Back to Journals » Drug Design, Development and Therapy » Volume 9
Pharmacokinetic interaction between udenafil and dapoxetine: a randomized, open-labeled crossover study in healthy male volunteers
Authors Kim YH, Choi HY, Lee SH, Jeon HS, Lim H, Bahng M, Bae K
Received 4 December 2014
Accepted for publication 30 December 2014
Published 23 February 2015 Volume 2015:9 Pages 1209—1216
DOI https://doi.org/10.2147/DDDT.S78713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Shu-Feng Zhou
Yo Han Kim,1 Hee Youn Choi,1 Shi Hyang Lee,1 Hae Sun Jeon,1 Hyeong-Seok Lim,1 Mi Young Bahng,2 Kyun-Seop Bae1
1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, College of Medicine, University of Ulsan, 2Clinical Development Department, Dong-A ST Co, Ltd, Seoul, Republic of Korea
Background: “Udenafil” is a phosphodiesterase-5 inhibitor indicated for erectile dysfunction. “Dapoxetine” is a serotonin transport inhibitor indicated for premature ejaculation. The aim of the study reported here was to investigate the pharmacokinetic drug interaction between udenafil and dapoxetine in healthy male subjects.
Methods: An open-label, three-treatment, six-sequence, three-period crossover study was performed in healthy male subjects. In varying sequences, each subjects received single oral doses of udenafil 200 mg, dapoxetine 60 mg, and both treatments. The periods were separated by a washout period of 7 days. Serial blood samples were collected up to 48 hours after dosing. The plasma concentrations of udenafil and dapoxetine were determined using a validated liquid chromatography-tandem mass spectrometry method. Pharmacokinetic parameters were obtained by non-compartmental analysis. Tolerability was assessed throughout the study.
Results: Twenty-three healthy subjects completed the study. The geometric mean ratios of the area under the plasma concentration–time curve from time 0 to last measurable time point and measured peak plasma concentration for udenafil were 0.923 (90% confidence interval [CI]: 0.863–0.987) and 0.864 (90% CI: 0.789–0.947), respectively. The geometric mean ratios of the area under the plasma concentration–time curve from time 0 to last measurable time point and measured peak plasma concentration for dapoxetine were 1.125 (90% CI: 1.044–1.213) and 0.837 (90% CI: 0.758–0.925), respectively. There were no serious adverse events reported, and none of the subjects dropped out due to adverse events.
Conclusion: Udenafil was found to have no clinically significant pharmacokinetic interactions with dapoxetine. The concurrent administration of udenafil and dapoxetine was generally well tolerated.
Keywords: drug interaction, pharmacokinetics, erectile dysfunction, premature ejaculation
Introduction
Erectile dysfunction (ED) and premature ejaculation (PE) are the two most prevalent male sexual dysfunctions.1,2 “ED” is defined as the inability to achieve or maintain an erection sufficient for satisfactory sexual performance, with a prevalence of approximately 5%–30%.3,4 “PE” is defined as earlier-than-desired ejaculation resulting from minimal stimulation that causes bother or distress, with a prevalence of approximately 20%–30%.5–7 ED and PE may be comorbid conditions in some men.8 A large survey that included 12,134 men from the USA, Germany, and Italy reported that 7.2% of men met the criteria for both ED and PE. Overall, 44% of men with ED also reported PE, whereas 32% of men with PE also reported ED.9
“Udenafil” is an oral phosphodiesterase (PDE)-5 inhibitor for the treatment of ED. It is absorbed with time to reach peak concentration (tmax) at 0.8–1.3 hours, then declined mono-exponentially with a terminal half-life (t1/2β) of approximately 7.3–12.1 hours.10 The absolute oral bioavailability in humans is not known but is 38.0%–55.6% in rats.11 It is metabolized primarily by cytochrome P450 (CYP) 3A4 into its active metabolite, DA-8164, which has approximately half the pharmacological activity of the parent compound12 (Figure 1). A meta-analysis of five trials involving 1,109 patients showed that the change from baseline in International Index of Erectile Function erectile-function domain score in the udenafil group was significantly greater than in the placebo group (mean difference 5.65, 95% confidence interval [CI] 4.41–6.89).13
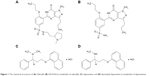  | Figure 1 The chemical structures of (A) Udenafil, (B) DA-8164 (a metabolite of udenafil), (C) dapoxetine, and (D) desmethyl dapoxetine (a metabolite of dapoxetine). |
“Dapoxetine” is a short-acting selective serotonin reuptake inhibitor marketed for the treatment of PE. It is absorbed with a tmax of 1.0–1.3 hours, and elimination is biphasic, with an initial half-life of approximately 1.4 hours and a t1/2β of approximately 20 hours.14 Dapoxetine is metabolized by multiple CYP isoenzymes including CYP3A4 and CYP2D6 to its active metabolite, desmethyl dapoxetine, which has similar pharmacological potency to the parent compound (Figure 1).15 An integrated analysis of five trials involving 6,081 patients showed that the PE profile measures improved significantly with dapoxetine as compared with placebo.16
Patients receiving udenafil for the treatment of ED could also potentially receive dapoxetine for the treatment of PE, and both of these molecules undergo CYP3A4 metabolism. Thus, the potential for interactions must be evaluated. The objective of this study was to investigate the pharmacokinetic interactions and tolerability of udenafil and dapoxetine in healthy volunteers.
Materials and methods
Subjects
Healthy male volunteers aged 20–45 years and with a body mass index of 19–27 kg/m2 were eligible for this study. Volunteers were considered to be in good health based on medical history, physical examinations, vital-sign measurements (blood pressure, heart rate, and body temperature), 12-lead electrocardiograms (ECGs), clinical laboratory tests (hematology, blood chemistry, and urinalysis), serology (hepatitis B surface antigen, hepatitis C virus antibody, and HIV antigen/antibody), and urine drug screening (for use of amphetamine, methamphetamine, barbiturate, cocaine, opiate, benzodiazepine, cannabinoid, and methadone) within 4 weeks before the first administration of the study drug. All subjects with known allergy or hypersensitivity to udenafil or dapoxetine, or with a history of drug abuse were excluded from the study.
Study design
The study was designed as a randomized, open-label, single-dose, three-treatment, three-period, six-sequence, crossover clinical trial. Subjects were randomly assigned to one of six sequences and received three different treatments: udenafil 200 mg (Treatment A), dapoxetine 60 mg (Treatment B), and co-administration of udenafil 200 mg and dapoxetine 60 mg (Treatment C). All treatments were given under fasting state with 240 mL of water. After the drug administration, the subjects were required to fast for 4 hours. Following a 1-week washout interval, subjects received alternate formulations (Figure 2).
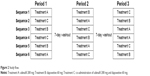  | Figure 2 Study flow. |
For each treatment period, subjects were admitted in the Clinical Trial Center (CTC) at the Asan Medical Center (AMC) from Day1 through Day 2 (24 hours after dosing). On Days 2 (32 hours after dosing) and 3 (48 hours after dosing), subjects visited the CTC to assess the tolerability and pharmacokinetics of udenafil or dapoxetine. The schedule for the second and third treatment period procedures was the same for the first period. Follow-up visits were performed within 5 to 9 days after the last treatment.
For pharmacokinetic analysis, sequential blood samples were collected prior to and at 0.25, 0.5, 0.75, and 1 hour, and 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 32, and 48 hours after dosing. All blood samples for the determination of udenafil, dapoxetine, DA-8164 (the metabolite of udenafil), and desmethyl dapoxetine (the metabolite of dapoxetine) concentrations were drawn into ethylenediaminetetraacetic acid (EDTA) tubes and separated by centrifugation at 1,800 g for 8 minutes at 4°C, then stored at −70°C until analysis.
Tolerability was assessed throughout the study using vital-sign measurements, 12-lead ECGs, clinical laboratory tests (hematology, blood chemistry, and urinalysis), physical examinations, and monitoring of adverse events (AEs). AEs were recorded in terms of symptoms and signs, duration, intensity, relationship to the study drug, action taken, outcome, and seriousness.
The study protocol was approved by the Ministry of Food and Drug Safety and the institutional review board of the AMC, Seoul, Republic of Korea. The study was conducted at the CTC of the AMC from September to November 2013. All subjects provided written informed consent before screening tests. The trial was registered with the identifier number NCT01928563 at ClinicalTrials.gov.
Determination of udenafil and DA-8164 concentrations
Plasma concentrations of udenafil and its active metabolite DA-8164 were determined using a validated ultra-performance liquid chromatography (UPLC) coupled with tandem mass spectrometry (MS) method, with the internal standards Udenafil-d7 and DA-8164-d3, respectively. The sample extracts were analyzed using an Acquity UPLC® System (Waters Corporation, Milford, MA, USA) and an Acquity UPLC BEH (ethylene bridged hybrid) C18 column (1.7 μm, 50.0*2.1 mm; Waters Corporation) with the mobile phase consisting of distilled water with 0.1% formic acid and methanol with 0.1% formic acid (50:50, v/v).
A Xevo TQ-S MS system (Waters Corporation) was operated in positive-ion electrospray mode with multiple-reaction monitoring. For udenafil and DA-8164, the precursor-to-production reactions monitored were m/z 517.58 → 283.25 and 406.39 → 364.26, respectively. Calibration curves covered the concentration range of 5.0–2,000.0 ng/mL udenafil (R 2>0.995) and 0.25–100.00 ng/mL DA-8164 (R2>0.995).
Using this assay, the accuracy of the calibration standard curve for udenafil was between 94.5% and 104.0%, and the coefficient of variation (CV) of the back-calculated concentration was <2.4%. The accuracy of the calibration standard curve for DA-8164 was between 98.5% and 102.0%, and the CV of the back-calculated concentration was <2.3%.
Determination of dapoxetine and desmethyl dapoxetine concentrations
Plasma concentrations of dapoxetine and its active metabolite desmethyl dapoxetine were determined using a validated UPLC-tandem MS method, with the internal standards dapoxetine-d4 and desmethyl dapoxetine-d7, respectively. The sample extracts were analyzed using the Acquity UPLC System and an Acquity UPLC BEH C18 column (1.7 μm, 50.0*2.1 mm; Waters Corporation) with the mobile phase consisting of distilled water with 0.1% formic acid and acetonitrile with 0.1% formic acid (60:40, v/v).
A Quattro Premier™ XE MS system (Waters Corporation) was operated in positive-ion electrospray mode with multiple-reaction monitoring. For dapoxetine and desmethyl dapoxetine, the precursor-to-production reactions monitored were m/z 306.20 → 157.02 and 292.17 → 157.04, respectively. Calibration curves covered the concentration range of 5.0–2,000.0 ng/mL dapoxetine (R2>0.995) and 0.25–100.0 ng/mL desmethyl dapoxetine (R2>0.995).
Using this assay, the accuracy of the calibration standard curve for dapoxetine was between 97.5% and 102.0%, and the CV of the back-calculated concentration was <2.9%. The accuracy of the calibration standard curve for desmethyl dapoxetine was between 97.0% and 103.2%, and the CV of the back-calculated concentration was <6.6%.
Pharmacokinetic assessment and statistical analysis
The plasma concentration–time profiles of udenafil, dapoxetine, DA-8164, and desmethyl dapoxetine of each subject were analyzed by a non-compartmental method using WinNonlin® software (v 6.1; Pharsight Corporation, Mountain View, CA, USA). All analyses were made using actual times of sampling. The peak plasma concentration (Cmax) and tmax were determined from the observed values. The terminal elimination rate constant (λz) was estimated by linear regression of the terminal log-linear portion of the plasma concentration–time curves. The area under the time–concentration curve from time 0 to the last measurable time (AUClast) was calculated by the trapezoidal rule and the area under the time–concentration curve extrapolated to infinity (AUC0~∞) was obtained AUClast + Clast/λz (Clast: the last quantifiable concentration). The apparent oral clearance (CL/F) was obtained as dose/AUC0~∞. The t1/2β was calculated for each participant as ln(2)/λz. To evaluate the metabolites of each component, DA-8164 and desmethyl dapoxetine, the metabolic ratio was calculated according to the ratio of the metabolite AUClast to the parent AUClast.
All statistical analyses were performed using SAS® software (v 9.3; SAS Institute, Cary, NC, USA) and Phoenix® WinNonlin (v 6.1; Pharsight Corporation). Demographic data and pharmacokinetic parameters were summarized using descriptive statistics. For the comparison of pharmacokinetic characteristics between monotherapy of udenafil or dapoxetine and co-administration of udenafil and dapoxetine, Cmax, AUClast, AUC0~∞, CL/F, t1/2β, and the metabolic ratio of each formulation were log-transformed and tested by a mixed-model analysis of variance. The mean differences and 90% CIs were back-transformed to obtain geometric mean ratios and CIs for those ratios. In addition, paired t-tests or Wilcoxon signed-rank tests were also used to compare the pharmacokinetic parameters.
Results
Study participants
A total of 25 healthy Korean volunteers were enrolled, and 23 volunteers were administered the study drugs and completed the study. Two subjects were dropped by the principal investigator: one subject showed abnormal lab result on Day1 and the other subject experienced an AE before drug administration. The mean (standard deviation) age of study participants was 27.65±4.54 years, the mean weight was 67.06±7.76 kg, and the mean height was 173.79±4.87 cm.
Pharmacokinetic analysis
To evaluate the pharmacokinetic drug–drug interactions between udenafil and dapoxetine, the pharmacokinetic profiles of udenafil, dapoxetine, DA-8164, and desmethyl dapoxetine were separately assessed. Figure 3 shows the plasma concentration–time profiles for udenafil and DA-8164. Figure 4 shows the plasma concentration–time profiles for dapoxetine and desmethyl dapoxetine.
Of the 23 subjects who completed the study, one subject was excluded from all pharmacokinetic analysis due to vomiting after co-administration of udenafil and dapoxetine, and two subjects were excluded from the pharmacokinetic analysis of dapoxetine due to vomiting after the administration of dapoxetine. Thus, 22 subjects were included in the pharmacokinetic analysis of udenafil, and 20 subjects were included in the pharmacokinetic analysis of dapoxetine.
The pharmacokinetic profile of udenafil was similar when it was administered alone and when it was co-administered with dapoxetine. It was rapidly absorbed, with a tmax of 1.0–2.0 hours, and then declined with a t1/2β of 10.81–11.02 hours. Plasma concentration of dapoxetine peaked at 1.0 hours, and elimination was rapid and biphasic, with a t1/2β of 15.34–16.23 hours.
The mean pharmacokinetic properties and the geometric mean ratios (combined/monotherapy) and 90% CIs of the AUClast and Cmax for udenafil, DA-8164, dapoxetine, and desmethyl dapoxetine are shown in Tables 1 and 2. For udenafil, the point estimates (PEs) (90% CI) of the AUClast and Cmax were 0.923 (0.863–0.987) and 0.864 (0.789–0.947), respectively. In the case of DA-8164, the PEs (90% CI) of the AUClast and Cmax were 0.898 (0.827–0.976) and 0.894 (0.816–0.980), respectively.
For dapoxetine, the PEs (90% CI) of AUClast and Cmax were 1.125 (1.044–1.213) and 0.837 (0.758–0.925), respectively. In the case of desmethyl dapoxetine, the PEs (90% CI) of AUClast and Cmax were 0.987 (0.921–1.056) and 0.879 (0.797–0.971), respectively.
Tolerability
Overall, udenafil and dapoxetine were well tolerated when administered alone or concomitantly. Seventeen subjects experienced a total of 69 AEs, among which 63 events in 16 subjects were considered “possibly related” to the study drug (Table 3). All AEs were mild or moderate in severity, and resolved without sequelae. No death or serious AE occurred during the entire course of the study. Likewise, there were no clinically significant abnormalities in laboratory tests, physical examinations, or ECGs.
  | Table 3 Summary of adverse drug reactions* |
Discussion
The study reported here investigated the potential for a pharmacokinetic interaction between udenafil and dapoxetine in healthy male subjects. In the study, a 200 mg dose of udenafil and 60 mg dose of dapoxetine were selected because they are the highest recommended dosage. Generally, these drugs are used on-demand;for this reason, this study was conducted after a single oral administration of each drug.2
In this study, the primary pharmacokinetic parameters were the Cmax and AUClast of udenafil and dapoxetine. The Cmax of udenafil and dapoxetine after the co-administration of udenafil and dapoxetine was slightly decreased compared with the administration of udenafil or dapoxetine alone. In terms of the AUClast, udenafil did not affect the pharmacokinetics of dapoxetine, nor did dapoxetine affect the pharmacokinetics of udenafil.
In addition, the other pharmacokinetic parameters of udenafil, dapoxetine, and their metabolites were also within 0.80–1.25 regardless of whether the drugs were administered alone or in combination. The exceptions were the tmax of udenafil and DA-8164, a metabolite of udenafil. After the co-administration of udenafil and dapoxetine, the tmax of udenafil and DA-8164 was prolonged by approximately 1 hour (from 1.0 and 2.0 hours) and 1.5 hours (from 2.0 and 3.5 hours), respectively.
The slight reduction in Cmax and delay in tmax indicate a decreased drug absorption rate of udenafil by dapoxetine. To evaluate these differences, the absorption rate constant (ka) of udenafil was obtained from a two-compartment structural model with first-order absorption using WinNonlin software. The ka of udenafil was 0.84±0.29 hours−1 and 0.50±0.17 hours−1 after monotherapy and combined therapy, respectively. These values are similar to a previously reported ka value (0.70±0.26 hours−1).17
As metabolism via CYP3A4 is the major elimination pathway for udenafil and one of the elimination pathways for dapoxetine, all inducers or inhibitors of CYP3A4 have the potential to interfere with systemic exposure and metabolism.12,15 A previous study reported that “ketoconazole”, a known CYP3A4 inhibitor, affects the pharmacokinetics of udenafil; the mean Cmax and AUClast of udenafil increased 1.9-fold and 3.2-fold, in the co-admiministration of ketoconazole, respectively. The metabolic area under the concentration–time curve (AUC) ratio was 1.71 when udenafil was administered alone, and the value decreased to 0.19 when udenafil was dosed in combination with ketoconazole.18 Ketoconazole also increased dapoxetine exposure to a greater extent. There was a 23% increase in Cmax and 88% increase in the AUC of active moiety and a 35% and 99% increase in parent drug Cmax and AUC, respectively.15 However, other drug interaction studies on dapoxetine and other PDE-5 inhibitors, including tadalafil and sildenafil, have reported no clinically significant pharmacokinetic interactions.19
The most common AEs were nausea and dizziness in the dapoxetine alone and dapoxetine with udenafil groups. These are well-known AEs of dapoxetine.20 Dapoxetine is absorbed rapidly and decreases to approximately 5% of its peak concentration by 24 hours after drug administration.14 Likewise, these AEs were started about 1 hour after drug administration and resolved within next day.
The findings from this study support the combined use of udenafil and dapoxetine for the treatment of PE and ED. Therefore, further long-term studies in patients are needed to evaluate the tolerability and clinical efficacy of combination treatment with udenafil and dapoxetine.
Conclusion
Our study indicates that udenafil has no significant pharmacokinetic interactions with dapoxetine. Dapoxetine did not affect the pharmacokinetics of udenafil. The concurrent administration of udenafil and dapoxetine was generally well tolerated.
Acknowledgments
The study was funded by Dong-A ST (Seoul, Republic of Korea), a manufacturer of udenafil. This study conducted by AMC was supported by the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (no HI07C0001).
Disclosure
The present work was presented as a poster at the 2014 annual meeting of the Korean Society for Clinical Pharmacology and Therapeutics (November 13–14, Busan, Republic of Korea). The authors report no other conflicts of interest in this work.
References
Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537–544. | ||
Hatzimouratidis K, Amar E, Eardley I, et al; European Association of Urology. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57(5):804–814. | ||
Kubin M, Wagner G, Fugl-Meyer AR. Epidemiology of erectile dysfunction. Int J Impot Res. 2003;15(1):63–71. | ||
Nicolosi A, Moreira ED Jr, Shirai M, Bin Mohd Tambi MI, Glasser DB. Epidemiology of erectile dysfunction in four countries: cross-national study of the prevalence and correlates of erectile dysfunction. Urology. 2003;61(1):201–206. | ||
Montague DK, Jarow J, Broderick GA, et al; AUA Erectile Dysfunction Guideline Update Panel. AUA guideline on the pharmacologic management of premature ejaculation. J Urol. 2004;172(1):290–294. | ||
Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol. 2007;51(3):816–823; discussion 824. | ||
Laumann EO, Nicolosi A, Glasser DB, et al; GSSAB Investigators’ Group. Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17(1):39–57. | ||
Jannini EA, Lombardo F, Lenzi A. Correlation between ejaculatory and erectile dysfunction. Int J Androl. 2005;28 Suppl 2:40–45. | ||
Shabsigh R, Perelman MA. Men with both premature ejaculation (PE) and erectile dysfunction (ED) experience lower quality of life than men with either PE or ED alone. Paper presented at the XVII World Congress of Sexology, Montreal, Canada, July 10–15, 2005. | ||
Kim BH, Lim HS, Chung JY, et al. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol. 2008;65(6):848–854. | ||
Shim HJ, Kim YC, Park KJ, et al. Pharmacokinetics of DA-8159, a new erectogenic, after intravenous and oral administration to rats: hepatic and intestinal first-pass effects. J Pharm Sci. 2003;92(11):2185–2195. | ||
Ji HY, Lee HW, Kim HH, et al. Role of human cytochrome P450 3A4 in the metabolism of DA-8159, a new erectogenic. Xenobiotica. 2004;34(11–12):973–982. | ||
Ding H, Du W, Wang H, et al. Efficacy and safety of udenafil for erectile dysfunction: a meta-analysis of randomized controlled trials. Urology. 2012;80(1):134–139. | ||
Modi NB, Dresser MJ, Simon M, Lin D, Desai D, Gupta S. Single- and multiple-dose pharmacokinetics of dapoxetine hydrochloride, a novel agent for the treatment of premature ejaculation. J Clin Pharmacol. 2006;46(3):301–309. | ||
Läkemedelsverket Medical Products Agency. Priligy: Dapoxetine Hydrochloride Film-Coated Tablets, 30 and 60 mg. Public Assessment Report [and] Scientific Discussion SE/H/718/01-02/DC. Uppsala: Läkemedelsverket Medical Products Agency; 2008. Available from: https://docetp.mpa.se/LMF/Priligy%20film-coated%20tablet%20ENG%20PAR.pdf. Accessed November 6, 2014. | ||
McMahon CG, Althof SE, Kaufman JM, et al. Efficacy and safety of dapoxetine for the treatment of premature ejaculation: integrated analysis of results from five phase 3 trials. J Sex Med. 2011;8(2):524–539. | ||
Shin KH, Kim JR, Cho JY, et al. [Comparison of the pharmacokinetics between udenafil 100 mg tablets and 200 mg tablets in healthy volunteers.] Journal of Korean Society for Clinical Pharmacology and Therapeutics. 2008;16(1):45–52. Korean. | ||
Shin KH, Chung YJ, Kim BH, et al. Effect of ketoconazole on the pharmacokinetics of udenafil in healthy Korean subjects. Br J Clin Pharmacol. 2010;69(3):307–310. | ||
Dresser MJ, Desai D, Gidwani S, Seftel AD, Modi NB. Dapoxetine, a novel treatment for premature ejaculation, does not have pharmacokinetic interactions with phosphodiesterase-5 inhibitors. Int J Impot Res. 2006;18(1):104–110. | ||
Buvat J, Tesfaye F, Rothman M, Rivas DA, Giuliano F. Dapoxetine for the treatment of premature ejaculation: results from a randomized, double-blind, placebo-controlled phase 3 trial in 22 countries. Eur Urol. 2009;55(4):957–967. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




