Back to Journals » Clinical Ophthalmology » Volume 13
Novel Technique For The Management Of Macroperforation During Deep Anterior Lamellar Keratoplasty
Authors Nasr Hashem A, Tolees SS, Samir A
Received 31 August 2019
Accepted for publication 8 October 2019
Published 23 October 2019 Volume 2019:13 Pages 2075—2080
DOI https://doi.org/10.2147/OPTH.S229249
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ayman Nasr Hashem,1 Sherif Said Tolees,2 Ahmed Samir3
1Eye City Hospital, Cairo, Egypt; 2Magrabi Eye and Ear Hospital, Jeddah, KSA; 3Faculty of Medicine, Zagazig University, Zagazig, Egypt
Correspondence: Ayman Nasr Hashem
Eye City Hospital, Fifth Settlement, Cairo, Egypt
Tel +2 01009769598
Email [email protected]
Purpose: To describe the outcomes of a case series of patients who underwent a novel technique for managing macroperforation during deep anterior lamellar keratoplasty (DALK).
Methods: Nine eyes of 9 patients with advanced keratoconus who underwent DALK comprised the case series. A macroperforation occurred in all eyes during DALK. The stromal patch suturing technique was used to manage all cases; a large piece of the previously excised stromal tissue was sutured to the edge of the trephine to seal the perforation. The technical aspects of the procedure are described and data are presented on the visual outcomes, endothelial microscopy, and postoperative complications at last postoperative visit (average 18 months; range, 7 to 23 months).
Results: All 9 cases were managed successfully with the stromal patch suturing technique. Postoperatively, the graft was clear in all cases. At day 1, seven eyes had a Descemet’s membrane detachment, 3 of these eyes resolved spontaneously within 2 weeks and 4 required air bubble injection for successful reattachment. At last follow up seven eyes had a corrected distance visual acuity of 20/30 or better and 2 eyes had 20/40 vision. The average endothelial cell count was 2162 cell/mm2. There were no cases with excessive inflammation or vascularisation during the postoperative follow-up.
Conclusion: The stromal patch suturing technique was successfully used to manage intraoperative macroperforation during DALK. This technique resulted in endothelial changes that were consistent with uncomplicated DALK and function visual acuity of 20/40 or better in all cases with no postoperative sequelae.
Keywords: DALK, keratoplasty, corneal transplant, stromal patch suturing technique, macroperforation
Introduction
Deep anterior lamellar keratoplasty (DALK) is a full thickness or near-full thickness stromal resection down to or close to Descemet’s membrane (DM).1 DALK is currently the procedure of choice for restoring transparency and curvature in corneal stromal diseases with healthy endothelium. Preserving the endothelium avoids endothelial rejection and the endothelial cell count remains stable, facilitating good long-term graft survival.2
DALK techniques can be divided into two main categories based on the depth of dissection: the descemetic procedure (dDALK), involving complete stromal excision, baring DM or the pre-Descemet layer3,4 and; pre-descemetic procedure (pdDALK) that leaves variable layers of stroma adherent to DM.
The main challenge is to avoid perforation of DM. The incidence of DM perforation during DALK ranges from 4% to 39.2%5–9 and appears to depend on surgeon experience, indications for keratoplasty and surgical techniques. Ruptures can vary considerably. Microperforation is a small DM lesion that usually occurs during the pdDALK approach when the surgeon tries to go “deeper and deeper” with the spatula. The management DM microperforation includes, intracameral air injection for internal tamponade of DM.10,11 This technique allows completion of the stromal dissection down to a satisfactory pre-descemetic plane (pdDALK) in the majority of cases, especially when performed by an expert surgeon.11
Larger perforations that cannot be adequately sealed with an air bubble in the anterior chamber can be sealed with a small piece of stromal tissue, in most cases this is enough to allow proceeding with the dissection. Large perforations that cannot be sealed with air or with a stromal patch may be sealed with a tissue adhesive (fibrin sealant).12 Macroperforation is a DM rupture that is usually more than 1 mm, causing an anterior chamber collapse.10 Management of macroperforation of DM during DALK remains controversial with many recommending conversion to PKP.12–15 For cases of DM macroperforation where conventional methods fail to reform and maintain the anterior chamber, completion of stromal dissection might be very difficult and converting PKP is very likely to be needed.17 The case becomes even more complicated when a large DM tear occurs at an early stage of stromal dissection, before complete baring of DM or before reaching a satisfactory pre-descemetic plane. Dissecting the remaining thick residual stromal layer will apply more traction forces on the edges of the tear making the conversion to PKP very likely.
Adequate repair of ruptures increase the success of DALK. In this study, we report a new technique to complete the lamellar dissection in the presence of macroperforation.
Materials And Methods
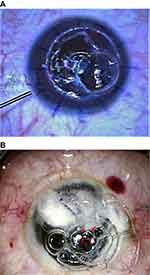
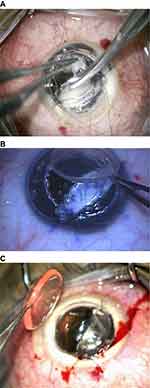
This case series evaluated 9 eyes of 9 patients with advanced keratoconus who underwent DALK. This study adheres to the principles of the Declaration of Helsinki and received the approval of the ethics committee of Magrabi Eye Hospital. Preoperatively and postoperatively all patients underwent an ophthalmic examination including, measurement of corrected distance visual acuity (CDVA), corneal topography, slit lamp examination and endothelial microscopy. Data are presented for the last postoperative visit on CDVA, endothelial microscopy, slit lamp examination and postoperative complications. All cases were planned for a deep anterior lamellar keratoplasty with the big bubble technique16 during which a large air bubble is formed between the DM and the stroma after injection of air deeply in the posterior stroma with 27 Gage needle, after that lamellar keratectomy is usually done then puncture of the bubble is done using a blade to expose the DM. After complete baring of DM, the graft is secured in place after peeling of DM. In all cases in this series creation of the big bubble has failed with intra-stromal air injection. Layer-by-layer dissection was initiated aiming for stromal dissection until reaching very thin smooth pre-Descemetic layer using a Barraquer spatula (Katena, Denville, NJ, USA). In all cases a large DM tear occurred during dissection with subsequent loss of anterior chamber (AC) perforations varied in size but all were larger than 1 mm in diameter. All perforations occurred in the peripheral and mid-periphery zone except one case with central perforation. All attempts at reformation of the AC with air failed even after external tamponade with previously excised stromal tissue (Figure 1). A large piece of previously excised stromal tissue was sutured to the edge of the trephine using 10/0 Nylon sutures (Alcon Inc., Fort Worth, TX, USA) an already available previously excised stromal tissue was used or trimming of larger pieces was needed in sometimes to make it more fitting. Two sutures one on each side of the perforations were usually sufficient to seal the perforation. The sutured stromal tissue was oriented to cover the DM break then subsequently; AC formation with air was possible (Figure 2).
After successful filling of the AC with air, the stromal dissection was restarted targeting a near DM plane using the same spatula (Figure 3). In some cases, air escape and partial or complete AC collapse that occurred during dissection was managed by re-injection of air. In some cases the sutured stromal tissue was reoriented in a manner to allow dissection of the underlying stromal tissue and this was obtained through applying new sutures at different locations on the trephine edge and removing the previous ones. Caution was used to avoid an excess traction force during dissection with the spatula while approaching the edge of the DM break so that the break was not inadvertently extended. So in such situations, the dissection was completed in with a sharp crescent knife or scissors (Figure 3A).
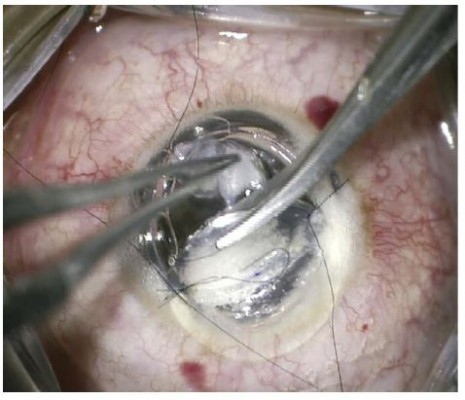
After reaching a satisfactory pre-Descemetic plane (Figure 3B and C), the graft was secured in place with some interrupted sutures while the sutured stromal patch remained in place. Subsequently, the AC was further inflated with air to ensure good internal tamponade before removing the stromal patch. The sutures fixing the stromal patch were cut and the stromal patch was pulled out through the trephine edge in a direction coaxial with the DM break to ensure that no shearing force was applied to the edges of the DM break (Figure 4).
The graft suturing was completed with interrupted sutures. Application of cyclopentolate 1% was initiated during suturing to start pupillary dilation to prevent against pupillary block. Air was partially removed from the AC at the end of the procedure. Postoperative medications included; frequent topical steroid for 1 week to be reduced to 4 times daily for 3 months before tapering, Topical antibiotic for 2 weeks, Cyclopentolate eye drops 1% for 3 days. https://vimeo.com/357668971 is available.
Results
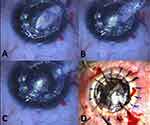
At the last follow up visit (average 18 months; range, 7 to 23 months) all cases had clear corneal graft tissue. On the first postoperative day, 7 eyes showed detachment of the very thin host stromal layer (Figure 5A), 3 of these eyes had spontaneous resolution within 2 weeks while 4 required air bubble injection for successful reattachment (Figure 5B). At last follow up visit Seven eyes had a corrected distance visual acuity of 20/30 or better and 2 eyes had 20/40 vision. The average endothelial cell count was 2162 cell/mm2. There was no excessive inflammation or vascularisation observed over the duration of postoperative follow-up. There was no occurrence of increase of intra-ocular pressure due to pupillary block in the immediate postoperative period.
Discussion
In the current study, the endothelial cell count was superior to a series of DALK cases complicated with microperforations and comparable to non-complicated DALK cases.17 In our series of patients, postoperative CDVA was comparable to non-complicated cases of DALK.18 The location of the macroperforation may affect the final CDVA as noted in our series where two cases with central macroperforations resulted in 20/40 CDVA postoperatively.
Den et al17 reported that all the patients with DM macroperforation had pseudochamber formation postoperatively and 2 developed endothelial decompensation. In our study 7 out of 9 eyes (78%) had a pseudochamper formation, 4 of these eye required air bubble injection for DM attachment. However, there were no cases of endothelial decompensation, likely due to the better DM seal with our technique. Additionally, better AC stability intraoperatively allowed the completion of stromal dissection, obtaining a smooth bed with better adherence to the donor cornea. The sutured stromal patch has reduced the frequency of AC collapse intraoperatively and the need of frequent air injections into the AC. Repeat air injections in the AC may negatively affect the endothelial cell count. In the current study, there were no cases of excessive inflammation or vascularisation over the duration of follow up. On long term follow up we do not expect other complications other than those with standard pdDALK yet we need to look at this in the future.
There are some limitations in this study the first is the limited number of participants and the relative short period of follow up but being a report of surgical technique we thought that reported time of follow up overs the immediate postoperative complication that might be related to this technique. One more thing is the possible impact on CDVA especially if the macroperforation were located centrally, in case of central perforation this will be left for the surgeon to decide based on the size and location of the perforation and the residual stromal bed whether to convert to PK or to use this technique but in some conditions with high rejection rate we might choose the side of using this technique even with the possible impact on vision rather than converting to PK, for example, with large graft, children, patient with chronic VKC and so on.
In summary, stromal patch suturing is a valid, reproducible technique to seal DM macroperforation allowing for completion of DALK without conversion to PKP. The postoperative endothelial cell differences did not vary compared to other modalities of management of microperforation and had no negative impact on final CDVA. A larger sample size of patients is required to confirm the benefits of the novel technique described in this study. This technique describes a valid management for DM macroperforation where conventional intracameral air injection and other options like applying external stromal plug failed to seal the perforation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saini JS, Jain AK, Sukhija J, Saroha V. Indications and outcome of optical partial thickness lamellar keratoplasty. Cornea. 2003;22:111–113. doi:10.1097/00003226-200303000-00005
2. Tan DT, Ang LP. Automated lamellar therapeutic keratoplasty for post-PRK corneal scarring and thinning. Am J Ophthalmol. 2004;136:1067–1069. doi:10.1016/j.ajo.2004.06.062
3. Reinhart WJ, Musch DC, Jacobs DS, et al. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118:209–218. doi:10.1016/j.ophtha.2010.11.002
4. Sarnicola V, Toro P, Gentile D, Hannush SB. Descemetic DALK and predescemetic DALK: outcomes in 236 cases of keratoconus. Cornea. 2010;29:53–59. doi:10.1097/ICO.0b013e3181a31aea
5. Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81:184–188. doi:10.1136/bjo.81.3.184
6. Tsubota K, Kaido M, Yu M, et al. A new surgical technique for deep lamellar keratoplasty with single running suture adjustment. Am J Ophthalmol. 1998;126:1–8. doi:10.1016/s0002-9394(98)00067-1
7. Fogla R, Padmanabham P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2006;141:254–259. doi:10.1016/j.ajo.2005.08.064
8. Coombes AG, Kirwan JF, Rostron CK. Deep lamellar keratoplasty with lyophilised tissue in the management of keratoconus. Br J Ophthalmol. 2001;85:788–791. doi:10.1136/bjo.85.7.788
9. Melles GR, Remeijer L, Geerards AJ, et al. The future of lamellar keratoplasty. Curr Opin Ophthalmol. 1999;10:253–259. doi:10.1097/00055735-199908000-00006
10. Leccisotti A. Descemet’s membrane perforation during deep anterior lamellar keratoplasty: prognosis. J Cataract Refract Surg. 2007;33:825–829. doi:10.1016/j.jcrs.2007.02.016
11. Fogla R, Sridhar U, Gupta A. Recent advances in lamellar keratoplasty. In: Gupta A, editor. Clinical Ophthalmology: Contemporary Perspectives.
12. Anwar HM, El-Danasoury A, Hashem AN. The use of fibrin glue to seal descemet membrane microperforations occurring during deep anterior lamellar keratoplasty. Cornea. 2012;31:1193–11969. doi:10.1097/ICO.0b013e318242fd94
13. Amayem AF, Anwar M. Fluid lamellar keratoplasty in keratoconus. Ophthalmology. 2000;107:76–80. doi:10.1016/s0161-6420(99)00002-0
14. Anwar M, Teichmann KD. Deep lamellar keratoplasty: surgical technique for lamellar keratoplasty with and without baring of descemet’s membrane. Cornea. 2002;21:374–383. doi:10.1097/00003226-200205000-00009
15. Watson SL, Ramsay A, Dart JK, et al. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patient with keratoconus. Ophthalmology. 2004;111:1676–1682. doi:10.1016/j.ophtha.2004.02.010
16. El-Danasoury A, Hashem A. Deep lamellar keratoplasty with baring of descemet membrane: the big bubble technique. Tech Ophthalmol. 2007;33:825–829.
17. Den S, Shimmura S, Tsubota K, Shimazaki J. Impact of the descemet membrane perforation on surgical outcomes after deep lamellar keartoplasty. Am J Ophthalmol. 2007;143:750–754. doi:10.1016/j.ajo.2007.01.053
18. Noble BA, Agrawal A, Collins C, Saldana M, Brogden PR, Zuberbuhler B. Deep anterior lamellar keartoplast (DALK): visual outcome and complication for heterogenous group of corneal pathologies. Cornea. 2007;26:59–64. doi:10.1097/01.ico.0000240080.99832.f3
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.