Back to Journals » Clinical Ophthalmology » Volume 14
Photoactivated Chromophore for Keratitis-Corneal Collagen Cross-Linking (PACK-CXL) Improves Outcomes of Treatment-Resistant Infectious Keratitis
Authors Gulias-Cañizo R , Benatti A, De Wit-Carter G, Hernández-Quintela E, Sánchez-Huerta V
Received 28 September 2020
Accepted for publication 4 November 2020
Published 21 December 2020 Volume 2020:14 Pages 4451—4457
DOI https://doi.org/10.2147/OPTH.S284306
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Rosario Gulias-Cañizo,1 Andres Benatti,2 Guillermo De Wit-Carter,3 Everardo Hernández-Quintela,3 Valeria Sánchez-Huerta3
1Universidad Anahuac Mexico, Huixquilucan, Estado de Mexico, Mexico; 2Cordoba Eye Clinic, Cornea and Refractive Surgery Department, Cordoba, Argentina; 3Asociación para Evitar la Ceguera en México, Hospital Dr. Luis Sanchez Bulnes, Cornea Department, Mexico City 04030, Mexico
Correspondence: Valeria Sánchez-Huerta
Asociación para Evitar la Ceguera en México, Hospital Dr. Luis Sanchez Bulnes, Cornea Department, Vicente García Torres 46, San Lucas, Mexico City 04030, Mexico
Tel +5215525599999
Fax +5215510841404
Email [email protected]
Purpose: To investigate the efficacy of photoactivated chromophore corneal collagen cross-linking (PACK)-CXL in the management of treatment-resistant infectious keratitis.
Design: Observational cohort study.
Participants: Forty-two eyes from 41 patients with treatment-resistant infectious keratitis.
Methods: Eyes underwent PACK-CXL treatment with the Dresden modified protocol in addition to standard antimicrobial therapy. The primary endpoint was the size of the corneal ulcer. Descriptive statistics, Wilcoxon rank test, McNemar test and Spearman correlation coefficient were used for statistical analysis, and p values lower than 0.05 were considered statistically significant.
Results: Success rate at third postoperative month was of 90.5%. Statistical analyses showed a significant effect of (PACK)‑CXL with standard antimicrobial therapy to reduce corneal ulcer size (p=0.031).
Conclusion: As adjuvant therapy to standard antimicrobial treatment, PACK-CXL improves the outcomes in patients with treatment-resistant corneal ulcers.
Keywords: cross-linking, PACK-CXL, resistant keratitis
Introduction
Infectious keratitis is associated with a risk of permanent and devastating visual loss worldwide, especially in developing countries.1–5 Treatment comprises topical broad-spectrum antibiotics, but if therapy is delayed, it is estimated that only 50% of the eyes will have a good visual outcome; actually, infectious keratitis can lead to corneal perforation or endophthalmitis.6
To improve treatment outcomes and enhance microbial eradication with fewer side effects, new approaches like corneal cross-linking (CXL) have been proposed. This technique was introduced in the late 1990s as a primary treatment for corneal ectasia, for which is widely used. It is a simple and non-invasive technique that combines ultraviolet A radiation and a chromophore (riboflavin) to stiffen corneal tissue through collagen fiber photopolymerization.7
CXL is proposed to be effective for treating infectious keratitis based on the antibacterial properties of photoactivated chromophore and UVA light. Its mechanism of action includes inhibition of microbial replication, DNA and RNA damage,8,9 intercalation of the chromophore with microbial nucleic acids,10 direct damage to the pathogen cell walls and oxidation of nucleic acid residues by reactive oxygen species,11,12 as well as increased resistance of the stiffened cornea to enzymatic damage from the microorganisms.13 Other potential advantages of UVA and riboflavin application over antibiotics include eliminating ocular surface toxicity and avoiding adherence issues associated with the need for frequent eyedrop administration, among others.14
In 2000, Schnitzler et al reported the use of CXL for stabilization of non‑infectious corneal melting in four patients.15 The melting process stopped in three of four patients, delaying surgical treatment. This early trial showed the efficacy of CXL in biomechanical stabilization of structurally altered corneas without inducing ectasia. Other case studies about the effect of CXL on melting corneas with advanced and treatment-resistant keratitis showed similar results and proved to be effective not only in stabilizing melting but also in eliminating several pathogens.16–19
To encourage the exploration of different applications of CXL and modifications to the Dresden protocol,20 the ninth CXL congress in 20138,21 established separate designations to distinguish between CXL for ectasia and CXL for infectious keratitis. The latter is also known as photo-activated chromophore for infectious keratitis (PACK)‑CXL.22
We present an observational cohort study of antibiotic-resistant infectious keratitis treated with PACK-CXL plus standard antibiotic treatment in a tertiary eye care center in Mexico City.
Materials and Methods
The participants were enrolled from the Cornea Department of the “Asociación para Evitar La Ceguera en Mexico I.A.P., Hospital Dr. Luis Sánchez Bulnes”, a tertiary eye care center located in Mexico City, from November 2014 to January 2015. The ethics committee of the “Asociación para Evitar La Ceguera en Mexico I.A.P., Hospital Dr. Luis Sánchez Bulnes”, approved this observational cohort study and all research followed the tenets of the Declaration of Helsinki. A written informed consent was obtained from all participants.
Patients eligible for the study were male and female adult patients (18 years or older) with treatment-resistant infectious keratitis. A patient was considered treatment resistant when after one week with treatment, there was a worsening of clinical presentation (increased epithelial defect). Treatment consisted in one of the following therapies: 1) If a bacterial infection was suspected or documented, a fluoroquinolone and an aminoglycoside were prescribed 2) If a fungal infection was suspected or documented, natamycin was prescribed 3) For suspected polymicrobial etiology, both strategies were used. Exclusion criteria were diagnosis of rheumatologic disease, perforation or a descemetocele with high risk of corneal perforation, endophthalmitis, and pregnancy.
All patients underwent a comprehensive ophthalmic examination that included uncorrected visual acuity (UCVA), slit lamp biomicroscopy, fundoscopy and intraocular pressure. The severity of keratitis was graded by slit lamp examination and the longest diameter of the ulcer in the first exam (initial size) was registered. Patients were classified according to the size of the ulcer in 3 categories: ulcers with size <3 mm, 3–6 mm and >6 mm. Ulcer size was defined as the corneal epithelial defect, regardless of infiltrating size. A minimum 400-micron corneal stromal thickness was considered safe to perform CXL, obtaining pachymetry by Pentacam® rotating Scheimpflug camera topography.
After obtaining corneal scrapings, they were sent for standard microbiology culture and antibiotic susceptibility testing, as following: after tetracaine instillation, a sample of the desired area of the cornea was taken with a spatula or cotton swab, proceeding to spread C-shaped streaks on chocolate agar, blood agar (5% ram blood), staphylococcus 110, Sabouraud agar, Sabouraud-Emmons medium for fungal growth, and Biggy agar for Candida.
For microscopic observation, the sample is placed in an area marked with a circle with a diamond pencil to prepare the following stains: Gram for positive and negative bacteria, Ziehl-Neelsen for acid-alcohol-fast bacilli, Periodic acid-Schiff (PAS) and calcofluor white (CFW) for Acanthamoeba and fungi under fluorescent light.
(PACK)–CXL with ultraviolet A and riboflavin was applied under topical anesthesia on the day of diagnosis (day 0). According to the Dresden modified protocol, riboflavin 0.1% solution was administered to the cornea every minute for 15 minutes, followed by exposure to 370-nm UVA light (with a fluence of 3 mW/cm2) from a distance of 1 cm for 30 minutes.
After PACK-CXL treatment, the eye was rinsed with saline followed by bandage contact lens placement; post-operative fluorometholone acetate 0.1% drops were given 4 times a day for 2 days and then 3 times a day for one week. Contact lens was removed one day after placement. Patients received topical antibiotic eyedrops based on the antibiogram results and artificial tear eyedrops until epithelial healing was observed, and as part of the postoperative care, patients were advised to wear UV protection glasses. The primary endpoint was the size of the ulcer. Treatment success was defined as the complete closure of the epithelial defect, with no corneal stromal infiltrates and lack of symptoms.
Standard antimicrobial treatment was initiated as monotherapy with fourth-generation fluoroquinolones and subsequently modified according to clinical response, gram stains culture and antibiotic susceptibility results. Antimicrobials given to the patients were moxifloxacin 0.5% ophthalmic solution (Vigamoxi®, 17 eyes, 40.47%); ofloxacin 0.3% ophthalmic solution (Ocuflox®, 8 eyes, 19.04%), gatifloxacin 0.5% ophthalmic solution (Zymar®, 15 eyes, 35.71%), natamycin 0.5% ophthalmic suspension (Pimaricin®, 7 eyes, 16.66%), netilmicin 0.3% ophthalmic solution (Netira®, 26 eyes, 61.9%), tobramycin 0.3% ophthalmic solution (Trazil®, 3 eyes, 7.14%), ceftazidime 5% ophthalmic solution (500 mg/2mL solution; 2 mL of this solution are added to 8 mL of hypromellose; 2 eyes, 4.76%), sulfacetamide 10% ophthalmic solution (Blef®-10, 1 eye, 2.38%), chloramphenicol 0.5% ophthalmic (Cloran®, 2 eyes, 4.76%) solution and/or voriconazole 1% ophthalmic solution (Vozole®, 8 eyes, 19.04%).
Statistical analysis was performed using descriptive statistics with the software IBM SPSS Statistics for Windows, Version 23.0 Armonk, NY: IBM Corp. Since the data followed a normal distribution (verified by Kolmogorov–Smirnov test), for quantitative variables we used measures of central tendency and dispersion. Qualitative variables were expressed as absolute and relative frequencies, using tables to present the results. For inferential statistics, the Wilcoxon rank test was used to compare changes in corneal ulcer size, considering that the size was categorized on an ordinal scale. McNemar test was used to compare treatment response; both tests were used to evaluate initial and each follow-up time-point after treatment, always comparing with the previous immediate visit. Finally, Spearman´s correlation coefficient was used to correlate treatment stages with the percentage of healed eyes. For all tests, an α level of significance was assumed if the p-value was <0.05.
Results
We analyzed 42 eyes corresponding to 41 patients; the average age of the patients was 48.24 years; 52.38% were male and 47.62% female. All patients were Mexican Mestizos (a mixture of indigenous and European ancestry). Of the eyes analyzed, 26 were OD and 16 were OS. Corneal scraping results showed Gram-positive organisms as the more frequent isolated strains followed by Gram-negative and fungal. There were also nine negative culture results. Staphylococcus epidermidis was the most frequent isolated strain (14 eyes, 33.33%) followed by Klebsiella (4 eyes, 9.52%), Fusarium dimerum (3 eyes, 7.14%), Serratia marcescens (2 eyes, 4.76%), Enterobacter cloacae (2 eyes, 4.76%) and Bacillus (2 eyes, 4.76%). The initial size (Day 0) of the corneal ulcer was of <3 mm in 21.43%, 3 to 6 mm in 47.62% and >6 mm in 30.95% (Table 1).
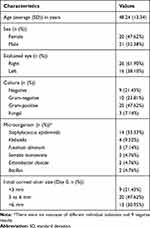 |
Table 1 Clinical Characteristics of Patients with Infectious Keratitis |
Corneal ulcer size was evaluated at different time-points after treatment, always comparing with the immediately preceding time-point. The results showed significant differences in the size of the corneal ulcer on the first postoperative day (Day 1) compared to the initial size (p-value = 0.008), where 16.67% of the eyes showed an increased size of the ulcer, while 83.33% maintained the initial size. At this point, four eyes needed surgery due to imminent perforation, so two underwent tectonic corneal transplant, one underwent conjunctivoplasty (conjunctival flap) and one was eviscerated. It is worth mentioning that these four cases were referred to our center and had been previously treated with steroids, which may explain the negative outcomes. Three of these eyes (75%) showed a mycotic isolate by culture and the other was negative. These eyes were maintained as failures (corneal ulcer or increased size of the ulcer) for the rest of analyses. For the remaining eyes, between Day 1 and Week 1, there were also significant size differences with a p-value = 0.000. In this case, 76.19% of the eyes showed a decrease in the size of the ulcer and 14.28% maintained the size. When comparing Week 1 with Month 1, we observed significant differences in the size of the ulcer (p-value = 0.000), where 85.71% of the eyes showed a decrease in the size of the ulcer or healing. At Month 3 compared to Month 1, significant differences (p-value = 0.020) were detected, with 14.28% showing a decrease of the ulcer size or healing, and the remaining 84.21% unchanged at Month 3, corresponded to patients that at the Month 1 time-point had already healed (Table 2).
 |
Table 2 Comparison of Ulcer Size by Time-Points |
Treatment response (healed or presence of corneal ulcer) was compared between the different time-points after treatment, always making comparisons with the previous time-point. The results showed that between Day 1 and Week 1 after treatment, no significant differences were observed, with treatment response in a few percentage of the cases. Between Week 1 and Month 1, significant differences were observed with a high percentage of the eyes healing and a few percentage with the corneal ulcer remaining. Finally, between Month 1 and Month 3, significant differences were also observed, achieving healing of all treated eyes except the four cases that needed emergency surgery (Table 3).
 |
Table 3 Comparison of Treatment Response by Time-Points |
When correlating each time-point with the percentage of healed eyes, a direct linear relationship was observed (p-value = 0.005, Spearman´s correlation coefficient 0.975), indicating that treatment effect increased with time. Regarding this result, it is important to highlight that the percentage of healing eyes increases dramatically between one week and one month of treatment (from 4.77% to 76.20%).
Discussion
Some authors have reported the use of CXL as a treatment for advanced corneal melting in treatment-resistant cases of infectious keratitis23–25 alone or in combination with surgery.26 A small cohort of five patients,23 unresponsive to standard topical and systemic antimicrobial therapy, prompted the use of CXL as a rescue measure, observing that melting stopped in four of five patients. This confirmed previous results from Schnitzler and colleagues15 and proposed that CXL may be effective for the treatment of infectious corneal ulcers. Several case series, two randomized controlled trials,22,27 a systematic review and meta-analysis28 and one meta-analysis29 showed similar findings.
The PACK-CXL protocol, widely described in the literature, uses conventional 365 nm UVA light at 3 mW/cm2 applied for 15, 30 or 45 minutes followed by 9 mW/cm2 accelerated UVA light for 10 minutes. In our institution, we follow the Dresden protocol modified by Wolensack20 which is the standard in most institutions, with 30 minutes of UVA light exposition.
Causative microorganisms of infectious keratitis found in our study were similar to those reported in the literature, with bacteria being prominent. We did not identify Acanthamoeba trophozoites or cysts on Gram, Giemsa–Wright or hematoxylin and eosin stains. Although other authors have described viral etiologies (eg, herpesvirus), the clinical evolution of our patients suggested a bacterial or fungal etiology, and additionally we do not have the laboratory resources to perform specific tests like immunofluorescence assays (IFA) or polymerase chain reactions (PCR), so we cannot exclude that negative cultures may have been of viral origin. We found a low incidence of fungal infections, consistent with a 30-year survey exploring causative organisms in infectious corneal ulcers that reported that only 1% of the cases were fungal.30
Papaioannou et al29 reported a healing rate of 87.2% (159 of 175 eyes) in a systematic review and meta-analysis of 2 randomized controlled clinical trials, 13 case series, and 10 case reports, and we found a similar rate in our study (90.50%). They also showed a low success rate in fungal infections consistent with our findings in which from the four eyes that needed surgery due to imminent perforation, three (75%) had a fungal isolate by culture. This finding is consistent with other studies31–34 where bacterial keratitis cases show improvement after PACK-CXL treatment, whereas fungal keratitis show less or no improvement at all,35 like in our study, where the 3 cases with a fungal isolate had a torpid evolution and needed urgent surgery to avoid organ loss, which unfortunately could not be prevented in one case. Nevertheless, there are studies that report a high success rate for fungal ulcers.36 The late application of PACK-CXL in our cases may explain the huge reduction in effectiveness and the low healing rate leading to treatment failure. Also, Zhang´s37 observations regarding lack of effect in deep fungal keratitis should be taken into consideration; perhaps our cases corresponded to deep infections and this would explain the bad outcomes. Finally, the efficacy of PACK-CXL correlates with the causative agent, a factor that should be considered when deciding to use this treatment in an individualized manner.
It is important to highlight that between Day 1 and Week 1 after treatment, the ulcer size does not decrease or even increases slightly compared to baseline and then decreases after Week 1. This should not be a reason for considering that the treatment is not successful, since this response is due to the mechanical de-epithelialization of the ulcer margins for CXL application and not a sign of deterioration. The slow healing rate in the first week (consistent with re-epithelialization) contrasts with the strong and sustained response to treatment showed by the decreased ulcer size and the healing response after this time-point, reaching a maximum at Month 3.
An important contribution of this work is the results achieved with the early use of fluorometholone in all patients. It is recommended to start fluorometholone only after re-epithelialization is complete; nevertheless, we have observed that post-CXL inflammation may induce corneal lysis and hence complications, but in our experience, the early use of fluorometholone helps to avoid negative lysis-related outcomes in bacterial cases.
This study was conducted in patients with resistant forms of corneal infections in whom antimicrobial therapy was administered for a long time without response; usually this cases progress and cause serious damage to the cornea and as a consequence, devastating visual or organ loss. The healing rates obtained with PACK-CXL are unprecedented positive results for this patient population, hence the importance of sharing our results to prompt a wider use of this procedure in cases unresponsive to standard therapy.
Also, as microbial resistance to antibiotics increases, new lines of treatment are needed. PACK-CXL may be a promising new alternative and its use is recommended due to the potential benefit obtained by controlling infection regardless of drug resistance, stopping the melting process, avoiding emergency keratoplasty and decreasing the possibility of performing lamellar grafts for visual rehabilitation.
Funding
The authors declare that they did not receive funding for this work.
Disclosure
Rosario Gulias-Cañizo reports she is an employee of Alcon Laboratories, outside the submitted work. Everardo Hernández-Quintela reports consultant fees from Allergan, Sifi and Thea Laboratories, outside the submitted work. The authors declare that there is no other conflicts of interest.
References
1. Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis: predisposing factors and morbidity. Ophthalmology. 2006;113(1):109–116. doi:10.1016/j.ophtha.2005.08.013
2. Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87(7):834–838. doi:10.1136/bjo.87.7.834
3. Wong T, Ormonde S, Gamble G, McGhee CN. Severe infective keratitis leading to hospital admission in New Zealand. Br J Ophthalmol. 2003;87(9):1103–1108. doi:10.1136/bjo.87.9.1103
4. McLeod SD, LaBree LD, Tayyanipour R, Flowers CW, Lee PP, McDonnell PJ. The importance of initial management in the treatment of severe infectious corneal ulcers. Ophthalmology. 1995;102(12):1943–1948. doi:10.1016/S0161-6420(95)30771-3
5. Ibrahim YW, Boase DL, Cree IA. Epidemiological characteristics, predisposing factors and microbiological profiles of infectious corneal ulcers: the Portsmouth corneal ulcer study. Br J Ophthalmol. 2009;93(10):1319–1324. doi:10.1136/bjo.2008.151167
6. Jones DB. Decision-making in the management of microbial keratitis. Ophthalmology. 1981;88(8):814–820. doi:10.1016/S0161-6420(81)34943-4
7. Randleman JB, Khandelwal SS, Hafezi F. Corneal cross-linking. Surv Ophthalmol. 2015;60(6):509–523. doi:10.1016/j.survophthal.2015.04.002
8. Tabibian D, Richoz O, Hafezi F. PACK-CXL: corneal cross-linking for treatment of infectious keratitis. J Ophthalmic Vis Res. 2015;10(1):77–80. doi:10.4103/2008-322X.156122
9. Goodrich RP. The use of riboflavin for the inactivation of pathogens in blood products. Vox Sang. 2000;78:211–215.
10. Naseem I, Ahmad M, Hadi SM. Effect of alkylated and intercalated DNA on the generation of superoxide anion by riboflavin. Biosci Rep. 1988;8(5):485–492. doi:10.1007/BF01121647
11. M V K, Yoneda T, Hiramatsu M. Scavenging activity of “beta catechin” on reactive oxygen species generated by photosensitization of riboflavin. Biochem Mol Biol Int. 1996;38(6):1163–1170.
12. Kumar V, Lockerbie O, Keil SD, et al. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochem Photobiol. 2004;80(1):15–21. doi:10.1562/2003-12-23-RA-036.1
13. Spoerl E, Wollensak G, Seiler T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr Eye Res. 2004;29(1):35–40. doi:10.1080/02713680490513182
14. Letko E. Collagen crosslinking for corneal infection. Refract Eyecare. 2012;16(7):1–3.
15. Schnitzler E, Spörl E, Seiler T. Irradiation of cornea with ultraviolet light and riboflavin administration as a new treatment for erosive corneal processes, preliminary results in four patients. Klin Monbl Augenheilkd. 2000;217(3):190–193. doi:10.1055/s-2000-10344
16. Alio JL, Abbouda A, Valle D, Del Castillo JM, Fernandez JA. Corneal cross linking and infectious keratitis: a systematic review with a meta-analysis of reported cases. J Ophthalmic Inflamm Infect. 2013;3(1):47. doi:10.1186/1869-5760-3-47
17. Del Buey MA, Cristóbal JA, Casas P, et al. Evaluation of in vitro efficacy of combined riboflavin and ultraviolet A for Acanthamoeba isolates. Am J Ophthalmol. 2012;153(3):399–404. doi:10.1016/j.ajo.2011.07.025
18. Galperin G, Berra M, Tau J, Boscaro G, Zarate J, Berra A. Treatment of fungal keratitis from fusarium infection by corneal cross-linking. Cornea. 2012;31(2):176–180. doi:10.1097/ICO.0b013e318221cec7
19. Richoz O, Gatzioufas Z, Hafezi F. Corneal collagen cross-linking for the treatment of acanthamoeba keratitis. Cornea. 2013;32(10):e189. doi:10.1097/ICO.0b013e31829a689e
20. Wollensak G, Spoerl E, Seiler T. Riboflavin/ultraviolet-a-induced collagen crosslinking for the treatment of keratoconus. Am J Ophthalmol. 2003;135(5):620–627. doi:10.1016/S0002-9394(02)02220-1
21. Tabibian D, Mazzotta C, Hafezi F. PACK-CXL: corneal cross-linking in infectious keratitis. Eye Vis (Lond). 2016;3:11.
22. Said DG, Elalfy MS, Gatzioufas Z, et al. Collagen cross-linking with photoactivated riboflavin (PACK-CXL) for the treatment of advanced infectious keratitis with corneal melting. Ophthalmology. 2014;121(7):1377–1382. doi:10.1016/j.ophtha.2014.01.011
23. Iseli HP, Thiel MA, Hafezi F, Kampmeier J, Seiler T. Ultraviolet A/riboflavin corneal cross-linking for infectious keratitis associated with corneal melts. Cornea. 2008;27(5):590–594. doi:10.1097/ICO.0b013e318169d698
24. Sorkhabi R, Sedgipoor M, Mahdavifard A. Collagen cross-linking for resistant corneal ulcer. Int Ophthalmol. 2013;33(1):61–66. doi:10.1007/s10792-012-9633-2
25. Sağlk A, Uçakhan OO, Kanpolat A. Ultraviolet A and riboflavin therapy as an adjunct in corneal ulcer refractory to medical treatment. Eye Contact Lens. 2013;39(6):413–415. doi:10.1097/ICL.0b013e3182960fdf
26. Mattila JS, Korsbäck A, Krootila K, Holopainen JM. Treatment of Pseudomonas aeruginosa keratitis with combined corneal cross-linking and human amniotic membrane transplantation. Acta Ophthalmol. 2013;91(5):e410–411. doi:10.1111/aos.12115
27. Bamdad S, Malekhosseini H, Khosravi A. Ultraviolet A/riboflavin collagen cross-linking for treatment of moderate bacterial corneal ulcers. Cornea. 2015;34(4):402–406. doi:10.1097/ICO.0000000000000375
28. Ting DSJ, Henein C, Said DG, Dua HS. Photoactivated chromophore for infectious keratitis - Corneal cross-linking (PACK-CXL): a systematic review and meta-analysis. Ocul Surf. 2019;17(4):624–634. doi:10.1016/j.jtos.2019.08.006
29. Papaioannou L, Miligkos M, Papathanassiou M. Corneal collagen cross-linking for infectious keratitis. Cornea. 2016;35(1):62–71. doi:10.1097/ICO.0000000000000644
30. Asbell P, Stenson S. Ulcerative keratitis: survey of 30 years’ laboratory experience. Arch Ophthalmol. 1982;100(1):77–80. doi:10.1001/archopht.1982.01030030079005
31. Escarião ACSL, Ribeiro ES, Jorge PA, Leite ECS, Brandt CT. Therapeutic effect of corneal crosslinking on infectious keratitis. Rev Bras Oftalmol. 2013;72:366–372. doi:10.1590/S0034-72802013000600003
32. Vajpayee RB, Shafi SN, Maharana PK, Sharma N, Jhanji V. Evaluation of corneal collagen cross-linking as an additional therapy in mycotic keratitis. Clin Exp Ophthalmol. 2015;43(2):103–107. doi:10.1111/ceo.12399
33. Uddaraju M, Mascarenhas J, Das MR, et al. Corneal cross-linking as an adjuvant therapy in the management of recalcitrant deep stromal fungal keratitis: a randomized trial. Am J Ophthalmol. 2015;160(1):131–134. doi:10.1016/j.ajo.2015.03.024
34. Makdoumi K, Mortensen J, Sorkhabi O, Malmvall BE, Crafoord S. UVA-riboflavin photochemical therapy of bacterial keratitis: a pilot study. Graefe’s Arch Clin Exp Ophthalmol. 2012;250(1):95–102. doi:10.1007/s00417-011-1754-1
35. Prajna NV, Radhakrishnan N, Lalitha P, et al. Cross-linking-assisted infection reduction: a randomized clinical trial evaluating the effect of adjuvant cross-linking on outcomes in fungal keratitis. Ophthalmology. 2020;127(2):159–166. doi:10.1016/j.ophtha.2019.08.029
36. Li Z, Jhanji V, Tao X, Yu H, Chen W, Mu G. Riboflavin/ultraviolet light-mediated crosslinking for fungal keratitis. Br J Ophthalmol. 2013;97(5):669–671. doi:10.1136/bjophthalmol-2012-302518
37. Zhang Z. Corneal cross-linking for the treatment of fungal keratitis. Cornea. 2013;32(2):217–218. doi:10.1097/ICO.0b013e3182732d62
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
