shifting light conditions. Over the years, new devices have been developed to help offset these limitations, significantly improving the repeatability of pupil measurement and diagnostic accuracy in the clinic.9 There is, however, a lack of consensus on the threshold of anisocoria that is considered clinically significant. There is also no consensus on whether anisocoria that disappears under varied lighting conditions is considered physiologic or random pupillary noise.2–4,7,10,11 Meyer used a “perceptible” difference in pupil size as a threshold for anisocoria and was among the first to record the prevalence of physiologic anisocoria in a healthy population.12 Loewenfeld later used a criterion of >0.4 mm difference in pupil diameter as a threshold for physiologic anisocoria in a healthy population and found the prevalence to be about 20%.1 Lam was among the first to record the prevalence of physiologic anisocora under varying lighting conditions.2,4 He noted that 41% of his subjects exhibited anisocoria >0.4 mm in at least 1 light setting, while only 3% exhibited anisocoria in every setting.
There is also no consensus on the threshold of physiologic anisocoria above which investigation into underlying pathology is warranted. Gross et al have reported that anisocoria under 1 mm can be considered physiologic and warrants no further workup.13 Suh et al reported that children with Horner syndrome had an average pupillary difference of >1.3 mm, while only 3% of healthy children without Horner syndrome had >1.3 mm pupillary difference.8 Given the variation in thresholds and lighting conditions used, it is not surprising that there is a wide range of reported prevalence of physiologic anisocoria in the literature.2,3,7,10,13
The rise of digital infrared pupillometers has allowed providers to obtain more accurate, repeatable measurements of physiologic anisocoria with controlled illumination.14 These devices use advanced software and infrared videography to account for pupillary noise and vertex distance variance. They also use controlled lighting to produce consistent and precise illumination.10 There have been several studies reporting the prevalence of physiologic anisocoria using these newer devices, yet these studies lack procedural standardization and utilize inconsistent luminance levels.3,6,7,10,15
This lack of standardization in the measurement of anisocoria, particularly the luminance levels of the examination room, prevents comparison of previous studies and compromises the accuracy of anisocoria as a clinical diagnostic test.
This study seeks to evaluate the measurement of anisocoria in a group of subjects with healthy eyes using a standardized protocol in scotopic, mesopic, and photopic lighting conditions. An additional goal is to study the prevalence of anisocoria with varying cut-offs to allow for cross comparison.
Materials and methods
Subjects
One hundred and twenty-six subjects (40 male and 86 female) with no ocular pathology or history of ocular trauma were recruited for the study. The study was approved by the Institutional Review Board at the Western University of Health Sciences and conducted in accordance with the tenets of the Declaration of Helsinki. All participants were explained the study procedures and signed an informed consent form to participate in the study. All participants underwent distance visual acuity measurements using an Early Treatment Diabetic Retinopathy Study chart and individuals with visual acuity <20/20 in both eyes were excluded. The study participants underwent an ophthalmic evaluation that included a slit-lamp examination, fundus photography, and posterior segment evaluation using optical coherence tomography (Topcon OCT 2000 Fourier Domain OCT, Oakland, NJ, USA) using the macula and the optic disc protocols to evaluate any other subtle abnormalities. All study participants qualified for the study and met the inclusion criteria. The mean age of the study participants was 30.5 years, SD 7.8.
VIP-200 pupilometer
The VIP-200 pupillometer (NeurOptics, Irvine, CA, USA) is a monocular infrared camera that captures 30 pupil positions over a 2-second scanning period and automatically detects pupil size, producing the weighted average pupil size and SD. The device uses a small white LED light in the eyecup to produce pre-set illumination settings. Two measurement modes are available: photopic and variable. Photopic measurements do not utilize the LED and captures 30 pupil measurements in the standard 2-second scanning period. Variable mode captures the same pupil measurements using 3 light levels produced by the LED light: scotopic (0 lux), low mesopic (0.3 lux), and high mesopic (3 lux).
Pupil measurements under photopic conditions
All subjects were measured during business hours to prevent variation based on alertness. Accommodation was controlled by asking participants to fixate at a distance target placed 11 feet away. Subjects were instructed to look straight ahead at the target and instructed to keep both eyes open. Right and left pupil diameters were measured twice using the pupillometer in photopic conditions produced by overhead room lighting (675 lux measured at 1 m distance from the floor).
Pupil measurements under other lighting conditions
A sub-group of 51 individuals had pupil measurements that were performed twice under 3 additional lighting conditions: scotopic (0 lux), low mesopic (0.3 lux), and high mesopic (3 lux). Subjects were seated in a dark room and underwent a 5-minute dark adaption phase. Each participant was measured using the pre-set illumination settings on the pupillometer in increasing order of lux: scotopic, then low mesopic, and finally, high mesopic. Sufficient time interval of 2 minutes was provided for recovery between measurement of right and left eyes.
Results
Anisocoria was defined as ≥0.4 mm difference in mean pupil diameter as described in previous studies.1,2 Prevalence of anisocoria under varying light conditions is summarized in Table 1. In the larger group under photopic conditions, 23.8% (n=30) exhibited anisocoria. In the smaller subset of individuals measured under 4 lighting conditions, anisocoria was present in 27.4% (n=14) in photopic, 43.1% (n=22) in scotopic, 43.1% (n=22) in low mesopic, and 47.1% (n=24) in high mesopic conditions. In this subset, 72.5% (n=37) of the subjects exhibited anisocoria in at least 1 illumination setting, and 7.8% (n=4) had anisocoria in all illumination settings. Nine subjects had anisocoria ≥1 mm, but each subject only exhibited this difference in 1 or 2 light conditions. The range of observed anisocoria was 0–1.55 mm.
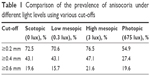  | Table 1 Comparison of the prevalence of anisocoria under different light levels using various cut-offs |
Considering alternate cut-offs for physiologic anisocoria
When anisocoria was defined as ≥0.2 mm, the prevalence of anisocoria in the large group under photopic conditions was 51.6% (n=65). In the subset of 51 individuals, the prevalence of anisocoria was 72.5% (n=37) in scotopic, 70.6% (n=36) in low mesopic, 76.5% (n=39) in high mesopic, and 54.9% (n=28) in photopic conditions. Every subject in this subset had ≥0.2 mm of anisocoria in at least 1 illumination condition, while 16 subjects (31.4%) had anisocoria in all conditions. Table 2 summarizes how adjusting the cut-off affects the prevalence of subjects exhibiting anisocoria under all light conditions.
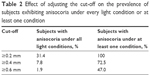  | Table 2 Effect of adjusting the cut-off on the prevalence of subjects exhibiting anisocoria under every light condition or at least one condition |
When anisocoria was defined as ≥0.6 mm, the prevalence of anisocoria under photopic conditions was 14.3% (n=18). In the subset of 51 individuals, the prevalence was 19.6% (n=10) in scotopic, 15.7% (n=8) in low mesopic, 21.6% (n=11) in high mesopic, and 19.6% (n=10) in photopic conditions. Approximately half (47%, n=24) of subjects in this subset had ≥0.6 mm of anisocoria in at least 1 illumination condition, while only 1 subject (1.9%) had anisocoria in all conditions.
Discussion
The results of this study are in agreement with the previously reported prevalence of physiologic anisocoria under photopic settings.2,3,12 Also, as reported previously, the prevalence of anisocoria increases under dim illumination levels, with anisocoria being 1.5 times greater at scotopic light levels compared with the photopic light levels.1,4,7 This may be in part due to an imbalance in resting sympathetic tone, or due to unequal fibrous iris stroma that increases resistance as the eye dilates, causing larger differences in pupil diameter under dim illumination levels.16,17
We also demonstrated that by using a cut-off of ≥0.4 mm, almost three-quarters of the subjects exhibited anisocoria in at least 1 illumination setting, while a mere 7.8% exhibited anisocoria across all lighting conditions. This phenomenon was reported previously by Ettinger et al and Lam et al and indicates that anisocoria is not consistent across various lighting conditions.2,3 In addition, 9 subjects exhibited anisocoria ≥1 mm, and this difference was only present in 1 or 2 light conditions per patient. These subjects did not exhibit anisocoria in the range close to 1 mm in other lighting conditions. It would be highly unusual for subjects with true anisocoria >1 mm to not exhibit anisocoria at other lighting levels, leading us to believe that these measurements are either user or device error. In these patients, the inconsistency of marked anisocoria may lead to an unwarranted and extensive neurological workup in the clinic, and clinicians should be particularly aware of this possibility.
When the cut-off for anisocoria was lowered to ≥0.2 mm, every subject in the smaller subset exhibited anisocoria in at least 1 setting, demonstrating that this threshold is either too stringent to be clinically useful or might be too low for the accuracy of the VIP-200 pupillometer.
When the cut-off for anisocoria was raised to ≥0.6 mm, the prevalence of anisocoria became more consistent across all light conditions, with ~20% of subjects exhibiting anisocoria in each condition. This is closer to the prevalence observed by previous studies using a lower threshold of ≥0.4 mm.3,4,6 This could be explained by differences in pupillometer accuracy or ambient light levels. Prior investigations into the appropriate threshold of anisocoria have yielded similarly variable results when the threshold was shifted to 0.2 or 0.6 mm.3,4 It is important to note that most studies to date on the prevalence of physiologic anisocoria have had varying sample sizes ranging from 50 to 1,300 subjects,3,6 and this could be contributing to the varying study results despite strict protocols. There is a lack of extensive research into physiologic anisocoria in a large population and this has limited our ability to clearly define a limit of physiologic anisocoria. Using a higher cut-off of ≥0.6 mm effectively reduces the number of healthy individuals who exhibit anisocoria in every light setting to zero, and may reduce error due to physiologic variation and improve comparison of studies. We recognize, however, that this cut-off is likely subject to significant variation based on a number of factors as explained below.
There are various systematic errors that can lead to erroneous anisocoria measurements. Variation from study to study may be largely due to differences in the measurement device and protocol. It may also be due to pupillary unrest or accommodation when moving from eye to eye with a monocular device. However, the software of the NeurOptics pupillometer in part negates this with 30 repeat measurements over a 2-second interval, which are then averaged to avoid the effect of pupillary unrest. Future studies may benefit from the use of binocular devices to exclude the possibility of this error. Lowenstein described the effect of attention on pupillary diameter stating that when a subject is fatigued, they exhibit large amplitude pupillary oscillations, and when a subject is in emotional distress, sympathetic input overrides the external stimulus from light.18 Our clinical exam did not perform any screening test for attention or distress; however, the exam was performed only during common business hours and there was no observable fatigue or distress.3,19 Although we performed all the examinations in a dark room, we cannot rule out variation in ambient illumination during the exam. This is especially true in scotopic levels where the light emitted from the device display prevented complete darkness. It is also important to note that although we allowed a consistent time interval between measurements, asymmetrically sluggish pupils may alter measurements taken in succession as noted in a recent case report and discussed in detail elsewhere.9,20 Physiologic anisocoria, as studied here, should not be significantly affected by sluggish pupils.21 Accommodation may also have been a source of variation because patients focused on a target about 11 feet (3.35 m) away. This translates to ~0.3 diopters of accommodation. The variation in pupil diameter caused by this accommodation is likely limited because Heine reported that near fixation has little to no effect on pupil diameter at distances >1 m.22
In conclusion, health care providers using digital pupillometers should be aware of the prevalence of physiologic anisocoria in a healthy population. In this study, we complement the current database describing the prevalence of physiologic anisocoria in healthy participants under photopic, mesopic, and scotopic conditions. Based on our data, physiologic anisocoria is more prevalent under varied lighting conditions, and clinicians and researchers may benefit from capturing pupil measurements in standardized light conditions. Furthermore, using a cut-off for anisocoria of ≥0.6 mm, and insisting on its presence under all lighting conditions, decreases the prevalence of anisocoria in healthy individuals. However, the protocol and device differences between studies greatly limit our ability to set a standard threshold to define physiological anisocoria. Future studies using standardized protocols and larger sample size are needed to establish a more robust cut-off for physiologic anisocoria.
Disclosure
The authors report no conflicts of interest in this work.
References
Loewenfeld IE. “Simple central” anisocoria: a common condition, seldom recognized. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1977;83(5):832–839. | ||
Lam BL, Thompson HS, Corbett JJ. The prevalence of simple anisocoria. Am J Ophthalmol. 1987;104(1):69–73. | ||
Ettinger ER, Wyatt HJ, London R. Anisocoria. Variation and clinical observation with different conditions of illumination and accommodation. Invest Ophthalmol Vis Sci. 1991;32(3):501–509. | ||
Lam BL, Thompson HS, Walls RC. Effect of light on the prevalence of simple anisocoria. Ophthalmology. 1996;103(5):790–793. | ||
Hashemi H, Yazdani K, KhabazKhoob M, Mehravaran S, Mohammad K, Fotouhi A. Distribution of photopic pupil diameter in the tehran eye study. Curr Eye Res. 2009;34(5):378–385. | ||
Silbert J, Matta N, Tian J, Singman E, Silbert DI. Pupil size and anisocoria in children measured by the plusoptiX photoscreener. J AAPOS. 2013;17(6):609–611. | ||
Rickmann A, Waizel M, Kazerounian S, Szurman P, Wilhelm H, Boden KT. Digital pupillometry in normal subjects. Neuroophthalmology. 2017;41(1):12–18. | ||
Suh SH, Suh DW, Benson C. The degree of anisocoria in pediatric patients with horner syndrome when compared to children without disease. J Pediatr Ophthalmol Strabismus. 2016;53(3):186–189. | ||
Wilhelm H, Wilhelm B. Clinical applications of pupillography. J Neuroophthalmol. 2003;23(1):42–49. | ||
Colard S. Prevalence of physiological anisocoria and influence of iris corneal parameters a in an adult population. Invest Ophthalmol Vis Sci. 2016;57(12):4560. | ||
Rosen ES, Gore CL, Taylor D, Chitkara D, Howes F, Kowalewski E. Use of a digital infrared pupillometer to assess patient suitability for refractive surgery. J Cataract Refract Surg. 2002;28(8):1433–1438. | ||
Meyer BC. Incidence of anisocoria and difference in size of palpebral fissures in five hundred normal subjects. Arch Neurol Psychiatry. 1947;57(4):464–468. | ||
Gross JR, McClelland CM, Lee MS. An approach to anisocoria. Curr Opin Ophthalmol. 2016;27(6):486–492. | ||
Bremner F. Pupil evaluation as a test for autonomic disorders. Clin Auton Res. 2009;19(2):88–101. | ||
Schallenberg M, Bangre V, Steuhl KP, Kremmer S, Selbach JM. Comparison of the colvard, procyon, and neuroptics pupillometers for measuring pupil diameter under low ambient illumination. J Refract Surg. 2010;26(2):134–143. | ||
Rosenberg ML. Physiologic anisocoria: a manifestation of a physiologic sympathetic asymmetry. Neuro Ophthalmol. 2008;32(3):147–149. | ||
Loewenfeld IE, Newsome DA. Iris mechanics. I. Influence of pupil size on dynamics of pupillary movements. Am J Ophthalmol. 1971;71(1 Pt 2):347–362. | ||
Lowenstein O, Feinberg R, Loewenfeld IE. Pupillary movements during acute and chronic fatigue a new test for the objective evaluation of tiredness. Invest Ophthalmol Vis Sci. 1963;2(2):138–157. | ||
Ludtke H, Wilhelm B, Adler M, Schaeffel F, Wilhelm H. Mathematical procedures in data recording and processing of pupillary fatigue waves. Pathophysiologie des Sehens und Neuroophtalmologie. 1998;38(19):2889–2896. | ||
Kramer CL, Rabinstein AA, Wijdicks EF, Hocker SE. Neurologist versus machine: is the pupillometer better than the naked eye in detecting pupillary reactivity. Neurocrit Care. 2014;21(2):309–311. | ||
Pilley SF, Thompson HS. Pupillary “dilatation lag” in Horner’s syndrome. Br J Ophthalmol. 1975;59(12):731–735. | ||
Heine C, Yazdani F, Wilhelm H. Pupillenweite in Alltagssituationen [Pupillary diameter in every day situations]. Klin Monbl Augenheilkd. 2013;230(11):1114–1118. German. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
