Back to Journals » International Journal of Nanomedicine » Volume 12
Nanoparticle-based hyperthermia distinctly impacts production of ROS, expression of Ki-67, TOP2A, and TPX2, and induction of apoptosis in pancreatic cancer
Authors Ludwig R, Teran FJ, Teichgraeber U, Hilger I
Received 16 March 2016
Accepted for publication 16 June 2016
Published 7 February 2017 Volume 2017:12 Pages 1009—1018
DOI https://doi.org/10.2147/IJN.S108577
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Robert Ludwig,1 Francisco J Teran,2,3 Ulf Teichgraeber,1 Ingrid Hilger1
1Department of Experimental Radiology, Institute for Diagnostic and Interventional Radiology, Jena University Hospital – Friedrich Schiller University Jena, Jena, Germany; 2iMdea-Nanociencia, Campus Universitario de Cantoblanco, 3Nanobiotecnología (iMdea-Nanociencia), Unidad Asociada al Centro Nacional de Biotecnología (CSIC), Madrid, Spain
Abstract: So far, the therapeutic outcome of hyperthermia has shown heterogeneous responses depending on how thermal stress is applied. We studied whether extrinsic heating (EH, hot air) and intrinsic heating (magnetic heating [MH] mediated by nanoparticles) induce distinct effects on pancreatic cancer cells (PANC-1 and BxPC-3 cells). The impact of MH (100 µg magnetic nanoparticles [MNP]/mL; H=23.9 kA/m; f=410 kHz) was always superior to that of EH. The thermal effects were confirmed by the following observations: 1) decreased number of vital cells, 2) altered expression of pro-caspases, and 3) production of reactive oxygen species, and 4) altered mRNA expression of Ki-67, TOP2A, and TPX2. The MH treatment of tumor xenografts significantly (P≤0.05) reduced tumor volumes. This means that different therapeutic outcomes of hyperthermia are related to the different responses cells exert to thermal stress. In particular, intratumoral MH is a valuable tool for the treatment of pancreatic cancers.
Keywords: iron oxide nanoparticles, magnetic hyperthermia, heat dose, nanomedicine, proliferation marker
Introduction
With the aim to circumvent the harmful side effects related to most conventional oncologic therapeutic modalities and increase the survival rate after cancer diagnosis, intensive research is being performed to assess the therapeutic potential of raising the tumor temperature (ie, hyperthermia) in order to kill proliferating cancer cells.1–3 In this context, different means to induce hyperthermia have been suggested. Thus, water bath, infrared radiation, focused ultrasound, and micro- or radiowaves are some examples of heat sources placed outside the body (external heating sources).4,5 Additionally, the progress of nanotechnology has brought a minimally-invasive approach based on the use of biocompatible6 iron oxide magnetic nanoparticles (MNPs) as heating mediators when subjected to alternating magnetic fields (AMFs). Under these conditions, heat dissipation occurs due to magnetization reversal processes of MNP magnetic moments.7–10 Being easily internalized into cells by endocytotic mechanisms,11 MNPs become efficient local heaters distributed along the cytoplasm.12 The thermal stress induced by MNPs tightly depends on the nanoparticle load and their heating efficiency.13
The heat dose applied to cancer cells is known to exhibit a distinct impact on cellular functions, in particular in relation to DNA stability,14 protein conformation,15 and/or expression.16 All these molecular alterations manifest themselves in the cell viability.17 Furthermore, the formation of reactive oxygen species (ROS) is known to be induced via hyperthermia.18 ROS can induce apoptosis.19–21 Moreover, the cytotoxic effects of hyperthermia might differ among different tumor cells.22,23
Besides the promising prospective of hyperthermia, in clinical studies, the therapeutic outcome was revealed to be rather heterogeneous.5,24 Whereas the extrinsic heating (EH) of organs or tissues benefits tumor regression when their temperature is maintained between 40°C and 42°C for short times,17 magnetic heating (MH) reveals that their heat dissipation may efficiently reduce cell viability.25
Therefore, in the present study, we sought to compare the cellular responses caused by distinct heat generation modalities such as by an internal heat source represented by MNPs deposited in the target region (ie, tumor) and exposure to an AMF (magnetic hyperthermia, MH) or via an external heat source (EH) applied from outside the body (such as hot air). Both modalities are expected to exert different effects,26 which are still poorly understood. We analyzed the “anti-cancer” effects of both hyperthermia modalities in terms of cell viability, apoptosis induction, the formation of ROS, and the expression of proliferation markers, which are expressed in different cell cycle phases, for example, Ki-67 is present in all phases of the cell cycle but not in the resting one (G0); topoisomerase 2-α (TOP2A) controls the topologic states during DNA transcription and its gene was shown to be amplified in cancer cells;27 and the expression of TPX2 (a microtubule-associated protein) in cancer cells is associated with vessel invasion and metastasis.28 Additionally, we examined the transferability of the cellular effects of MH observed in vitro to the in vivo conditions.
Material and methods
Cell culture
PANC-1 and BxPC3-cells (human pancreatic adenocarcinoma) were cultivated at 37°C and 5% CO2 using DMEM and RPMI 1640 media (Thermo Fisher Scientific, Waltham, MA, USA).
Magnetic nanoparticles
Superparamagnetic iron oxide nanoparticles (MF66) were obtained from Liquids Research Limited (Bangor, Gwynedd, UK). MNP synthesis was performed by co-precipitation technique as described elsewhere.29 The average core size of the MNPs was 12±3 nm, hydrodynamic diameter 85 nm, and specific absorption rate value 900 W/g Fe (corresponding to intrinsic loss power values of 8.7 nHm2*kg−1). The ζ-potential was −41 mV.29
In vitro extrinsic hyperthermia (EH)
PANC-1 and BxPC-3 cells were incubated for 60 minutes at different temperatures (41°C, 43°C, and 47°C). Temperatures were monitored using fiber optic temperature probe and thermometer (TS5 & FOTEMPMK-19; Optocon AG, Dresden, Germany). MNP untreated cells at 37°C were used as controls. The recorded temperature data were used to calculate the thermal dose as cumulative equivalent minutes above 43°C (CEM43) as described elsewhere.30
In vitro (intrinsic) magnetic hyperthermia
PANC-1 and BxPC-3 cells were incubated with MNP (100 μg/mL) for 24 hours at 37°C. Afterwards, cells were exposed to AMF (H=23.9 kA/m, f=410 kHz) for 60 minutes so as to induce temperatures of 41°C, 43°C, or 47°C. Temperatures were monitored and cells were processed for further analysis as described earlier. Uptake of cellular MNP at 24 hours after incubation was quantified by atomic absorption spectroscopy (AAS 5 FL; Analytik Jena AG, Jena, Germany) as described elsewhere and was found to be of 22±6 pg Fe/cell (PANC-1) and 67±17 pg Fe/cell (BxPC-3), respectively, in a prototype experiment.29
Determination of cell viability
To determine the amount of vital, apoptotic, and necrotic PANC-1 and BxPC-3 cells after hyperthermia treatments (ie, 24 or 48 hours after MH or EH), cells were washed with HBSS and incubated with CellEvent® Caspase-3/7 Green Flow Cytometry Assay Kit (Molecular Probes®) in accordance with the manufacturer’s protocol. Cell viability was analyzed using a FACSCalibur (BD Bioscience, San Jose, CA, USA; λexc=488 nm, λem=533 nm/647 nm). Cells declared as vital were not stained by either dye (Cell Event Caspase 3/7 Green nor SYTOX AADvanced dead cell stain), early apoptotic cells were positively stained only with Cell Event® Caspase 3/7 Green, late apoptotic cells were positively stained with Cell Event® Caspase 3/7 Green and SYTOX® AADvanced™ dead cell stain, and necrotic cells were positively stained only with SYTOX® AADvanced™.
Western blot analysis
PANC-1 cells were harvested after treatment, washed, and lysed with RIPA lysis buffer containing protease inhibitors (Hoffman-La Roche Ltd., Basel, Switzerland). Protein concentration was determined using the Bradford protein assay. Protein extracts were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis. Membranes were blocked and incubated with primary Bax (mouse monoclonal, clone 2D2, 1:500; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), Bcl-xL (rabbit polyclonal, 1:500; Cell Signaling Technology Inc., Danvers, MA, USA), caspase-3 (mouse monoclonal, clone 3G2, 1:500; Cell Signaling Technology Inc.), caspase-8 (mouse monoclonal, clone 1C12, 1:500; Cell Signaling Technology Inc.), cPARP (rabbit anti cPARP, 1:700; Cell Signaling) heat shock protein 70 (HSP70) (mouse anti-HSP70, 1: 1,200; StressMarq Biosciences Inc.), or β-actin-HRP antibody (mouse monoclonal, clone 8226, 1:25,000; Abcam, Cambridge, UK) before incubation with secondary anti-mouse IgG-HRP antibody or anti-rabbit IgG-HRP antibody (1:25,000; both from Santa Cruz Biotechnology Inc., Dallas, TX, USA). Immunosignals were detected using chemoluminescence HRP substrate (EMD Millipore, Billerica, MA, USA).
Determination of ROS production
ROS formation was assessed by incubating harvested and washed cells with 100 μM DCFH-DA (OxiSelect™ Intracellular ROS Assay Kit, Cell Biolabs Inc., USA). ROS formation was analyzed by flow cytometry (λexc=488 nm, λem=530 nm). The fluorescence levels obtained were further normalized to the amount of vital cells.
Proliferation marker expression after hyperthermia
Total RNA was extracted and reverse RNA transcription was performed. For quantitative real-time reverse transcription polymerase chain reaction analysis of Ki-67, TOP2A, TPX2, and β-2-microglobulin (B2M; reference gene) transcript expression, splice junction crossing primers were designed.31 The relative expression of genes of interest was calculated by normalization of the mRNA expression after treatment to that of the reference gene B2M and by subtraction of the relative mRNA expression of non-treated cells.
In vivo magnetic hyperthermia of xenograft models
All experiments were conducted according to the national and EU norms guidelines on the ethical use of animals and were approved by the regional animal care committee (Freistaat Thüringen, Landesamt für Verbraucherschutz). Animals were anesthetized with isoflurane. PANC-1 cells were implanted subcutaneously into female athymic nude mice (Hsd:Athymic Nude-Foxn1nu; Harlan Laboratories, Venray, the Netherlands). Experiments were started when tumors reached volumes between 60 and 470 mm3. Animals were divided into four independent treatment groups: group 1 animals (animals that underwent treatment) were treated with MH (intratumoral injection of 0.2 mg Fe in 100 mm3 volume of colloidal MNP dispersion and application of AMF for 60 minutes twice with a time interval of 6 days; parameters of the magnetic field: H=15.4 kA/m, f=435 kHz); group 2 (MNP control group) received intratumoral injection of 0.2 mg Fe in 100 mm3 volume of colloidal MNP dispersion (control of MNP influence); group 3 (AMF control group; exposure to AMF two times for 60 minutes each and a time interval of 6 days) and group 4 (tumor control group) received intratumoral injection of ddH2O in a volume of 100 mm3. Group 3 (AMF control group) was additionally exposed to AMF to assess the impact of AMF on tumor growth. Treatment of PANC-1 xenografts with MH, monitoring of temperature, and calculation of the thermal dose CEM43T90 covering at least 90% (T90) of tumor surface were performed as described elsewhere.29 High temperatures were applied during MH so that even the outermost tumor regions were exposed to temperatures of 43°C. To determine treatment outcome, tumor volume (caliper) and body weight were analyzed every 3–4 days. Blood count of MH-treated animals and controls was analyzed as described elsewhere.29
Statistics
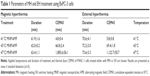
In order to assess the heating effects (i.e. temperature, time, therapeutic modality etc. see Figure 1), the corresponding groups (cell samples or animals) were compared via the utilization of the Mann–Whitney U-test as indicated in the figures. P-values ≤0.05–0.001 were considered to be significantly different.
Results
Impact of hyperthermia on cell viability and apoptosis induction
Cell viability
As shown for BxPC-3 cells, the heat dose supplied by MH distinctly decreased cell viability at temperatures of 43°C or higher (Figure 1, upper and lower left). The induction of necrotic cells was particularly prominent. BxPC-3 cells responded to EH only at a temperature of 47°C (late apoptosis and sparse amount of necrotic cells, Figure 1, upper and lower right). For the corresponding reference temperature, the exposure to MH or EH led to comparable heat doses as revealed by calculations of CEM43 (Table 1).
Protein expression
Western blot analysis revealed the potential of MH to induce cell death in investigated pancreatic cells.
MH in PANC-1 cells
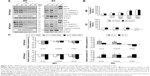
After exposure to MH, no distinct impact on the expression of the pro-apoptotic and anti-apoptotic proteins Bax and Bcl-xL, respectively, was detected in PANC-1 cells (Figure 2A, left). The expression of pro-caspases 3 and 8 decreased. No effects on the expression of cPARP (marker for irreversible apoptosis) were found.
EH in PANC-1 cells
In contrast, the heat dose supplied by EH to PANC-1 cells distinctly increased Bax and Bcl-xL expression, reduced pro-caspases 3 and 8 expression, and increased HSP70 expression (Figure 2A, 48 hours, right). Again, no distinct effects on cPARP were found.
MH in BxPC-3 cells
BxPC-3 cells treated with MH showed almost no changes in the expression of Bax and Bcl-xL (24 hours of post-treatment). Only at a later time point (48 hours), temperatures of 41°C and 43°C led to an increased expression of these proteins (Figure S1A, upper left). The expression of pro-caspases 3 and 8 was decreased on increasing the temperature to 47°C (Figure S2A, lower left). No effects on c-PARP, but a tendency for an increased expression of HSP70, were detected.
EH in BxPC-3 cells
In contrast, EH treatment of BxPC-3 cells exhibited an impact on the expression of pro- and anti-apoptotic proteins only after these cells were exposed to the highest temperature. The expression of the protein cPARP was prominent (highest temperature, 48 hours post-treatment, HSP70 not (Figure S1A).
Impact of hyperthermia on the formation of ROS
MH exhibited a marked impact on the cellular ROS content of PANC-1 cells. ROS formation increased with increasing temperatures compared to the non-treated cells (Figure 2B). Exposure of PANC-1 cells to MNP alone (100 μg/mL) slightly increased the formation of ROS. However, EH treatment of PANC-1 had almost no effect on the production of ROS (Figure 2B).
Regarding BxPC-3 cells, MH led to a similar response in the formation of ROS as observed for PANC-1 cells (Figure S3B). In contrast, the exposure of these cells to EH led to ROS production only at a temperature of 47°C (Figure S2B).
Impact of hyperthermia on the expression of proliferation markers
Analysis of the mRNA expression of the proliferation markers Ki-67, TOP2A, and TPX2 revealed a marked reduction in their expression after PANC-1 cells were treated with MH compared to the non-treated cells (Figure 2C, upper left). MNP incubation (100 μg/mL) exerted a comparably lower impact. The highest impact on mRNA expression was observed for MH treated cells at a temperature of 43°C.
In contrast, PANC-1 cells exposed to EH showed an increased mRNA expression of Ki-67, TOP2A, and TPX2 (Figure 2C).
Similar to PANC-1 cells, MH treatment of BxPC-3 cells or MNP controls (100 μg/mL) revealed a significant (P≤0.05) reduction in the mRNA expression of proliferation markers Ki-67, TOP2A, and TPX2 (Figure S1C, 24 hours of post-treatment). MH at a temperature of 43°C showed the highest impact. At later post exposure times to MH (or MF66 MNP alone) mRNA expression was almost comparable to the non-treated cells (Figure S2C, lower left). In contrast to MH exposure, EH treatment of BxPC-3 cells resulted in an increased mRNA expression of all the three markers at 24 hours of post-treatment (Figure S1C, upper right). After 48 hours, the expression levels were almost comparable to the non-treated cells (Figure S1C, lower right); however, EH at a temperature of 43°C still distinctly affected the TOP2A expression.
In vivo potential of magnetic hyperthermia in pancreatic cancer therapy using PANC-1 xenografts
MH treatment of PANC-1 tumor-bearing animals (thermal dose CEM43T90: 137±118 min) significantly reduced (P≤0.05) tumor volumes from day 8 to day 28 (Figure 3A). At the end of the observation period, animals displayed distinctly lower relative tumor volume compared to non-treated animals. Intratumoral injection of MNP in combination with AMF led to the application of comparable heat doses achieving similar hyperthermic temperatures. In most cases, a smaller tumor area got affected when subjected to MH treatment for the second time compared to the first time. The first MH treatment often led to variation of the MNP location inside the tumor tissue and/or the loss of MNP with respect to the initial intratumoral injection of MNP. Figure 3B shows the local changes on the surface of tumor xenografts that underwent treatment, such as the formation of eschars, followed by the development of emarginations, the loss of tumor tissue, and the formation of scar tissue. Furthermore, intratumoral MNP injection resulted in an inhomogeneous MNP distribution within the tumor, thus resulting in the formation of local heat spots and temperature gradients within the AMF (Figure 3C). Contrary to the MH treatment, the presence of MNP or the application of AMF (controls) did not result in any detectable temperature increase at the tumor surface of the xenograft models (Figure 3C). Thus, MH treatment progressively and continuously decreased tumor volumes as seen in Figure 3A, whereas the tumor volumes of control animal groups increased as expected. Interestingly, the presence of MNP attenuated the increase in tumor volumes compared to AMF and tumor control groups.
Hematological parameters such as the number of white and red blood cells and the hemoglobin concentration of animals exposed to MH displayed no differences compared to control groups (Figure S2A). White blood cell count exhibited a high variability between different animal groups. However, the overall hematological parameters of the animal groups were not far from the blood counts reported by the supplier of the animal models (black line in Figure S1A). Furthermore, the body weight of mice remained stable and was, therefore, not affected by either treatment (Figure S2B). Finally, it is worth to mention that intratumoral injection of MNP and subsequent MH treatment did not lead to the accumulation of MNP in other organs and/or tissues of group 1 animals 28 days after the first application of AMF (Figure S3).
Discussion
In general, our in vitro data revealed the superiority of MH over EH in killing cancer cells and inhibiting tumor cell proliferation. Intracellular MH mainly increased the number of cells in necrosis. MH triggered ROS formation and reduced the expression of the proliferation markers Ki-67, TOP2A, and TPX2 at mRNA level. EH prominently increased the number of cells in apoptosis; ROS formation was less pronounced and the expression of the proliferation markers and cell protective proteins (HSP70) increased in some cases. Despite the differences between both hyperthermia modalities (MH and EH), we also detected distinct responses depending on the cell line. The MH treatment of tumor-bearing xenografts inhibited tumor growth compared to untreated animals.
In particular, the strong effect of MH treatment on the induction of cell necrosis and ROS formation may be related to the large heat doses in the intracellular MNP surroundings.32 The fact that a decreased cell viability for different cell lines after MH was reported, though no rise in temperature of the surrounding medium could be detected in other studies,25 further reinforces our observations. The by-passing of thermotolerance induction due to the inhibition of HSP expression as well as increased cellular stress due to MNP rotation within the AMF could be two further explanations for the higher impact of MH exposure with respect to EH.
The fact that no distinct signs of apoptosis (eg, cell fraction analysis, expression of increased Bax and decreased Bcl-xL and pro-caspases 3 and 8, no cPARP and HSP70) were observed on protein expression after MH in both cell lines is an indication that the impact of the produced intracellular temperatures resulted in an immediate destruction of cells (cell necrosis). This is in agreement with our data from cell population analysis. The effect of EH is comparatively lower since cells do die in a retarded manner via the induction of metabolically programmed process of apoptosis, in particular at higher temperatures. Consequently, this means that MH is superior to EH in inactivating tumor cells. The in vivo situation, particularly the MH treatment, shows that the induction of necrosis or apoptosis depends on the degree of internalization of the nanoparticles after their application to the tumor area and the formation of intracellular heating spots capable of triggering cellular death as our in vitro results show. The fact that magnetic hyperthermia of tumors decreases cell viability (in terms of proliferation) has been emphasized by a decreased presence of cells expressing Ki67.29 Hence, these complex relationships indicate that the hyperthermia treatment modality, the heat dose, and the phenotype of the cancer cell line determine the regulation of molecular processes associated with cell death.
Interestingly, there was a tendency detectable for a higher sensitivity of PANC-1 compared to BxPC-3 to MH, but not for EH. This means that MH might well have a higher impact on cell-specific differences in metabolism compared to EH. In particular, the pattern of protein expression involved in apoptosis (Bax, Bcl-xL, pro-caspases 3 and 8, PARP; see below) was very different in both cell lines and depended upon whether MH or EH was applied. The same holds true for the expression of Ki-67, TOP2A, and TPX2. Different thermosensitivity could also be due to alterations in the expression of p53 and pRb as well as differential expression of HSP70 and HSP90.33–35 This means that the response of tumor cells to thermal stress is cell-line dependent as well as from the therapeutic strategy. From a clinical translation perspective, there will be a need to characterize the tumor entities for their therapeutic ability of MH. Our data show that the concept of precision medicine seems to be valid for nanotherapies too.
Exposure to MH treatment dramatically increased the formation of cellular ROS in both cell lines, whereas EH treatment showed almost no impact (up to a temperature of 43°C). Therefore, we can assume that the ROS-based response to hyperthermic temperatures does not depend on the cell line. ROS can severely damage cellular structures like DNA, proteins, lipids, and cofactors of enzymes due to oxidation and, therefore, induce apoptosis. Moreover, it is known that high levels of oxidative stress can destroy tumor cells or at least arrest tumor growth.36 In this context, the increased amount of ROS with increasing temperatures correlated well with the induction of apoptosis and the viability of the cells after either treatment.
Interestingly, the exposure of cells to bare MNP also led to an increased formation of ROS. One reason could be that the partial degradation of internalized MNP is known to take place in endolysosomes. Iron in its elemental state will then be exposed to the cell, which can participate in the Fenton reaction leading to the generation of ROS.37 Accordingly, ROS formation after MH is a consequence, at least in parts, of the presence and degradation of the MNP within the cells. It is conceivable that the MNP-associated ROS production acts as secondary stress factor during and after MH treatment.
Furthermore, MH led to a reduction in the mRNA expression of the proliferation markers Ki-67, TOP2A, and TPX2 compared to the non-treated cells. This indicates that a higher percentage of cells from the population corresponds to the resting (Go) phase of the cell cycle (Ki-67); moreover, DNA-transcription activity (TOP2A) and vessel invasion potential and metastasis (TPX2) are reduced. This observation underlines the effects of MH on the tumor cells. In contrast, EH mainly increased marker expression. This finding might be associated to hormesis, that is, the adaptive response of cells to the comparatively “moderate” heat stress induced by EH. It was shown that the molecular mechanisms involved in hormesis include the increased production of cytoprotective and restorative proteins and protein chaperones.38 In this view, one could also explain the expression of the antagonistic proteins regulating apoptosis (Bax and Bcl-xL) in response to EH (ie, at higher temperatures). In consequence, the impact of EH on the expression of the tested proliferation markers is less prominent than of MH.
The differential expression pattern of Ki-67, TOP2A, and TPX2 in both cell lines (considering MH vs EH) indicates that their short-term response pattern on the expression of the investigated proliferation markers seems not to be cell line specific, whereas the period of time after which the impact of hyperthermia is still detectable is a rather cell-specific response. Hence, in the view of a clinical translation, hyperthermia treatment of tumors derived of the same organ could result in a slightly increased or reduced effectiveness of the respective treatment due to the metabolic heterogeneity of the tumor cells. As a result, tumor cells of a same entity exhibit a different response in terms of proliferation marker expression with consideration of the heating modality, sensitivity against heat, and time after therapy.
The in vivo treatment of tumors via MH using a heating dose of 137±118 min CEM43T90 led to a significant (P≤0.05) reduction in tumor volume and inhibition in tumor growth compared to animals treated with AMF, MNP, or untreated ones. Earlier studies reported a significant inhibition of tumor growth and a prolonged survival of xenografts bearing murine pancreatic cancer cells (MPC-83) after treatment with MH using temperatures between 47°C and 51°C for up to 30 minutes.39 Despite the comparably much lower magnetic material dosing (0.2 mg Fe vs 49.6 mg Fe) used in the present study, the treatment of tumors with physiological amounts of MNP still leads to a marked impact on tumor growth.39
The reason as to why the presence of MNP per se led to a reduction in tumor volumes in comparison to the untreated controls is speculative. Our in vitro data showed that the MNPs were not cytotoxic to tumor cells. On the other hand, it may well be conceivable that the presence of MNP in the extracellular tumor compartments led to some impairment of nutrient supply that resulted in a retarded tumor cell proliferation.
MH treatment of PANC-1 xenografts led to a reduction in tumor volume. BxPC-3 xenografts treated in the same manner showed a regrowth in tumor volume starting at day 14 after the first MH treatment.29 These differences in tumor regrowth were due to a lower magnetic material dosing (0.1 mg Fe in a volume of 100 mm3) in BxPC-3 xenografts with a high interstitial fluid pressure within the tumors compared to PANC-1 xenografts (0.2 mg Fe in a volume of 100 mm3) as well as a higher proliferating potential of BxPC-3 cells as indicated by a tumor growth rate of 8.3%±3.4%/day of untreated animals compared to untreated PANC-1 xenografts (4.9%±4.0%/day). Furthermore, a lower thermal dose was applied to BxPC-3 xenografts (CEM43T90: 1±1) compared to PANC-1 xenografts (CEM43T90: 137±118). A clear tendency toward the inhibition of tumor growth caused by MH was visible. Treating BxPC-3 xenografts with a magnetic material dosing and heat dosages as high as those applied for PANC-1 xenografts very likely would have resulted in a complete inhibition of tumor relapse, at least during the observation period of 28 days. Nevertheless, as observed in the in vitro experiments, various metabolisms of cancer cells and individual tumors could slightly influence the outcome of hyperthermia treatment when using MH. With consideration of the aforementioned results, we also expect that the in vivo treatment of tumors by EH exposure would lead to a lower therapeutic outcome than MH. In addition, neither MNP nor AMF exposure led to significant anticancer effects on the tumor volume growth.
No damage to organs or interference with their functions was observed after in vivo treatment of tumors via MH. The fact that MH, MNP, or AMF exposure did not influence the blood count or the body weight of the xenograft models further proves the high biocompatibility of MH and the employed MNP. In consequence, our in vivo results rise up the evidence that the MH therapeutic modality is highly biocompatible.
Potential limitations of this study are that the observed effects of MH and EH are not based on in situ studies of isolated tumors but on pancreatic tumor cells in culture. Moreover, our data on proliferation markers are based on the mRNA expression levels. Due to post-translational regulations, the expression on the protein level could be different.
Conclusion
In conclusion, our data showed that MH treatment was superior in inducing thermal stress in comparison to EH. MH exposure triggers cell death more aggressively (increased cell necrosis) and is more efficient in producing ROS and in reducing the expression of the proliferation markers Ki-67, TOP2A, and TPX2 on mRNA level compared to EH. Additionally, the presence of MNP in tumor cells might secondarily promote the efficiency of MH due to their core degradation process that favors oxidative stress reactions (Fenton reactions). In most cases, there was an increased degree of complexity in the variability hyperthermia therapeutic outcome when considering the applied temperature, thermal sensitivity of the tumor cells, and temporal response after treatment. With consideration of the clinical situation, these findings outline the preference for the induction of intracellular temperatures via MH together with the necessity to identify those pancreatic tumor entities with high ability to respond to MH at defined temperatures, as demonstrated by the different sensitivity of the used cell lines. In this context, MH may also be subjected to the concept of precision medicine in order to optimize its therapeutic efficacy.
Acknowledgments
This work was supported by European Commission (MULTIFUN, number 262943), as well as in parts by the Spanish Ministry of Economy and Competitiveness (MAT2013-47395-C4-3-R) and Comunidad de Madrid (NANOFRONTMAG-CM, S2013/MIT-2850). We kindly thank H Dähring, MM Sanhaji, and M Stapf for scientific assistance and J Göring for technical assistance. IH and FJT acknowledge the European COST Action TD1402 (RADIOMAG). FJT acknowledges the financial support from Ramon y Cajal subprogram (RYC-2011-09617).
Disclosure
The authors report no conflict of interest in this work.
References
Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE. Cellular responses to combinations of hyperthermia and radiation. Radiology. 1977;123(2):463–474. | ||
Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg. 1957;146(4):596–606. | ||
Mackowiak PA, Wasserman SS, Levine MM. A critical-appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268(12):1578–1580. | ||
Guthkelch AN, Carter LP, Cassady JR, et al. Treatment of malignant brain tumors with focused ultrasound hyperthermia and radiation: results of a phase I trial. J Neurooncol. 1991;10(3):271–284. | ||
Wust P, Hildebrandt B, Sreenivasa G. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002;3(8):487–497. | ||
Soenen SJ, De Cuyper M. Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects. Nanomedicine (Lond). 2010;5(8):1261–1275. | ||
Kallumadil M, Tada M, Nakagawa T, et al. Suitability of commercial colloids for magnetic hyperthermia. J Magn Magn Mater. 2009;321(10):1509–1513. | ||
Ludwig R, Stapf M, Dutz S, Müller R, Teichgräber U, Hilger I. Structural properties of magnetic nanoparticles determine their heating behavior – an estimation of the in vivo heating potential. Nanoscale Res Lett. 2014;9(1):602. | ||
Périgo EA, Hemery G, Sandre O, et al. Fundamentals and advances in magnetic hyperthermia. Appl Phys Rev. 2015;2. | ||
Salas G, Camarero J, Cabrera D, et al. Modulation of magnetic heating via dipolar magnetic interactions in monodisperse and crystalline iron oxide nanoparticles. J Phys Chem C. 2014;118(34):19985–19994. | ||
Gratton SE, Ropp PA, Pohlhaus PD, et al. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105(33):11613–11618. | ||
Guardia P, Di Corato R, Lartigue L, et al. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano. 2012;6(4):3080–3091. | ||
Di Corato R, Espinosa A, Lartigue L, et al. Magnetic hyperthermia efficiency in the cellular environment for different nanoparticle designs. Biomaterials. 2014;35(24):6400–6411. | ||
Krawczyk PM, Eppink B, Essers J, et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci U S A. 2011;108(24):9851–9856. | ||
Hadley KC, Borrelli MJ, Lepock JR, et al. Multiphoton ANS fluorescence microscopy as an in vivo sensor for protein misfolding stress. Cell Stress Chaperones. 2011;16(5):549–561. | ||
Wang HY, Fu JC, Lee YC, Lu PJ. Hyperthermia stress activates heat shock protein expression via propyl isomerase 1 regulation with heat shock factor 1. Mol Cell Biol. 2013;33(24):4889–4899. | ||
Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43(1):33–56. | ||
Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998;431(2):285–286. | ||
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. | ||
Katschinski DM, Boos K, Schindler SG, Fandrey J. Pivotal role of reactive oxygen species as intracellular mediators of hyperthermia-induced apoptosis. J Biol Chem. 2000;275(28):21094–21098. | ||
Trieb K, Sztankay A, Amberger A, Lechner H, Grubeck-Loebenstein B. Hyperthermia inhibits proliferation and stimulates the expression of differentiation markers in cultured thyroid-carcinoma cells. Cancer Lett. 1994;87(1):65–71. | ||
Jordan A, Wust P, Scholz R, et al. Cellular uptake of magnetic fluid particles and their effects on human adenocarcinoma cells exposed to ac magnetic fields in vitro. Int J Hyperthermia. 1996;12(6):705–722. | ||
Rampersaud EN, Vujaskovic Z, Inman BA. Hyperthermia as a treatment for bladder cancer. Oncology (Williston Park). 2010;24(12):1149–1155. | ||
Hurwitz MD. Today’s thermal therapy: not your father’s hyperthermia: challenges and opportunities in application of hyperthermia for the 21st century cancer patient. Am J Clin Oncol. 2010;33(1):96–100. | ||
Connord V, Clerc P, Hallali N, et al. Real-time analysis of magnetic hyperthermia experiments on living cells under a confocal microscope. Small. 2015;11(20):2437–2445. | ||
Rabin Y. Is intracellular hyperthermia superior to extracellular hyperthermia in the thermal sense? Int J Hyperthermia. 2002;18(3):194–202. | ||
de Resende MF, Vieira S, Chinen LT, et al. Prognostication of prostate cancer based on TOP2A protein and gene assessment: TOP2A in prostate cancer. J Transl Med. 2013;11:36. | ||
Warner SL, Stephens BJ, Nwokenkwo S, et al. Validation of TPX2 as a potential therapeutic target in pancreatic cancer cells. Clin Cancer Res. 2009;15(21):6519–6528. | ||
Kossatz S, Ludwig R, Dähring H, et al. High therapeutic efficiency of magnetic hyperthermia in xenograft models achieved with moderate temperature dosages in the tumor area. Pharm Res. 2014;31(12):3274–3288. | ||
Sapareto SA, Dewey WC. Thermal dose determination in cancer-therapy. Int J Radiat Oncol Biol Phys. 1984;10(6):787–800. | ||
Brizova H, Kalinova M, Krskova L, Mrhalova M, Kodet R. A novel quantitative PCR of proliferation markers (Ki-67, topoisomerase IIalpha, and TPX2): an immunohistochemical correlation, testing, and optimizing for mantle cell lymphoma. Virchows Arch. 2010;456(6):671–679. | ||
Gordon RT, Hines JR, Gordon D. Intracellular hyperthermia: a biophysical approach to cancer-treatment via intracellular temperature and biophysical alterations. Med Hypotheses. 1979;5(1):83–102. | ||
Li GC, Mak JY. Induction of heat shock protein synthesis in murine tumors during the development of thermotolerance. Cancer Res. 1985;45(8):3816–3824. | ||
van Bree C, van der Maat B, Ceha HM, Franken NA, Haveman J, Bakker PJ. Inactivation of p53 and of pRb protects human colorectal carcinoma cells against hyperthermia-induced cytotoxicity and apoptosis. J Cancer Res Clin Oncol. 1999;125(10):549–555. | ||
Walsh N, Larkin A, Swan N, et al. RNAi knockdown of Hop (Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down regulation. Cancer Lett. 2011;306(2):180–189. | ||
Fang J, Seki T, Maeda H. Therapeutic strategies by modulating oxygen stress in cancer and inflammation. Adv Drug Deliv Rev. 2009;61(4):290–302. | ||
Foy SP, Labhasetwar V. Oh the irony: iron as a cancer cause or cure? Biomaterials. 2011;32(35):9155–9158. | ||
Mattson MP. Hormesis defined. Aging Res Rev. 2008;7(1):1–7. | ||
Wang L, Dong J, Ouyang W, Wang X, Tang J. Anticancer effect and feasibility study of hyperthermia treatment of pancreatic cancer using magnetic nanoparticles. Oncol Rep. 2012;27(3):719–726. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.