Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Omentum flap as a salvage procedure in deep sternal wound infection
Authors Spindler N, Etz CD, Misfeld M, Josten C, Mohr FW, Langer S
Received 16 February 2017
Accepted for publication 4 May 2017
Published 23 August 2017 Volume 2017:13 Pages 1077—1083
DOI https://doi.org/10.2147/TCRM.S134869
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Nick Spindler,1 Christian D Etz,2 Martin Misfeld,2 Christoph Josten,1 Friedrich-Wilhelm Mohr,2 Stefan Langer1
1Department of Orthopedic Surgery, Traumatology and Plastic Surgery, University Hospital Leipzig, 2Department of Cardiac Surgery, Leipzig Heart Center, University of Leipzig, Leipzig, Germany
Introduction: Deep sternal wound infections (DSWIs) are rare but devastating complication after median sternotomy following cardiac surgery. Especially in the presence of artificial material or inadequate preliminary muscle flaps, the pedicled omentum flap is due to its immunological properties, the predetermined flap in salvage procedures.
Methods: We treated 14 patients suffering a mediastinitis and open thorax using a pedicled omentoplasty as a salvage procedure because of persisting DSWIs. Omentoplasty was performed in combination with a split skin graft and the wound was closed by a vacuum-assisted therapy for 7 days. The patients’ sex and comorbid risk factors supporting DSWIs as well as the postoperative complications were recorded.
Results: Retrospective analysis of 14 patients (10 males and four females) after a follow-up time of 24 months was performed. The average age was 75 years (range: 67–83). Heart surgery took place electively in eight cases, in three cases urgently and three for emergency reasons. The preoperative Euro Score was 16 (range 3.51–42.58). We had no flap loss in any patients. The skin graft showed a full take in all patients. Two patients needed revision of an abdominal wound dehiscence after laparotomy and one patient developed hernia in the late outcome.
Discussion: The greater omentum flap has, over many years, become an ideal partner in the coverage and treatment of DSWIs. Especially due to its immunologic capacity and amorphous structure, it has the ability to fill up cavities and cover infected artificial material so residual infections can be controlled.
Keywords: deep sternal wound infection, omentum flap, reconstruction of the thorax, mediastinitis, DSWI
A Letter to the Editor has been received and published for this article.
Introduction
With an incidence rate of 0.5%–4% and mortality up to 50%, deep sternal wound infections (DSWIs) are a rare but devastating complication after median sternotomy following cardiac surgery.1,2 Due to infection and compromised perfusion of the sternum, the bone weakens, K-wires get ripped out of the bone by the permanent motion of the two sternum halves and an open thorax results. Furthermore, these infections may lead up to a fulminant mediastinitis. Immediate radical debridement, removal of artificial material and coverage by muscle flaps are the standard treatment. However, in some cases the infection persists in the anterior mediastinum and severe healing disorders set in. In case that artificial material, for instance, drive lines of left ventricular heart assist device or infected aortic prosthesis, cannot be removed, the local biofilm raises the risk for the persistence of the infection immensely. In these cases, immediate revision of the wound needs to be performed and the cavity should be filled with a pedicled omentum flap.
The greater omentum is a very potent tissue in wound healing. A good vascularization pattern and the immunological properties of the greater omentum flap build the basis of its capacity of cellular proliferation and repair function. By transporting the greater omentum flap into the deep sternal defect, the infection can be controlled and simultaneously covered.
This paper presents our experience in managing DSWIs after median sternotomy because of cardiac intervention using a pedicled greater omentum flap as a salvage procedure.
Methods
From May 2012 until August 2016, we treated 14 patients suffering from a mediastinitis and open thorax using a pedicled omentum plastic as a salvage procedure because of persisting DSWIs.
The patients’ sex and comorbid risk factors supporting DSWIs were recorded. Duration of the DSWIs and its delay to the primary cardiac intervention, as well as the interval to debridement and reconstruction of the thoracic wall by a pedicled omentoplasty was recorded. Omentoplasty was majorly performed in combination with a split skin graft and the wound was closed by a vacuum-assisted therapy (VAT) for 7 days. This retrospective analysis has been approved by the local ethics committee.
Surgical technique
In all patients, a radical debridement of the infected and necrotic soft tissue and bone material was carried out until a well-perfused and vascularized tissue had been exposed. Therefore, partial to total resection of the sternum had to be performed. Free-lying artificial material was macroscopically exempted from any adhesive tissue and a water-jet lavage of 5 L of Ringer’s lactate solution was carried out. The wound was then packed with abdominal bandages containing octenisept solution and covered up by a foil dressing. After midline incision from the upper to middle part of the abdomen, the greater omentum was divided from the colon transversum opening up the bursa omentalis. Preparation of the left and right gastroepiploic arteries follows. In general, the right artery has a larger diameter and higher flow, so the right flap is normally pedicled. Harvesting the flap by dissecting the greater omentum from the great curvature of the stomach, pedicled by the right gastroepiploic artery, the perfusion is secured by the arc of Barkow. After making an incision in the medial-frontal part of the diaphragm, the flap can be transferred to the sternal defect in the anterior mediastinum. Care has to be taken not to twist the pedicle. Here the flap will, due to its amorphous structure, fill the cavity and can be laid around any type of artificial structure or prosthesis. The omentum will be covered with a split graft or a muscle flap. Also, the direct skin closure is a standardized technique. Two Robinson drains were placed in the peritoneal cavity prior to closure.
A stabilization of the remaining parts of the sternum or rib-ends has in no case been performed.
Ethics
The study has been approved by the Ethical Committee at the Medical Faculty, Leipzig University.
The study data and information as well as the pictures of the case report are fully anonymous, therefore, the ethical committee of University of Leipzig required no specific patient consent statement. Regarding the case report, the patient gave written informed consent to allow publication of case report details and the accompanying pictures.
Results
Retrospective analysis of 14 (10 male and 4 female) patients after a follow-up time of 24 months was performed. The average age was 75 years (range: 67–83 years). Risk factors for DSWIs were similar to those reported by others (Table 1).3 Heart surgery took place electively in eight cases, in three cases urgently and three for emergency reasons. The preoperative Euro Score was 16 (range: 3.51–42.58).
  | Table 1 Risk factors of the described population |
Omentum coverage was performed 60 days (range: 18–136 days) after initial cardiac surgery. Surgery for valvular disease alone or in combination with coronary surgery was performed in eight patients, and five patients received an aortic bow replacement including replacement of the aortic valve. One patient received an external heart device and two patients multiple valve replacement (Table 2).
Postoperatively, two patients needed reanimation; two patients were treated with an extracorporeal membrane oxygenation in case of acute lung failure. Arrhythmia was seen postoperatively in nine patients, six patients needed hemodialysis and five patients thorax revision because of bleeding. Postoperatively, ventilation failure appeared in 11 cases, and six patients needed a tracheostomy and extended ventilation. All patients showed persistent purulent secretion and wound dehiscence and ended up with mediastinitis and an open thorax. The width between the two sternum halves averaged 5.6 cm. All patients demonstrated not only local, but additional symptomatic infections. Leukocytosis >10,000/μL and fever >38°C were observed in all patients. However, following the initial debridement, there was a spontaneous regression to a marginal normal level of the leukocytes. This has been attributed to the infectious potential of the prosthesis.
We had no flap loss in any patients. The skin graft showed a full take in all patients. The mediastinitis came to a stop immediately represented by a decline of the local and systemic inflammation parameters up to normal values. Two patients needed a revision of an abdominal wound dehiscence after laparotomy. One patient developed a hernia in the late outcome.
Nine patients died, averaging 98 days after cardiac surgery (min 44 days, max 176 days). Hospital mortality was 21% (three patients). In two patients, death was due to multiple organ dysfunction syndrome (MODS), and in seven patients due to cardiovascular failure. There were no signs of late complications related to mediastinitis or the omentum flap.
Case report
We present the case of a 76-year-old female patient suffering from a DSWI with an open thorax following implantation of an aortic valve, an aortic arch replacement (Gore-Tex) as well as a triple aortocoronary bypass. After radical debridement of the sternal bone and soft tissue, the mediastinitis was under control, and coverage of the 22×6.5 cm measuring wound was achieved by a pedicled latissimus dorsi flap. Due to the superinfected Gore-Tex aortic arch prostheses, local and systemic inflammation returned and the wound had to be revised. Because of the impossibility of replacing the aortic prosthesis, we indicated a renewed local debridement.
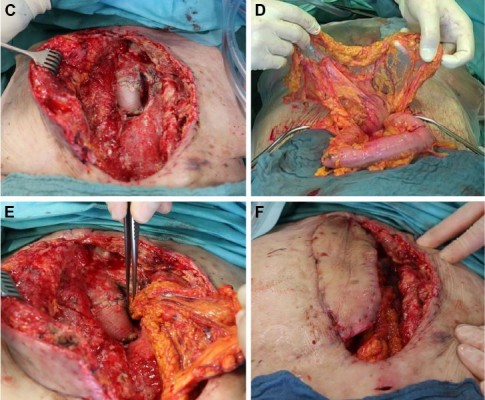
After reopening of the wound, pus was observed under the flap and floating all around the prosthesis. We took a representative sample of the purulent material (Figure 1A) and performed a radical debridement with a scalpel as well as a jet lavage. Afterward, the situs was found to be macroscopically clean (Figure 1B and C). We performed an upper midline incision of the abdomen and divided the greater omentum from the colon transversum. The arborizing arteries deriving from the left gastroepiploic artery assure the perfusion of the flap (Figure 1D). Through a created tunnel in the diaphragm, the pedicled omentum could be shoved into the anterior mediastinum, be wrapped around the prosthesis and fill the dead space of the cavity. The preexisting latissimus dorsi flap was taken to secure a permanent wound closure (Figure 1E and F). Hereby, the immunologically active tissue could suppress the inflammation caused by the prosthesis, and the wound healing could be finished. Three weeks after the operation, the wound appeared clean without local signs of infection and the formerly exiting leukocytosis decreased to a physiological level (Figure 1G).
Discussion
DSWIs following cardiac and thoracic surgery are rare but devastating defects. The risk factors leading to these wound-healing problems are numerous. Patient-derived risk factors are influenced by increasing age >68 years, male sex, obesity and smoking as well as the surgery-associated risk factors; duration of surgery, necessity of reoperation or re-wiring as well as the utilization of both internal mammary arteries as bypass grafts have a great impact on the outcome in this multimorbid population.3
A wide spectrum of wound-healing programs for postoperative mediastinitis exist.4 The desired main goal is control of the infection, stability of the thorax wall as well as immediate sufficient soft tissue coverage.5 Open granulation wound healing has, due to its complication and long-lasting period until the wound is finally closed, been abandoned long ago.6 Long-lasting irrigation and drainage following debridement were producing too many complications and did not have the desired effects either.7,8 Substernal channels and exposed parts of the sternum were consectaneous. In the late 1990s, long-time VAT concepts were hoped to avoid edema, dispatch fluid from the wound, create a granulation tissue and prevent the sharp edges of the sternum from penetrating the ventricle.9 DSWI is defined as a bone-related infection, which needs a radical debridement.10,11 Therefore, VAT can only be a good bridge to prepare the situs for plastic reconstruction. Some authors suggest a survival benefit for patients, who have been treated with VAT.12,13 This might be the reason for this method being implemented in many heart centers to bridge the interval from debridement to the final wound closure.
The importance of radical debridement and coverage using pedicled muscle flaps was introduced by Jurkiewicz in 1980.14 Hereby the post-sternotomy incidence of medistinits could be reduced to 8%–15%.15–17 Pedicled or free myocutanious flaps as well as the use of the pedicled greater omentum are possible options.5,18 Nowadays, coverage by a muscle flap is still the gold standard. The simple way of rising the muscle flap, as well as the low donor-side morbidity are the reasons for preference of the muscle flap. However, defect coverage in DSWIs using a muscle flap can, in case of persistent or recurrent infection as well as remaining potentially infected artificial material, be insufficient. Re-inflammation, segregation of pus and urgent revision will follow. The following attempt to cover the defect by the already existing flap will become more difficult. Reason for that is the fact that muscle flaps will lose their flexibility soon after its transplantation and transform to a firm scar-like structure. In that sense a muscle flap will bring a strong stability to the thorax wall in case of revision it becomes more and more difficult to replace it in the defect zone. Therefore, a tissue is needed, which will be an active aggressor against remaining infection.
Due to its immunologic capacity and its anti-infection activity, the greater omentum was destined to play an integral role in this type of wound treatment.19,20 The use of the pedicled greater omentum for coverage in case of DSWIs was published in 1976 by Lee et al and is a well-established therapy option.21 Size and the amorphous structure of the tissue allow a sufficient filling of cavities and dead spaces as well as total coverage of artificial material, for instance, drive lines from external heart assistance devices and Gore-Tex prosthesis, or even treatment of florid infection.20,22 In these cases, the use of the pedicled greater omentum has proved successful.
Opening up the abdomen next to a potentially infectious area has always been feared. Occurrence of superficial wound infection or insufficiencies of the abdominal suture are typically recorded complications.23 Secondary hernia, at rates of up to 21%, is the most frequently reported donor-side morbidity.24 Intraperitoneal abscess formations, gastric outlet and partial small bowl obstruction are unusual but can have a significant influence on the patient.23
Along with the open preparation of the omentum by laparotomia of the abdomen, the laparoscopic approach is an established alternative.25,26 Application of the laparoscopic techniques has the potential to lower the donor-side morbidity by improved visualization, decreased length of the abdominal incision and a more rapid return of the bowl function.27,28 However, in some cases the laparoscopic approach can be contraindicated. Nevertheless, hemodynamic lability, severe pulmonary disease as well as limited ventilation of the lung or restricted cardiac output can, in this cardiologic high-risk population, be on top negatively influenced by an applied pneumoperitoneum.23 Therefore, risk and value of a laparoscopic approach have to be taken into appropriate consideration and be adapted to the risk profile of the individual patient. The high survival rate following greater omentum transfer after DSWIs, published by Krabatsch and Hetzer as well as Yasuura et al, approves, in contempt of the multimorbidity in this population, the omentum as a reliable option in therapy.7,29
The latissimus dorsi flap is the regularly applied flap in the author’s hospital for treatment of DSWIs. During the past 4 years, 219 patients have been treated successfully. Treatment consists of radical bone and soft tissue debridement and coverage using a latissimus dorsi as our preferred standard muscle flap. In all our patients, the omentum flap was performed as a salvage procedure following unsuccessful interventions as closed irrigation therapy, VAT or unsuccessful muscle flap. On behalf of superinfected artificial material, remaining and recurrent infection in this population, the greater omentum flap was utilized.
Patients with DSWIs are always in a life-threatening situation. Therefore, in this special subpopulation we did not restore the continuity of the bone chest wall. Knowing that the muscle flap gives a better stability to the thorax because of its change to a rigid scar-like structure, the goal of our approach was to bring the infection to an end quickly and cover the afflicted area, leading the patients out of their life-threatening situation.
We performed the omentoplasty on average 60 days (range 18–136 days) after the first cardiac intervention. In all cases, we used a VAT for wound closure for a period of 7 days giving extra stability to the thorax, assuring liquid evacuation as well as a seroma prophylaxis. In one case the formerly used muscle flap was taken for skin closure, in the rest of the cases the omentum was split-grafted. There was no flap loss in any patients. The skin graft showed a full take in all patients. The mediastinitis came to a stop immediately. In all cases, omentoplasty led to a complete wound healing. In our population, two patients (body mass index >35) needed revision because of abdominal wound dehiscence after laparotomy. There were no adverse events related to the built communication of the mediastinum and the abdomen. No patient showed any signs of peritoneal infection. However, one patient developed a hernia deemed unworthy of treatment in the late outcome. Harvest of the omentum flap did not influence the gastric function.
The in-house mortality was in three patients (21%) rather high and underlines the findings from Kenzo who showed that hemodialysis and ventilation support at the time of omentoplasty is a negative predictor to in-house mortality.29 Our population showed a ventilation failure in 11 out of 14 patients with an average ventilation time of 682 h (range 6–3,628). This demonstrated on the one hand the multimorbid state our population was in prior to the omentoplasty. On the other hand, it underlines that utilizing the omentum for coverage reason will gather control of the infection showing low signs of donor-side morbidity or wound healing.
Death in our population was not related to the omentum flap but to cardiovascular failure; one patient died due to sepsis and MODS. In all cases, the sternal wound had already healed. There was no recurrence of DSWI in our population. This demonstrates that omentoplasty leads to a complete wound healing despite the presence of potentially infected artificial material, a state we did not experience while utilizing muscle flaps. The reason for this is the immense immunologic capacity of the greater omentum. The direct contact and excellent perfusion of the omentum allows an improved local nutrition, filling of the dead space and penetration of antibiotics to surrounding tissue.
The omentum flap has, therefore, become indispensable in modern plastic surgery as it is able to cover the whole part of the sternum, fill out deep cavities and due to its immunologic capacity and anti-infection activity is predestinated for coverage and treatment.
In our opinion, the omentum flap is the most useful for salvage procedures. This is indicated not only by the severe cardiac risk and infectiousness, but, moreover, the multimorbid state the patient is in. The high mortality rate we experienced is, therefore, caused by the multimorbidity of the patient and is not a result of the omentoplasty itself. In case of severe infection of nonremovable artificial material, a muscle flap is an insufficient option. As a salvage procedure, omentoplasty is a reliable treatment procedure in this specific patient population.
Conclusion
The greater omentum flap has, over many years, become a reliable partner in the coverage and treatment of DSWIs.
Especially due to its immunologic capacity and amorphous structure, it has the ability to fill up cavities and cover infected artificial material so residual infections can be controlled.
Though the latissimus dorsi flap is in our hospital the first choice in DSWIs, the greater omentum flap leads to an eradication of infection and, therefore, has changed our plan of treatment in our clinic.
In case of remaining, potentially infected artificial material (drive lines, vascular prosthesis) or persisting infection especially after reconstruction of the valves, the patient will receive an additional radical debridement and a simultaneous coverage using a omentoplasty with a split graft + VAT for 7 days. In case of a preexisting muscle flap, it will be utilized for skin closure reason.
Disclosure
The authors verify that this article is original and does not infringe on any copyright of any third party. The article is not considered by any other journal and has not been previously published. The final manuscript has been read and approved by each author. The individual contribution of each author has been approved by the corresponding author. This study has been reviewed and authorized by the ethical committee of University of Leipzig.
All authors have acknowledged the conflict of interest, disclosures and declare no conflict of interest.
References
Mauermann WJ, Sampathkumar P, Thompson RL. Sternal wound infections. Best Pract Res Clin Anaesthesiol. 2008;22(3):423–436. | ||
Toumpoulis IK, Anagnostopoulos CE, Derose JJ Jr, Swistel DG. The impact of deep sternal wound infection on long-term survival after coronary artery bypass grafting. Chest. 2005;127(2):464–471. | ||
Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg. 1998;65(4):1050–1056. | ||
Schimmer C, Sommer SP, Bensch M, Elert O, Leyh R. Management of poststernotomy mediastinitis: experience and results of different therapy modalities. Thorac Cardiovasc Surg. 2008;56(4):200–204. | ||
Spindler N, Lehmann S, Steinau HU, Mohr FW, Langer S. Komplikationsmanagement nach Thoraxeingriffen: Tiefe sternale Wundinfekte [Complication management after interventions on thoracic organs: deep sternal wound infections]. Chirurg. 2015;86(3):228–233. German. | ||
Robicsek F, Fokin A, Cook J, Bhatia D. Sternal instability after midline sternotomy. Thorac Cardiovasc Surg. 2000;48(1):1–8. | ||
Krabatsch T, Hetzer R. Poststernotomy mediastinitis treated by transposition of the greater omentum. J Card Surg. 1995;10(6):637–643. | ||
Shumacker HB Jr, Mandelbaum I. Continuous antibiotic irrigation in the treatment of infection. Arch Surg. 1963;86:384–387. | ||
Obdeijn MC, de Lange MY, Lichtendahl DH, de Boer WJ. Vacuum-assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg. 1999;68(6):2358–2360. | ||
Stahle E, Tammelin A, Bergstrom R, Hambreus A, Nystrom SO, Hansson HE. Sternal wound complications-incidence, microbiology and risk factors. Eur J Cardiothorac Surg. 1997;11(6):1146–1153. | ||
Grossi EA, Culliford AT, Krieger KH, et al. A survey of 77 major infectious complications of median sternotomy: a review of 7949 consecutive operative procedures. Ann Thorac Surg. 1985;40(3):214–223. | ||
Petzina R, Hoffmann J, Navasardyan A, et al. Negative pressure wound therapy for post-sternotomy mediastinitis reduces mortality rate and sternal re-infection rate compared to conventional treatment. Eur J Cardiothorac Surg. 2010;38(1):110–113. | ||
Sjogren J, Gustafsson R, Nilsson J, Malmsjo M, Ingemansson R. Clinical outcome after poststernotomy mediastinitis: vacuum-assisted closure versus conventional treatment. Ann Thorac Surg. 2005;79(6):2049–2055. | ||
Jurkiewicz MJ, Bostwick J 3rd, Hester TR, Bishop JB, Craver J. Infected median sternotomy wound. Successful treatment by muscle flaps. Ann Surg. 1980;191(6):738–744. | ||
Jones G, Jurkiewicz MJ, Bostwick J, et al. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg. 1997;225(6):776–778. | ||
Castello JR, Centella T, Garro L, et al. Muscle flap reconstruction for the treatment of major sternal wound infections after cardiac surgery: a 10-year analysis. Scand J Plast Reconstr Surg Hand Surg. 1999;33(1):17–24. | ||
Ivert T, Lindblom D, Sahni J, Eldh J. Management of deep sternal wound infection after cardiac surgery–Hanuman syndrome. Scand J Thorac Cardiovasc Surg. 1991;25(2):111–117. | ||
Greig AV, Geh JL, Khanduja V, Shibu M. Choice of flap for the management of deep sternal wound infection–an anatomical classification. J Plast Reconstr Aesthet Surg. 2007;60(4):372–378. | ||
Ghazi BH, Carlson GW, Losken A. Use of the greater omentum for reconstruction of infected sternotomy wounds: a prognostic indicator. Ann Plast Surg. 2008;60(2):169–173. | ||
Sajjadian A, Valerio IL, Acurturk O, et al. Omental transposition flap for salvage of ventricular assist devices. Plast Reconstr Surg. 2006;118(4):919–926. | ||
Lee AB Jr, Schimert G, Shaktin S, Seigel JH. Total excision of the sternum and thoracic pedicle transposition of the greater omentum; useful strategems in managing severe mediastinal infection following open heart surgery. Surgery. 1976;80(4):433–436. | ||
Mathisen DJ, Grillo HC, Vlahakes GJ, Daggett WM. The omentum in the management of complicated cardiothoracic problems. J Thorac Cardiovasc Surg. 1988;95(4):677–684. | ||
Hultman CS, Carlson GW, Losken A, et al. Utility of the omentum in the reconstruction of complex extraperitoneal wounds and defects: donor-site complications in 135 patients from 1975 to 2000. Ann Surg. 2002;235(6):782–795. | ||
Weinzweig N, Yetman R. Transposition of the greater omentum for recalcitrant median sternotomy wound infections. Ann Plast Surg. 1995;34(5):471–477. | ||
Salameh JR, Chock DA, Gonzalez JJ, Koneru S, Glass JL, Franklin ME Jr. Laparoscopic harvest of omental flaps for reconstruction of complex mediastinal wounds. JSLS. 2003;7(4):317–322. | ||
van Wingerden JJ, Coret ME, van Nieuwenhoven CA, Totte ER. The laparoscopically harvested omental flap for deep sternal wound infection. Eur J Cardiothorac Surg. 2010;37(1):87–92. | ||
Domene CE, Volpe P, Onari P, et al. Omental flap obtained by laparoscopic surgery for reconstruction of the chest wall. Surg Laparosc Endosc. 1998;8(3):215–218. | ||
Corral CJ, Prystowsky JB, Weidrich TA, Harris GD. Laparoscopic-assisted bipedicle omental flap mobilization for reconstruction of a chest wall defect. J Laparoendosc Surg. 1994;4(5):343–346. | ||
Yasuura K, Okamoto H, Morita S, et al. Results of omental flap transposition for deep sternal wound infection after cardiovascular surgery. Ann Surg. 1998;227(3):455–459. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.