Back to Journals » Clinical Ophthalmology » Volume 13
Phacoemulsification: an alternative for prophylaxis of a glaucomatous crisis
Authors Stock RA , Röhrig MW , Mezzomo CD , Bonamigo EL
Received 17 July 2019
Accepted for publication 21 August 2019
Published 5 September 2019 Volume 2019:13 Pages 1721—1726
DOI https://doi.org/10.2147/OPTH.S223496
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ricardo Alexandre Stock, Mariéli Wobeto Röhrig, Camila Duranti Mezzomo, Elcio Luiz Bonamigo
Medical School of the University of the West of Santa Catarina (Universidade do Oeste de Santa Catarina – UNOESC), Joaçaba, SC, Brazil
Correspondence: Ricardo Alexandre Stock
Medical School, Universidade do Oeste de Santa Catarina, Belotto Stock Centro Oftalmológico, Rua Rio Branco, 589, Centro, CEP: 89.600-000, Joaçaba, SC, Brazil
Tel +55 493 522 0788
Fax +55 493 522 5059
Email [email protected]
Purpose: To evaluate the effectiveness of phacoemulsification for the prophylaxis of a glaucomatous crisis in the affected and contralateral eyes; to investigate the evolution of intraocular pressure levels after iridotomy and phacoemulsification; to assess the need for antiglaucoma medication after the proposed treatments; and to identify potential complications associated with phacoemulsification.
Patients and methods: This retrospective observational study evaluated 22 eyes of 12 patients between September 2006 and September 2018, with a minimum follow-up period of 9 months.
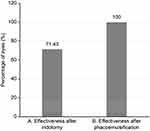
Results: After iridotomy, a persistent increase in intraocular pressure was observed in 42.85% of the cases, 100% of the patients required antiglaucoma medications, and recurrence of angle closure was observed in 28.57% of the cases. In contrast, during follow-up of phacoemulsification, the intraocular pressure levels in all eyes evaluated remained normal, without the need for medication, and no recurrence of the glaucomatous crisis or other complications was observed.
Conclusion: Phacoemulsification maybe consequently more effective than laser iridotomy for the resolution of angle-closure glaucoma and particularly for preventing its relapses.
Keywords: intraocular pressure, glaucoma, angle-closure glaucoma, phacoemulsification
Plain language summary
Glaucomatous crisis is an eye disease that is manifested as a sudden rise in intraocular pressure and is accompanied by intense and continuous pain due to blockade of the humor aqueous flow. This block occurs due to a narrowing of the angle between the iris and cornea, primarily due to cataract formation. The discovery of iridotomy for the treatment and prevention of glaucomatous crisis caused by narrow-angle glaucoma, as a minimally invasive procedure, greatly contributed to its acceptance and diffusion among ophthalmologists.
However, how to best treat patients with narrow-angle glaucoma and glaucomatous crisis remains controversial. This research, which was performed in a private clinic, suggests that cataract surgery with phacoemulsification is superior to peripheral iridotomy, a laser procedure in the iris aimed at avoiding glaucomatous crisis. Thus, a patient treated with phacoemulsification as the first choice will benefit from both greater comfort because they do not need to be subjected to multiple procedures and lower costs due to the dispersion of fewer medications after the procedure.
Introduction
Primary angle-closure glaucoma is an ocular neuropathy characterized by acute occlusion of the iridocorneal angle, leading to structural impairment of aqueous humor drainage via the trabecular meshwork.1,2,3 This condition occurs in anatomically predisposed eyes and causes severe pain in the eye and often a considerable increase in intraocular pressure.1,2 It is a major cause of preventable blindness worldwide.1
Conventional treatment, after stabilization of intraocular pressure levels, involves performing a peripheral iridotomy with laser.1,2 However, phacoemulsification has recently been presented as an alternative to iridotomy for the prophylaxis of angle closure and has demonstrated greater effectiveness in preventing a subsequent crisis, even in the contralateral eye.3
The aim of the present study was to describe the effectiveness of phacoemulsification for the prevention of acute glaucoma recurrence in previously affected and contralateral eyes in patients with predisposing factors. Moreover, we aimed to verify the evolution of intraocular pressure levels after the completion of iridotomy and phacoemulsification, identify the potential complications associated with phacoemulsification, and assess the need for medication after these procedures.
Materials and methods
This retrospective observational study evaluated the use of phacoemulsification for the prophylaxis of a glaucomatous crisis in predisposed patients. The study was performed by reviewing medical records. A total of 22 eyes from 12 patients treated and monitored at the BelottoStock Ophthalmological Center of Joaçaba, Santa Catarina, Brazil, were evaluated between September 2006 and September 2018. All patients with a history of glaucomatous crisis in at least one eye and with a minimum follow-up period of 9 months were included in the study. The project was approved by the Research Ethics Committee of the University of the West of Santa Catarina under protocol number 725.894/2014. Informed consent was waived because the research was done on medical records. The study was conducted in accordance with the principles set forth by the Declaration of Helsinki and the data were collected in strict confidentiality.
Four different therapeutic procedures were included in the study: sequential Argon-YAG laser iridotomy, phacoemulsification after sequential iridotomy, phacoemulsification after glaucomatous crisis, and prophylactic phacoemulsification in the contralateral eye. For the Argon-YAG laser iridotomy, it first used The Oculight Laser Iridex (Mountain View, CA, USA) and the procedure finalized with the Optimis YAG Laser, from Quantel Medical (Cournoun d´Auvergne, France). The phacoemulsification was performed with the Stelaris PC, Bausch-Lomb (Saint-Louis, Missouri, USA). The following variables were considered: existence of a glaucomatous crisis; use of medication prior to the crisis and after the completion of iridotomy and phacoemulsification; recurrence of angle closure; and measurement of intraocular pressure after iridotomy and phacoemulsification.
The collected data were entered in Microsoft Excel software and organized into graphs. Statistical analyses were performed using the Shapiro–Wilk test and Fisher’s exact test.
Results
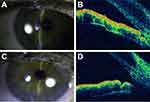
A total of 22 eyes from 12 patients were analyzed. The age of the participants varied between 47 and 82 years, with an average of 67.77±10.63 years. All patients were Caucasian women who presented with cataracts with a shallow anterior chamber and a closed angle on gonioscopy, as illustrated in Figure 1.
Phacoemulsification was performed in 3 groups of eyes (Figure 2). Phacoemulsification alone after a glaucomatous crisis was performed on 7 (31.81%) eyes. Prophylactic phacoemulsification was performed on 8 (36.36%) contralateral eyes due to their shallow anterior chamber and closed angle on gonioscopy, which predisposes the patients to acute angle closure. Sequential argon-YAG laser iridotomy was performed on 7 (31.81%) eyes.
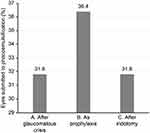 |
Figure 2 Eyes subjected to phacoemulsification. (A) After glaucomatous crisis. (B) As prophylaxis for glaucoma. (C) After iridotomy. |
Of all patients evaluated, 4 (18.18%) used antiglaucoma medication prior to the study, and 18 (81.81%) patients did not take any medication (Figure 3). After the procedures, all patients subjected to sequential argon-YAG laser iridotomy required antiglaucoma medication. During the follow-up, none of the patients subjected to phacoemulsification required medication. No complications resulting from the use of this technique were observed.
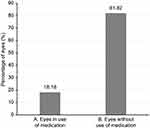 |
Figure 3 Use of antiglaucomatous medication prior to crisis. (A) Eyes in use of medication. (B) Eyes without use of medication. |
The mean intraocular pressure level was 22±9.14 mmHg after sequential iridotomy and 12.71±2.89 mmHg (P=0.0472) after phacoemulsification, as shown in Figure 4. We considered a normal intraocular pressure to be <21 mmHg4 and found that, of the 7 eyes subjected to iridotomy, a persistent increase in intraocular pressure occurred in 3 (42.85%) cases following the procedure, of which 2 cases presented a new glaucomatous crisis. All these 7 patients were subjected to phacoemulsification for prophylaxis of acute angle closure.
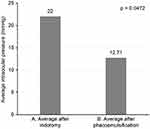 |
Figure 4 Average intraocular pressure levels after iridotomy and phacoemulsification. (A) Average after iridotomy. (B) Average after phacoemulsification. |
Iridotomy was effective for preventing a glaucomatous crisis in 5 (71.43%) of the 7 eyes subjected to this procedure. None of the eyes subjected to phacoemulsification exhibited recurrence (Figure 5).
Discussion
The patients consisted of Caucasian women, with a mean age of 67.77±10.63 years. These data are consistent with those found in the literature, which show that angle-closure glaucoma is prevalent in individuals older than 60 years2,5–7 and in women2,6,7 but uncommon among black individuals.2
It is already known, for long time that narrowing angle predisposes the patients to acute angle closure.8,9,10 The rationales for conducting phacoemulsification after iridotomy in the 7 eyes included the persistent increase in intraocular pressure in 2 eyes, the recurrence of a glaucomatous crisis despite iridotomy in 2 eyes, and prophylaxis of acute angle closure in 3 eyes.
After sequential argon-YAG laser iridotomy, all eyes required antiglaucoma therapy. This result is consistent with a previous study11 that evaluated the efficacy of phacoemulsification in acute glaucoma in a group of 32 patients subjected to iridotomy, of whom 10 required medication for the control of intraocular pressure after the procedure. For all 3 therapeutic indications for phacoemulsification, none of the patients treated required medication, and this result was similar to other studies6,7,9,11,13,14 that reported a decrease in the number of medications required for the control of intraocular pressure after phacoemulsification. One of these studies12 evaluated 58 eyes with angle-closure glaucoma and found that only 4 eyes required antiglaucoma therapy after phacoemulsification. Therefore, by decreasing the volume of the capsular sac and increasing the size of the iridocorneal angle, phacoemulsification corrects the pathophysiology of the disease and promotes pressure stability independent of medications, being superior to iridotomy.
Two studies compared phacoemulsification with iridotomy for the prevention of further increases in intraocular pressure and reported increases in ocular pressure of 46.7% and 38.6% after laser iridotomy.3,15 This result may be due to a residual angle closure produced as a result of an anteriorly placed ciliary process.3 Other possible alternatives include direct damage to the trabecular meshwork and development of peripheral anterior synechiae.3 In the present study, a persistent increase in intraocular pressure of 42.85% was observed after completion of sequential iridotomy, and this percentage was within the ranges described in the literature.
Following phacoemulsification, a decrease in intraocular pressure relative to the post-iridotomy period occurred, and intraocular pressure levels were maintained within desirable limits, with an average of 12.71±2.89 mmHg. A similar study16 evaluated 31 eyes and found that residual angle closure was common after iridotomy but was resolved after the extraction of the crystalline lens.
Cataract surgery significantly increased the depth of the anterior chamber and opened the angles (Figure 1C and D), thereby attenuating the anterior positioning of the ciliary process and decreasing the average intraocular pressure. These changes likely justify the findings of the present and other studies6,7,9,10,13,14 that have found a significant decrease in intraocular pressure after phacoemulsification; one study17 also found that these changes did not reverse during the 12-month postoperative follow-up period. Another study3 found a persistent increase in intraocular pressure of 3.3% (one patient) after phacoemulsification, which is consistent with the results of the present study.
Regarding the recurrence of a glaucomatous crisis after the procedure, 28.57% of the cases exhibited recurrence after iridotomy. In contrast, none of the eyes subjected to phacoemulsification showed recurrence. The occurrence of a glaucomatous crisis despite iridotomy could possibly be due to the angle closure that remains after iridotomy, wherein the angle is narrowed, predisposing the eye to a new acute closure.
In the present study, none of the eyes evaluated presented complications resulting from phacoemulsification, which is consistent with the findings of other studies.7,18 A previous study analyzed 37 patients with angle-closure glaucoma, of whom 18 were subjected to sequential argon-YAG laser iridotomy and 19 were subjected to phacoemulsification. The only postoperative complication in the group subjected to phacoemulsification was intraocular pressure greater than 30 mmHg in a single patient on the first day after the procedure. In the group subjected to iridotomy, one case of transient bleeding, one case of burning of the cornea, and three cases involving a second iridotomy procedure due to nonpatency were observed.5 Other complications due to iridotomy reported in another study11 included corneal edema, transient pressure peaks, fibrinoid reaction, and rupture of the posterior capsule; these complications have also been found after cataract surgery3,11 but to a lesser extent.
The success criteria were an intraocular pressure below 21 mmHg and the absence of the recurrence of a glaucomatous crisis. In our study, the success rate for phacoemulsification after iridotomy was 100%, and in all 3 cases that had maintained high intraocular pressure despite iridotomy, phacoemulsification lowered the pressure levels. This result is consistent with those of other studies, which have reported success rates of 90%, 93%, and 100%.5,12 It is also consistent with the results of another study,11 wherein the success rate, defined as an intraocular pressure bellow 21 mmHg without the need for further surgical intervention and/or drug therapy, was achieved in 72% of the eyes treated with phacoemulsification but in only 35% of the eyes subjected to iridotomy.
In the present study, the iridotomy group required antiglaucoma medication, and 65% required surgical reintervention; phacoemulsification was the first-line indication. Trabeculectomy after phacoemulsification is rare.19 Additionally, phacoemulsification is more cost-effective.20 After 24 months of follow-up in a similar study,5 of the 18 patients subjected to iridotomy, 6 were subsequently subjected to phacoemulsification, confirming the existence of a higher number of failures after iridotomy, compared to those observed after cataract surgery. However, phacoemulsification needs to be performed a few weeks before the attack.21
Conclusion
Patients with angle-closure glaucoma were Caucasian women and, in general, aged above 60 years. Phacoemulsification thoroughly controlled intraocular pressure, thereby eliminating the use of antiglaucoma medication during follow-up, in contrast to iridotomy, which did not maintain the pressure levels within the normal range and did not eliminate the need for drug therapy. Therefore, phacoemulsification maintained the adequate intraocular pressure for a longer period and favored the use of fewer interventions. Furthermore, phacoemulsification was more effective than laser iridotomy for increasing the dimensions of the anterior chamber in eyes with narrow angles, as this condition was directly involved in the pathophysiology of angle-closure glaucoma. In conclusion, phacoemulsification by decreasing capsular sac volume and increasing the iridocorneal angle corrects the pathophysiology of the disease and prevents relapses, being more effective than iridotomy and is the best choice for the treatment and prevention of a crisis in the affected and contralateral eyes. However, further studies with longer follow-up and a larger sample size are required to confirm the applicability of phacoemulsification for the prophylaxis of a glaucomatous crisis to provide better guidelines for therapeutic choices.
Ethics approval and informed consent
This study was reviewed and approved by the Research Ethics Committee of UNOESC under number 826.475/2014.
Author contributions
All authors contributed to the data analysis and article drafting and provided final approval of the version to be published and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Riordan-Eva P, Whitcher JP. Oftalmologia Geral de Vaughan & Asbury [General Ophthalmology of Vaughan & Asbury]. Porto Alegre: Artmed; 2011.
2. Kanski J. Oftalmologia Clínica: Uma Abordagem Sistemática [Clinical Ophthalmology: A Systematic Approach]. Rio de Janeiro: Elsevier; 2016.
3. Lam DS, Leung DY, Tham CC, et al. Randomized trial of early phacoemulsification versus peripheral iridotomy to prevent intraocular pressure rise after acute primary angle closure. Ophthalmology. 2008;115(7):1134–1140. doi:10.1016/j.ophtha.2007.10.033
4. Sakata K, Figueira ALM, Guimarães ACP, et al. Estudo da correlação entre pressão intra-ocular e espessura corneana central (projeto glaucoma) [Intraocular pressure vs central corneal thickness: a comparative study (glaucoma project)]. Arq Bras Oftalmol. 2000;63(5):355–358. doi:10.1590/S0004-27492000000500005
5. Husain R, Gazzard G, Aung T, et al. Initial management of acute primary angle closure: a randomized trial comparing phacoemulsification with laser peripheral iridotomy. Ophthalmology. 2012;119(11):2274–2281. doi:10.1016/j.ophtha.2012.06.015
6. Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology. 2009;116(4):725–731. doi:10.1016/j.ophtha.2008.12.054
7. Su WW, Chen PYF, Hsiao CH, Chen HSL. Primary phacoemulsification and intraocular lens implantation for acute primary angle-closure. PLoS One. 2011;6(5):e20056. doi:10.1371/journal.pone.0020056
8. Vasconcellos J, Sakata L, Da Silva M, Costa V. 2nd Consenso de Glaucoma Primário de Ângulo Fechado [2nd Closed-Angle Primary Glaucoma Consensus]. São Paulo: Alcon/Novartis; 2012.
9. Liu CJ, Cheng CY, Ko YC, Lau LI. Determinants of long-term intraocular pressure after phacoemulsification in primary angle-closure glaucoma. J Glaucoma. 2011;20(9):566–570. doi:10.1097/IJG.0b013e3181e07970
10. Shams PN, Foster PJ. Clinical outcomes after lens extraction for visually significant cataract in eyes with primary angle closure. J Glaucoma. 2012;21(8):545–550. doi:10.1097/IJG.0b013e31821db3c7
11. Jacobi PC, Dietlein TS, Luke C, Engels B, Krieglstein GK. Primary phacoemulsification and intraocular lens implantation for acute angle-closure glaucoma. Ophthalmology. 2002;109(9):1597–1603. doi:10.1016/S0161-6420(02)01123-5
12. Pachimkul P, Intajak Y. Effect of lens extraction on primary angle closure in a Thai population. J Med Assoc Thai. 2008;91(3):303–308.
13. Imaizumi M, Takaki Y, Yamashita H. Phacoemulsification and intraocular lens implantation for acute angle closure not treated or previously treated by laser iridotomy. J Cataract Refract Surg. 2006;32(1):85–90. doi:10.1016/j.jcrs.2005.11.014
14. Lee SJ, Lee CK, Kim WS. Long-term therapeutic efficacy of phacoemulsification with intraocular lens implantation in patients with phacomorphic glaucoma. J Cataract Refract Surg. 2010;36(5):783–789. doi:10.1016/j.jcrs.2009.11.023
15. Hayashi K, Hayashi H, Nakao F, Hayashi F. Changes in anterior chamber angle width and depth after intraocular lens implantation in eyes with glaucoma. Ophthalmology. 2000;107(4):698–703. doi:10.1016/S0161-6420(00)00007-5
16. Moghimi S, Lin S. Role of phacoemulsification in angle closure glaucoma. Eye Sci. 2011;26(3):121–131.
17. Nonaka A, Kondo T, Kikuchi M, et al. Cataract surgery for residual angle closure after peripheral laser iridotomy. Ophthalmology. 2005;112(6):974–979. doi:10.1016/j.ophtha.2004.12.042
18. Nonaka A, Kondo T, Kikuchi M, et al. Angle widening and alteration of ciliary process configuration after cataract surgery for primary angle closure. Ophthalmology. 2006;113(3):437–441. doi:10.1016/j.ophtha.2006.04.029
19. Chen PP, Lin SC, Junk AK, Radhakrishnan S, Singh K, Chen TC. The effect of phacoemulsification on intraocular pressure in glaucoma patients. a report by the American Academy of Ophthalmology. Ophthalmology. 2015;122(7):1294–1307. doi:10.1016/j.ophtha.2015.03.021
20. Azuara-Blanco A, Burr J, Ramsay C, et al. Effectiveness of early lens extraction for the treatment of primary angle-closure glaucoma (EAGLE): a randomised controlled trial. Lancet. 2016;388(10052):1389–1397. doi:10.1016/S0140-6736(16)30956-4
21. Moghimi S, Hashemian H, Chen R, Johari M, Mohammadi M, Lin SC. Early phacoemulsification in patients with acute primary angle closure. J Curr Ophthalmol. 2016;27(3–4):70–75. doi:10.1016/j.joco.2015.12.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.