Back to Journals » Clinical Interventions in Aging » Volume 14
Paraneoplastic neuromyelitis optica spectrum disorder associated with breast cancer
Authors Yuan J , Jia Z, Qin W, Hu W
Received 18 January 2019
Accepted for publication 24 April 2019
Published 6 June 2019 Volume 2019:14 Pages 1039—1044
DOI https://doi.org/10.2147/CIA.S202102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Junliang Yuan, Zejin Jia, Wei Qin, Wenli Hu
Department of Neurology, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, People’s Republic of China
Abstract: Neuromyelitis optica spectrum disorder (NMOSD) is a group of inflammatory disease involving the central nervous system. Although most commonly an idiopathic autoimmune condition, NMOSD may also occur as a paraneoplastic syndrome in rare instances. Herein, we report a rare case of a 60-year-old woman with paraneoplastic NMOSD associated with breast cancer. Our findings increase the recognition that NMOSD may present as a paraneoplastic neurological syndrome associated with breast cancer. Our case also raises awareness of an important complication of neurological complications of breast cancer. Early diagnosis of paraneoplastic NMOSD may be imperative for a better prognosis.
Keywords: neuromyelitis optica spectrum disorder, paraneoplastic syndrome, breast cancer
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a group of inflammatory disease involving the central nervous system and may cause severe disability.1 Paraneoplastic syndromes are remote effects of cancer caused by an autoimmune response triggered by tumor cells. However, although most commonly an idiopathic autoimmune condition,1 there were only a few cases of NMOSD presenting paraneoplastic syndromes, such as mature B-cell neoplasm,2 hepatic metastasis from a small-bowel neuroendocrine tumor,3 stomach carcinoid tumor,4 lung carcinoma,5,6 esophageal cancer,7 adenocarcinoma of the lung and breast,8 squamous cell lung, ovarian carcinoma and adrenocortical carcinoma,9 teratoma,10 and esophageal adenocarcinoma.1 As a result, NMOSD in elderly patients should raise the suspicion of paraneoplastic etiology.
To the best of our knowledge, there were only a few cases of NMOSD occasionally develop carcinomas, however, the underlying mechanism for these rare cases is not well characterized.9 Herein, we report a case of a 60-year-old woman with paraneoplastic NMOSD associated with breast cancer.
Case report
A 60-year-old woman presented with speech difficulties for 10 days after an upper respiratory infection. She was diagnosed with breast cancer three years ago and was treated with surgery, radiation and chemotherapy. Neurological examinations revealed speech difficulty, slight weakness of lower limbs, positive Hoffmann sign, hyperactive deep tendon reflexes on both sides. Left ankle clonus was positive.
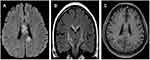
Brain magnetic resonance imaging (MRI) showed some abnormal T2 hyperintense signals involving in the splenium and the body of corpus callosum, midbrain and bilateral internal capsule (Figure 1A and B), with slight contrast enhancement (Figure 1C). Cervical MRI indicated T2 hyperintense lesions involving C3-T5 (Figure 2A and B). Contrast enhanced thoracic MRI showed T2 hyperintense lesions extending from T7-T12 (Figure 3A and B), with slight contrast enhancement (Figure 3C). High resolution CT of chest found not any lung cancer. PET-CT found that the right breast cancer.
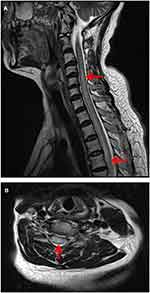 | Figure 2 Cervical MRI indicated T2 hyperintense lesions involving C3-T5. |
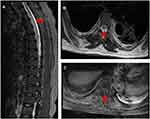 | Figure 3 Contrast enhanced thoracic MRI showed T2 hyperintense lesions extending from T7-T12, with slight contrast enhancement (C). |
The antibodies to human immunodeficiency virus and T. Pallidum were negative in serum, and the antibodies to T. Pallidum included rapid plasma regain, treponema pallidum particle agglutination, and toluidine red unheated serum test. The results of cerebrospinal fluid (CSF) were increased leucocyte count (38/µL, reference range 0–5/µL) and protein concentration (189mg/dl, reference range 15–45 mg/dl). CSF IgG was 16.30 mg/dl. The myelin basic protein both in serum and CSF were positive, however, the oligoclonalbands both in serum and CSF were negative. The Ig G index (0.9) and blood-brain barrier permeability were both increased. AQP-4 autoantibodies were detected both in serum and in CSF using the technique of cell based assay. The inflammatory, immune and infectious biomarkers of both CSF and serum were also unremarkable. The biomarkers of tumor in serum (such as cancer embryo antigen, alpha fetoprotein, prostate specific antigen, carbohydrate antigen 125, carbohydrate antigen 153, carbohydrate antigen 199, carbohydrate antigen 724, ferroprotein, neuron specific enolase, CYFRA21-1, squamous cell carcinoma antigen, pepsinogen I, pepsinogen II) and paraneoplasic syndrome (such as Hu, Yo, Ri, NMDAR, CV2, Amphiphysin, PNMA2, Recoverin, SOX1, Zic4, Tr and titin) both in serum and CSF were normal using the technique of immunoblotting. The antibodies of anti-ganglioside and autoimmune encephalitis both in serum or CSF were unremarkable.
The weakness of both lower limbs gradually decreased to I level after her admission. The D-dmier was 17.83(mg/L FEU), and lower extremity ultrasound showed obvious venous thrombosis in both lower limbs and right peroneal vein. The computed tomography pulmonary angiography found the pulmonary embolism. She was finally diagnosed as paraneoplastic NMOSD. She was treated with intravenous methylprednisolone 1 g daily (gradually reduced for 3–5 days) and intravenous immunoglobulin 25 g daily for 5 days. After 3 weeks, the patient’s speech difficulties were slightly improved. After 3 mouths follow up, the patient made a relatively good recovery with both clinical and radiological improvement. The weakness of both lower limbs gradually recovered to be III level, however, she could not stand by herself. However, the long-term immunosuppression was rejected by this patient.
Our study was approved by the Ethics Committee of Beijing Chaoyang Hospital, Capital Medical University. Written informed consent was obtained from the patient to publish the case details.
Discussion
NMOSD is one of demyelinating, autoimmune disease affecting the central nervous system. In most cases, NMOSD is associated with autoantibodies targeting AQP-4. Herein, we reported a rare case of a 60-year-old woman with paraneoplastic NMOSD associated with breast cancer.
Autoimmune diseases are sometimes associated with neoplasms. Patients with NMOSD occasionally develop carcinomas. Several case reports have demonstrated AQP-4 expression in tumor tissue, and the prevalence of cancer in patients with NMOSD associated with positive AQP4-IgG antibodies has been reported incidentally in 0.02%.9 There are quite rare cases presenting as a paraneoplastic syndrome in association with carcinomas,11 such as carcinoma of lung,5,6 esophageal,7 small-bowel neuroendocrine tumor,3 carcinoid tumor of the stomach,4 thymoma, breast carcinoma, lymphoma. As a result, the possibility of underlying malignancy should be considered in patients with the diagnosis of NMOSD, especially in the elderly. What triggers the production of AQP4-IgG antibodies in patients with malignancy has been poorly understood, but it has been speculated that such antibody production is triggered by an immune response against a patient’s cancer.1 However, AQP-4 is also expressed in neurologically normal patients’ tumors, suggesting additional factors are required for NMOSD induction in patients with cancer.12 AQP4-IgG may involve in the pathogenesis of paraneoplastic NMOSD, however, its clinical utility and underlying exact mechanism warrant further investigations.
Breast cancer has been proved to be related paraneoplastic endocrine syndromes with neurologic syndromes. As for the breast carcinoma found in NMOSD, there was an observation about 41 patients with positive NMO-IgG, six malignancies were identified in five NMOSD seropositive patients, including breast carcinoma (three cases), lymphoma, cervical carcinoma and leiomyosarcoma (in a patient who also had breast carcinoma).13 Furthermore, from another studies of 34 paraneoplastic NMOSD cases, 11 (32%) cases had breast carcinoma.14 We should attach importance to the detection of paraneoplastic etiology, especially the breast carcinoma in NMOSD cases.
The strengths of our case were showed as follows. Firstly, we reported a rare case of a 60-year-old woman with paraneoplastic NMOSD associated with breast cancer. According to the international consensus diagnostic criteria for NMOSD,15 the diagnosis of paraneoplastic NMOSD was based on the patient’s progressing neurological symptoms, MRI findings both in brain and spine, and breast cancer, the positive AQP-4 autoantibodies, and the effect of treatment. In spite of the diagnosis of paraneoplastic NMOSD was very likely, however, the possibility that NMO occurred independently of cancer can not be ruled out. Secondly, our case was diagnosed after the occurrence of breast cancer with a long interval of 3 years. As a result, clinical findings suggestive of NMOSD in elderly patients should raise the suspicion of paraneoplastic etiology and warrants thorough investigations for an underlying cancer, with long-term follow-ups, at least for several years. Besides, the patient was paraplegic and all paraplegic patients are in a risk of developing DVTs and pulmonary embolism, which should be attached great importance to in our clinical practice.
Conclusion
In summary, we herein reported a rare case of a 60-year-old woman with paraneoplastic NMOSD associated with breast cancer. Our case may extend the context that AQP-4 may have a paraneoplastic basis in some cases. Early diagnosis of paraneoplastic NMOSD is of great importance for a better outcome.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81301016) and the Beijing Municipal Administration of Hospitals Incubating Program (PX2019009). The two fundings helped us with data collection, analysis, and interpretation of data. Junliang Yuan and Zejin Jia are co-first authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wiener DC, Kaplan TB, Bravo-Iniguez CE, Miller J, Berkowitz AL, Jaklitsch MT. Paraneoplastic neuromyelitis optica spectrum disorder as presentation of esophageal adenocarcinoma. Ann Thorac Surg. 2018;105(3):e133–5. doi:10.1016/j.athoracsur.2017.10.015
2. Nakayama-Ichiyama S, Yokote T, Hiraoka N, et al. A paraneoplastic neuromyelitis optica spectrum disorder associated with a mature B-cell neoplasm. Leuk Res. 2011;35(7):e111–3. doi:10.1016/j.leukres.2011.02.019
3. Figueroa M, Guo Y, Tselis A, et al. Paraneoplastic neuromyelitis optica spectrum disorder associated with metastatic carcinoid expressing aquaporin-4. JAMA Neurol. 2014;71(4):495–498. doi:10.1001/jamaneurol.2013.6331
4. Al-Harbi T, Al-Sarawi A, Binfalah M, Dermime S. Paraneoplastic neuromyelitis optica spectrum disorder associated with stomach carcinoid tumor. Hematol Oncol Stem Cell Ther. 2014;7(3):116–119. doi:10.1016/j.hemonc.2014.06.001
5. Iorio R, Rindi G, Erra C, Damato V, Ferilli M, Sabatelli M. Neuromyelitis optica spectrum disorder as a paraneoplastic manifestation of lung adenocarcinoma expressing aquaporin-4. Mult Scler. 2015;21(6):791–794. doi:10.1177/1352458515572241
6. Annus A, Bencsik K, Obal I, et al. Paraneoplastic neuromyelitis optica spectrum disorder: A case report and review of the literature. J Clin Neurosci. 2018;48:7–10. doi:10.1016/j.jocn.2017.10.030
7. Kon T, Ueno T, Suzuki C, et al. Aquaporin-4 antibody positive neuromyelitis optica spectrum disorder associated with esophageal cancer. J Neuroimmunol. 2017;309:38–40. doi:10.1016/j.jneuroim.2017.05.009
8. Sepulveda M, Sola-Valls N, Escudero D, et al. Clinical profile of patients with paraneoplastic neuromyelitis optica spectrum disorder and aquaporin-4 antibodies. Mult Scler. 2018;24(13):1753–1759. doi:10.1177/1352458517731914
9. Beauchemin P, Iorio R, Traboulsee AL, Field T, Tinker AV, Carruthers RL. Paraneoplastic neuromyelitis optica spectrum disorder: a single center cohort description with two cases of histological validation. Mult Scler Relat Disord. 2018;20:37–42. doi:10.1016/j.msard.2017.12.012
10. Frasquet M, Bataller L, Torres-Vega E, et al. Longitudinally extensive transverse myelitis with AQP4 antibodies revealing ovarian teratoma.J. Neuroimmunol. 2013;263(1–2):145–147. doi:10.1016/j.jneuroim.2013.07.003
11. Tanaka M, Yanagida H, Suzumura A. Treatment for paraneoplastic neuromyelitis optica spectrum disorder (NMOSD): probable effects of tocilizumab for both cancer and NMOSD. Mult Scler Relat Disord. 2018;20:82–83. doi:10.1016/j.msard.2017.12.002
12. Armagan H, Tuzun E, Icoz S, et al. Long extensive transverse myelitis associated with aquaporin-4 antibody and breast cancer: favorable response to cancer treatment. J Spinal Cord Med. 2012;35(4):267–269. doi:10.1179/2045772312Y.0000000018
13. Ontaneda D, Fox RJ. Is neuromyelitis optica with advanced age of onset a paraneoplastic disorder? Int J Neurosci. 2014;124(7):509–511. doi:10.3109/00207454.2013.854208
14. Cai G, He D, Chu L, Dai Q, Xu Z, Zhang Y. Paraneoplastic neuromyelitis optica spectrum disorders: three new cases and a review of the literature. Int J Neurosci. 2016;126(7):660–668. doi:10.3109/00207454.2015.1054481
15. Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi:10.1212/WNL.0000000000001729
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.