Back to Journals » Clinical Ophthalmology » Volume 14
Optimum Refractive Target in Patients with Bilateral Implantation of Extended Depth of Focus Intraocular Lenses
Authors Jackson MA , Edmiston AM, Bedi R
Received 6 November 2019
Accepted for publication 22 January 2020
Published 18 February 2020 Volume 2020:14 Pages 455—462
DOI https://doi.org/10.2147/OPTH.S237457
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mitchell A Jackson,1 Anna M Edmiston,1 Raman Bedi2
1Jacksoneye, Lake Villa, IL, USA; 2IrisARC, Chandigarh, India
Correspondence: Mitchell A Jackson
Jacksoneye, 300 N Milwaukee Avenue Suite L, Lake Villa, IL 60046, USA
Tel +1-847-356-0700
Fax +1-847-356-0757
Email [email protected]
Purpose: To determine the refractive target of extended depth of focus (EDOF) intraocular lenses in dominant and non-dominant eyes which provides the best binocular vision at all ranges after cataract surgery.
Patients and Methods: This retrospective, single-center, non-comparative study included 47 patients who had undergone bilateral cataract surgery with implantation of EDOF IOLs (Tecnis Symfony or Tecnis Symfony Toric) targeting emmetropia in both eyes. Binocular uncorrected visual acuity at distance (UDVA), near (UNVA), intermediate (UIVA), and manifest refraction spherical equivalent (MRSE) were recorded between 1 and 3 months after the second-eye cataract surgery. Scattergrams for combined binocular UDVA, UIVA, UNVA and postoperative MRSE were plotted and the points of minima of the quadratic regression curve for the dominant and non-dominant eyes were considered as the optimum MRSE corresponding to the best overall visual acuity. Subgroup analysis of patients who achieved UDVA and UIVA ≥ 20/20 and UNVA ≥ 20/30 was also performed.
Results: For the overall group, the optimum MRSE was − 0.08 D for dominant and − 0.63 D for non-dominant eyes. In a subset of 17 patients who achieved excellent acuity at all distances, the mean MRSE for the dominant and non-dominant eyes was − 0.07 ± 0.14 D and − 0.21 ± 0.24 D, respectively.
Conclusion: Excellent visual acuity at all ranges can be achieved with bilateral EDOF intraocular lenses implanted after cataract surgery. Our results indicate the best results when the dominant eye is targeted at emmetropia and the nondominant eye is targeted between − 0.21D and − 0.63D, with excellent results shown with mild myopia of − 0.21 in the non-dominant eyes. Future studies with larger sample sizes and subjective patient-reported outcomes may validate current study outcomes.
Keywords: optimum refractive target, bilateral EDOF IOL implantation, best myopic target in non-dominant eyes, optimum visual outcomes across all ranges
Introduction
With rapid technological advances in intraocular lens (IOL) optics and changing practice patterns, cataract surgery has become a lens-based refractive surgery. Due to increased use of computers, tablets, and smart-phones for everyday activities, cataract patients desire better near and intermediate vision postoperatively.1 To address patient expectations and demand for presbyopia correction, several presbyopia-correcting IOLs based on different optical approaches are in use, which broadly include multifocal, accommodative and the extended depth of focus (EDOF) IOLs.2–5 Among these, EDOF IOLs have been available for only the past few years; best practices for obtaining optimal outcomes are still evolving.
The Tecnis Symfony EDOF IOL (Johnson & Johnson Vision, Santa Ana, CA) incorporates a diffractive echelette design which splits the light energy into an elongated focus, creating a continuous range of vision. Unlike multifocal IOLs that split the light into distinct focal point(s), the elongated focal zone of EDOF IOL reduces overlap of near and far images, thus generating a lower incidence of halos and glare.5 Additionally, the use of achromatic technology to correct longitudinal chromatic aberrations improves the visual performance of the EDOF IOL.6
EDOF IOLs have been shown to yield excellent distance and intermediate visual acuity.7,8 The defocus curve of the Tecnis Symfony IOL is a smooth, dome-shaped curve with an extended range of vision of about 1.5 D, which provides good quality distance and intermediate vision, but tapers off at reading distance. As such, it is believed that EDOF IOLs may work less efficiently for near vision compared to some multifocal IOLs.9–11
Several investigators have suggested that aiming for emmetropia in the dominant eye and a slightly myopic refraction ranging between 0.5 and 0.75 D in the nondominant eye may improve near vision and thereby increase patient satisfaction with EDOF IOLs.6,12 Although good postoperative near visual acuity has been reported with this approach, the desired spherical equivalent in each eye to achieve optimum visual outcomes across all ranges is unknown. The manufacturer recommends a plano target for both eyes, irrespective of dominance. The current study is a retrospective evaluation of outcomes of bilateral EDOF IOL implantation in a real-world practice setting. The goal was to identify the MRSE for the dominant and non-dominant eyes which produced the best overall binocular visual outcomes at far, intermediate and near distances.
Materials and Methods
In this retrospective, single-center, non-comparative study, we included patients who had undergone a consecutive bilateral cataract surgery at Lindenhurst Surgery Center in Lindenhurst, IL, between September 2016, and October 2018, with implantation of EDOF IOLs (Tecnis Symfony or Tecnis Symfony Toric) targeting emmetropia in both eyes. As part of routine clinical protocols, the risks, benefits, and alternatives to phacoemulsification with implantation of an EDOF IOL were explained to all patients prior to surgery and signed informed consent was obtained. Since the data were previously collected, de-identified, and compliant with the Health Insurance Portability and Accountability Act, approval from an Institutional Review Board was not required.
Other inclusion criteria were age ≥40 years and follow-up of at least 1 month following the second eye surgery. Also included were patients with prior corneal refractive procedures without thinning or ectasia on corneal topography. Patients with ocular comorbidities with a potential to affect visual outcomes were excluded. These included macular/retinal pathology, optic neuropathy, amblyopia, uncontrolled glaucoma, combined/past incisional glaucoma surgery, uveitis, corneal disease, irregular corneal astigmatism, corneal ectasia (including forme-fruste or diagnosed keratoconus), zonular or capsular weakness that may impede IOL centration, use of systemic alpha-1 antagonists such as tamsulosin and previous ocular trauma.
Forty-seven patients (25 females and 22 males) with a mean age 61.8 ± 8.3 years (range: 42–78 years) met the inclusion criteria. Preoperative parameters that included uncorrected (UDVA) and corrected (CDVA) distance visual acuity at 20 feet using Snellen’s chart, subjective refraction, intraocular pressure (IOP), slit-lamp anterior segment examination, dilated fundoscopic examination, biometry, corneal topography using Pentacam (Oculus Optikgeräte GmbH, Wetzlar, Germany) and Cassini (i-Optics BV, The Hague, The Netherlands), optical coherence tomography (Optovue, Inc., Freemont, CA), keratometry, and determination of eye dominance using the Porta test were retrospectively reviewed.
All patients underwent bilateral femtosecond laser-assisted cataract surgery (FLACS) using the Lensar laser system, with all procedures performed by a single surgeon (MAJ) under topical anesthesia. IOLMaster and multiple power calculation formulas were used for toric IOL power calculation. ORA intraoperative aberrometry (Alcon) was used in all patients to confirm the aphakic IOL power.
Preexisting astigmatism was addressed with either arcuate keratotomy (AK) or toric IOL implantation as applicable. The axis of toric IOL implantation/AK was guided by Cassini or Pentacam iris registration. The axis was marked with the femtosecond laser using intrastromal corneal arcuate marks (IntelliAxis-C; Lensar Inc., Orlando, FL) or anterior capsulotomy marks (IntelliAxis Refractive Capsulorhexis; Lensar Inc., Orlando, FL). When iris registration was not available, corneal marks were made by the surgeon with the patient in the upright position, and the desired IOL axis was confirmed by ORA intraoperative aberrometry. With lower amounts of regular corneal astigmatism, laser AIs were used to reduce astigmatism.
For this study, we analyzed the visual and refractive data that were recorded between 1 and 3 months after the second eye surgery. The following parameters were evaluated: Binocular UDVA by Snellen chart, binocular uncorrected near visual acuity (UNVA) measured at 40 cm using a Jaeger chart, binocular uncorrected intermediate visual acuity (UIVA) measured at 67 cm using reduced Snellen visual acuity chart, and manifest refraction spherical equivalent (MRSE). If visually significant posterior capsule opacification was present, visual and refractive data after Nd:YAG capsulotomy were recorded. Since the increased depth of focus of EDOF IOLs can cause autorefractors to overminus by up to 1.5D, manifest refraction was routinely confirmed with the maximum plus manifest refraction technique.
Statistical Analysis
Data analysis was performed using Microsoft Excel (Microsoft Inc., Redmond, WA) and SPSS version 17.0 (SPSS, Inc., Chicago, IL). The visual acuity values were converted to logarithm of the minimal angle of resolution (logMAR). Mean MRSE and binocular UDVA, UIVA, and UNVA were obtained, along with their corresponding standard deviations. The percentages corresponding to patients with UDVA of 20/25, UIVA of 20/20, UNVA of 20/30 or better and eyes within ± 0.5 D of MRSE were also calculated.
Scattergrams were plotted for postoperative binocular logMAR visual acuity at each distance (far, intermediate and near) and post-operative MRSE. Since the purpose of the study was to evaluate the MRSE in the dominant and the non-dominant eye that would provide the best overall visual outcomes at far, intermediate and near distances, scattergrams for combined binocular UDVA, UIVA, UNVA and postoperative MRSE were also plotted. The relationship between visual acuity and MRSE was evaluated using regression analysis, with logMAR visual acuity as the dependent variable and MRSE as the independent variable.
Results
Of the total 47 patients, 22 patients were implanted with bilateral Symfony toric IOLs; 25 were implanted with Symfony toric IOL in one eye and Symfony non-toric IOL in the contralateral eye. Six of 47 patients had a history of prior corneal refractive surgery (5 with LASIK/PRK and 1 with radial keratotomy); of these, 2 were implanted with bilateral Symfony toric IOLs and 4 with a combination of Symfony toric in one eye and Symfony non-toric IOL in the contralateral eye.
Of the 69 eyes implanted with toric IOLs, toric IOL alignment was performed by the surgeon with intrastromal corneal marks in 50 eyes, capsular marks in 15 eyes, and manual marks in 4 eyes. Of the 25 eyes implanted with non-toric IOLs, 7 eyes had femtosecond laser arcuate incisions for astigmatism reduction. None of the eyes with prior corneal refractive procedures underwent arcuate incisions for astigmatism reduction.
Postoperatively, the mean MRSE for all 94 eyes was −0.08 ± 0.41 D. The mean MRSE for the 47 dominant eyes was −0.01 ± 0.35 D and, for the 47 non-dominant eyes, −0.16 ± 0.45 D. The mean postoperative refractive astigmatism was 0.33D ± 0.39 D. Of the total 94 eyes, 84.04% of eyes were within ± 0.5 D of MRSE and 92.47% eyes had ≤0.75 D of residual cylinder. No eyes with toric IOLs needed repositioning for postoperative IOL rotation.
The postoperative mean binocular UDVA was 0.02 ± 0.05 logMAR. Of these, 95.74% (45/47) patients had UDVA of 20/25 or better. Mean binocular UIVA was −0.04 ± 0.11 logMAR and 85.11% (25/47) patients had UIVA of 20/20 or better. Mean binocular UNVA was 0.19 ± 0.11 logMAR and 53.19% (40/47) patients had UNVA of 20/30 or better.
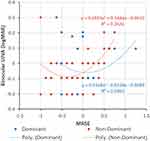
Figures 1–3 are scattergrams that show binocular UDVA, UIVA and UNVA plotted against MRSE of dominant and non-dominant eyes. The best fit second-order polynomial (quadratic) regression line with corresponding R2 and p values is also shown. When analyzing the combined (UDVA, UIVA & UNVA) visual acuities against MRSE (Figure 4), the quadratic regression line revealed a significant relationship for both dominant (R2 =0.053; p=0.02) and non-dominant eyes (R2 =0.05; p=0.039). The points of minima (lowest point on the parabola) of the quadratic regression curve for the dominant and non-dominant eyes were considered as the optimum MRSE corresponding to the best overall visual acuity. For the overall group, the point of minima was −0.08 D for dominant eyes and −0.63 D for non-dominant eyes.
There were 17 patients with excellent acuity at all distances (defined as UDVA and UIVA ≥20/20 and UNVA ≥20/30). The mean MRSE for the dominant eyes in this subset of 17 patients was −0.07 ± 0.14 D (range: −0.5 to 0.25 D; median: 0.00) and for the non-dominant eyes, it was −0.21 ± 0.24 D (range: −1.0 to 0.38 D; median: −0.25). The frequency distribution histogram of these eyes is presented in Figure 5A and B.
Discussion
Previous studies with Tecnis Symfony IOL implantation have demonstrated excellent distance and intermediate visual outcomes with a minimal level of dysphotopsia, high levels of spectacle independence and good patient satisfaction.6,13,14 However, visual outcomes at near have been reported to be relatively lower than those achieved with multifocal IOLs when emmetropia is the target.15–18 In the current study, all patients achieved excellent distance and intermediate visual acuity with good near visual acuity when implanted with EDOF toric or non-toric IOLs bilaterally.
In order to provide good functional visual acuity with bilateral EDOF IOL implantation, some investigators have studied whether blended vision can improve UNVA with bilateral implantation of EDOF IOLs.6,12,19,20 In blended vision, one eye is optimized for distance vision and the other eye is left slightly myopic to improve the near and intermediate visual acuity. In the CONCERTO study, the non-dominant eye was targeted for slight myopia of 0.5 to 0.75 D and the dominant eye for emmetropia.6 Later, some other investigators also used a similar approach: Ganesh et al chose a target of −0.75 D in the non-dominant eye;12 Sandoval et al and Tan et al preferred a −0.5 D target.19,20 Still others have advocated crossed monovision, that is, targeting emmetropia in the non-dominant eye and a small amount of myopia in the dominant eye.21 The ideal pair of refractive targets for EDOF IOLs is unknown.
In the current study, we chose a dataset where emmetropia was targeted in both eyes so that dominant and non-dominant eyes were provided equal opportunity to exhibit myopia and see better at near, thus allowing us to determine not only the optimum myopic target but also if it should be targeted in the dominant or the non-dominant eye. The best refractive target to achieve good visual outcomes across all distances, as obtained from the regression analysis computed separately for each distance, was −0.08 D in the dominant eye and −0.63 in the non-dominant eye. In a small subset of eyes with excellent acuity at all three distances (20/20 or better UDVA and UIVA and 20/30 or better UNVA), the ideal pair of refractive targets was −0.07 D for the dominant eye and −0.21 D for the nondominant eye, more closely matching the manufacturer’s recommendation for emmetropic refractive targeting.
With monovision, binocular summation plays an important role.22,23 It is well known that as the level of anisometropia in monovision increases, near visual acuity improves; however, binocular summation decreases, with a corresponding decrease in stereoacuity and contrast sensitivity.23–26 As such, there is a need to determine the level of myopia that would provide the right balance to improve near visual acuity without compromising binocular summation. Our study seems to suggest that inducing slight myopia of ~-0.6 D in the non-dominant eye may help achieve good overall visual acuity at distance, intermediate, and near with bilateral implantation of EDOF IOL. This small dioptric difference between the two eyes is unlikely to considerably affect the binocular summation.
In a subanalysis of the results of the CONCERTO study, Cochener et al evaluated the impact of different levels of achieved monovision after bilateral EDOF implantation.27 While the binocular UNVA was excellent in the subgroup with anisometropia >1.0 D, binocular UIVA was comparatively lower than the subgroups with lower levels of anisometropia. This could potentially be due to the decrease in binocular summation at intermediate distance with an increase in the level of anisometropia.
In the current study, there were 17 patients who achieved binocular UDVA and UIVA of 20/20 and UNVA of 20/30 or better. The mean postoperative MRSE of these 17 patients was −0.07 ± 0.14 D in the dominant eyes and −0.21 ± 0.24 D. It is possible that this represents the true “best pair” of refractive targets. However, it is also possible that other factors contributed to improved depth of focus in these eyes, including age, astigmatism, corneal multifocality, pupil diameter, IOL centration or corneal aberrations.28–30
Surgeons taking a blended vision approach with bilateral EDOF IOLs should be cognizant of patients’ specific visual needs and expectations. While blended vision can provide good functional vision at all distances, it is important to note that the stereoacuity may be reduced.24 Accordingly, this approach may not be suitable for patients who wish not to compromise stereopsis. Emmetropic targets for both eyes or a “personalized vision” approach of implanting an EDOF lens in the dominant eye and a mid-add multifocal IOL in the nondominant eye may be a better option.
The current study is limited by its retrospective nature and a small dataset. In addition, patient satisfaction and contrast sensitivity were not evaluated. Our results suggest that adopting blended vision targeting emmetropia in the dominant eye and inducing slight myopia in the non-dominant eye is a realistic approach to obtain good functional vision at all distances. While the regression analysis suggests a target of −0.6 D, from the subgroup analysis of eyes with excellent visual outcomes at all distances, a near-plano target of −0.2 D seemed to be the most effective in the non-dominant eyes. Future prospective studies with a larger sample size including metrics of quality of vision, patient-reported outcomes and stereopsis are warranted to establish the most precise myopic target in non-dominant eyes.
Acknowledgment
Jan Beiting (Wordsmith Consulting, Cary, North Carolina) provided editing assistance.
Disclosure
MAJ and AME received research grant from Johnson & Johnson Vision, Santa Ana, CA. MAJ is a consultant/speaker for Johnson & Johnson. RB is a consultant to Johnson & Johnson. The authors report no other conflicts of interest in this work.
References
1. Rocha KM. Extended depth of focus IOLs: the next chapter in refractive technology? J Refract Surg. 2017;33(3):146–149. doi:10.3928/1081597X-20170217-01
2. Bellucci R, Curatolo MC. A new extended depth of focus intraocular lens based on spherical aberration. J Refract Surg. 2017;33(6):389–394. doi:10.3928/1081597X-20170329-01
3. Epitropoulos AT. Visual and refractive outcomes of a toric presbyopia-correcting intraocular lens. J Ophthalmol. 2016;2016:7458210.
4. Gatinel D, Loicq J. Clinically relevant optical properties of bifocal, trifocal, and extended depth of focus intraocular lenses. J Refract Surg. 2016;32(4):273–280. doi:10.3928/1081597X-20160121-07
5. Hamid A, Sokwala A. A more natural way of seeing: visual performance of three presbyopia correcting intraocular lenses. Open J Ophthalmol. 2016;6(03):176. doi:10.4236/ojoph.2016.63025
6. Cochener B. Clinical outcomes of a new extended range of vision intraocular lens: International Multicenter Concerto Study. J Cataract Refract Surg. 2016;42(9):1268–1275. doi:10.1016/j.jcrs.2016.06.033
7. Sachdev GS, Ramamurthy S, Sharma U, Dandapani R. Visual outcomes of patients bilaterally implanted with the extended range of vision intraocular lens: a prospective study. Indian J Ophthalmol. 2018;66(3):407–410. doi:10.4103/ijo.IJO_813_17
8. Savini G, Schiano-Lomoriello D, Balducci N, Barboni P. Visual performance of a new extended depth-of-focus intraocular lens compared to a distance-dominant diffractive multifocal intraocular lens. J Refract Surg. 2018;34(4):228–235. doi:10.3928/1081597X-20180125-01
9. Cochener B, Boutillier G, Lamard M, Auberger-Zagnoli C. A comparative evaluation of a new generation of diffractive trifocal and extended depth of focus intraocular lenses. J Refract Surg. 2018;34(8):507–514. doi:10.3928/1081597X-20180530-02
10. de Medeiros AL, de Araujo Rolim AG, Motta AFP, et al. Comparison of visual outcomes after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of an extended depth of focus intraocular lens with a diffractive bifocal intraocular lens. Clin Ophthalmol. 2017;11:1911–1916. doi:10.2147/OPTH.S145945
11. Monaco G, Gari M, Di Censo F, Poscia A, Ruggi G, Scialdone A. Visual performance after bilateral implantation of 2 new presbyopia-correcting intraocular lenses: trifocal versus extended range of vision. J Cataract Refract Surg. 2017;43(6):737–747. doi:10.1016/j.jcrs.2017.03.037
12. Ganesh S, Brar S, Pawar A, Relekar KJ. Visual and refractive outcomes following bilateral implantation of extended range of vision intraocular lens with micromonovision. J Ophthalmol. 2018;2018:7321794.
13. Attia MSA, Auffarth GU, Kretz FTA, et al. Clinical evaluation of an extended depth of focus intraocular lens with the salzburg reading desk. J Refract Surg. 2017;33(10):664–669. doi:10.3928/1081597X-20170621-08
14. Gundersen KG. Rotational stability and visual performance 3 months after bilateral implantation of a new toric extended range of vision intraocular lens. Clin Ophthalmol. 2018;12:1269–1278. doi:10.2147/OPTH
15. Pandit RT. Monocular clinical outcomes and range of near vision following cataract surgery with implantation of an extended depth of focus intraocular lens. J Ophthalmol. 2018;2018:8205824.
16. Pedrotti E, Carones F, Aiello F, et al. Comparative analysis of visual outcomes with 4 intraocular lenses: monofocal, multifocal, and extended range of vision. J Cataract Refract Surg. 2018;44(2):156–167. doi:10.1016/j.jcrs.2017.11.011
17. Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi:10.1007/s00417-018-4052-3
18. Ruiz-Mesa R, Abengozar-Vela A, Aramburu A, Ruiz-Santos M. Comparison of visual outcomes after bilateral implantation of extended range of vision and trifocal intraocular lenses. Eur J Ophthalmol. 2017;27(4):460–465. doi:10.5301/ejo.5000935
19. Sandoval HP, Lane S, Slade S, Potvin R, Donnenfeld ED, Solomon KD. Visual and refractive clinical outcomes with an extended depth of focus toric intraocular lens targeted for binocular emmetropia or slight myopia in the non-dominant eye. J Cataract Refr Surg. 2019;45:1398–1403.
20. Tan J, Qin Y, Wang C, Yuan S, Ye J. Visual quality and performance following bilateral implantation of TECNIS Symfony intraocular lenses with or without micro-monovision. Clin Ophthalmol. 2019;13:1071. doi:10.2147/OPTH.S202380
21. Zhang F, Sugar A, Arbisser L, Jacobsen G, Artico J. Crossed versus conventional pseudophakic monovision: patient satisfaction, visual function, and spectacle independence. J Cataract Refract Surg. 2015;41(9):1845–1854. doi:10.1016/j.jcrs.2015.10.013
22. Azen SP, Varma R, Preston-Martin S, Ying-Lai M, Globe D, Hahn S. Binocular visual acuity summation and inhibition in an ocular epidemiological study: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2002;43(6):1742–1748.
23. Handa T, Shimizu K, Mukuno K, Kawamorita T, Uozato H. Effects of ocular dominance on binocular summation after monocular reading adds. J Cataract Refract Surg. 2005;31(8):1588–1592. doi:10.1016/j.jcrs.2005.01.015
24. Hayashi K, Ogawa S, Manabe S, Yoshimura K. Binocular visual function of modified pseudophakic monovision. Am J Ophthalmol. 2015;159(2):232–240. doi:10.1016/j.ajo.2014.10.023
25. Pardhan S, Gilchrist J. The effect of monocular defocus on binocular contrast sensitivity. Ophthalmic Physiol Opt. 1990;10(1):33–36. doi:10.1111/opo.1990.10.issue-1
26. Zheleznyak L, Sabesan R, Oh JS, MacRae S, Yoon G. Modified monovision with spherical aberration to improve presbyopic through-focus visual performance. Invest Ophthalmol Vis Sci. 2013;54(5):3157–3165. doi:10.1167/iovs.12-11050
27. Cochener B. Influence of the level of monovision on visual outcome with an extended range of vision intraocular lens. Clin Ophthalmol. 2018;12:2305–2312. doi:10.2147/OPTH
28. Finkelman YM, Ng JQ, Barrett GD. Patient satisfaction and visual function after pseudophakic monovision. J Cataract Refract Surg. 2009;35(6):998–1002. doi:10.1016/j.jcrs.2009.01.035
29. Hayashi K, Hayashi H. Stereopsis in bilaterally pseudophakic patients. J Cataract Refract Surg. 2004;30(7):1466–1470. doi:10.1016/j.jcrs.2003.12.030
30. Nanavaty MA, Vasavada AR, Patel AS, Raj SM, Desai TH. Analysis of patients with good uncorrected distance and near vision after monofocal intraocular lens implantation. J Cataract Refract Surg. 2006;32(7):1091–1097. doi:10.1016/j.jcrs.2006.03.021
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.