Back to Journals » International Journal of Nanomedicine » Volume 14
Nanoformulations of natural products for management of metabolic syndrome
Authors Taghipour YD , Hajialyani M , Naseri R, Hesari M , Mohammadi P , Stefanucci A, Mollica A , Farzaei MH , Abdollahi M
Received 30 April 2019
Accepted for publication 25 June 2019
Published 16 July 2019 Volume 2019:14 Pages 5303—5321
DOI https://doi.org/10.2147/IJN.S213831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Yasamin Davatgaran Taghipour,1–3 Marziyeh Hajialyani,4 Rozita Naseri,5 Mahvash Hesari,6 Pantea Mohammadi,6 Azzurra Stefanucci,7 Adriano Mollica,7 Mohammad Hosein Farzaei,4,6 Mohammad Abdollahi8
1Department of Medical Nanotechnology, School of Advanced Medical Sciences, Tabriz University of Medical Sciences, Tabriz, Iran; 2Phytopharmacology Interest Group (PPIG), Universal Scientific Education and Research Network (USERN), Tehran, Iran; 3Student Research Committee, Tabriz University of Medical Sciences, Tabriz, Iran; 4Pharmaceutical Sciences Research Center, Health Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran; 5Internal Medicine Department, Faculty of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran; 6Medical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran; 7Department of Pharmacy, G. d’Annunzio University of Chieti-pescara, Chieti 66100, Italy; 8Toxicology and Diseases Group, the Institute of Pharmaceutical Sciences (TIPS) and Faculty of Pharmacy, Tehran University of Medical Sciences (TUMS), Tehran, Iran
Abstract: Metabolic syndrome is a common metabolic disorder which has become a public health challenge worldwide. There has been growing interest in medications including natural products as complementary or alternative choices for common chemical therapeutics regarding their limited side effects and ease of access. Nanosizing these compounds may help to increase their solubility, bioavailability, and promisingly enhance their efficacy. This study, for the first time, provides a comprehensive overview of the application of natural-products-based nanoformulations in the management of metabolic syndrome. Different phytochemicals including curcumin, berberine, Capsicum oleoresin, naringenin, emodin, gymnemic acid, resveratrol, quercetin, scutellarin, stevioside, silybin, baicalin, and others have been nanosized hitherto, and their nanosizing method and effect in treatment and alleviating metabolic syndrome have been reviewed and discussed in this study. It has been discovered that there are several pathways or molecular targets relevant to metabolic disorders which are affected by these compounds. Various natural-based nanoformulations have shown promising effect in treatment of metabolic syndrome, and therefore can be considered as future candidates instead of or in conjunction with pharmaceutical drugs if they pass clinical trials successfully.
Keywords: nanoformulation, natural products, metabolic syndrome, diabetes
Introduction
Metabolic syndrome (MetS) is a health disorder that includes a cluster of medical conditions occurring together. MetS increases the risk of progressing atherosclerotic cardiovascular diseases (CVDs), central obesity, insulin resistance, systemic hypertension, cerebrovascular accident, and atherogenic dyslipidemia.1,2 Diverse definition has been suggested by the International Diabetes Federation, National Cholesterol Education Program’s Adult Treatment Panel III, and the WHO. All mentioned international agencies concentrate on five medical conditions as a diagnosis guideline which include waist circumference more than 35 inches in women and 40 inches in men, enhanced fasting glucose of l00 mg/dL or greater, increased triglycerides (TGs) 150 mg/dL or greater, declined high-density lipoprotein cholesterol (HDL) less than 50 mg/dL in women and less than 40 mg/dL in men, and blood pressure values of systolic 130 mmHg or higher and/or diastolic 85 mmHg or higher.3 Chronic inflammation has been associated with insulin resistance and visceral obesity, which results in creation of abnormal adipocytokines including IL-1, IL-6, TNF-α, adiponectin, and leptin.4 In the case of MetS, lifestyle modification should be included along with pharmacological treatment for those who have high levels of risk factors. An ordinary treatment guideline for MetS consists of prescribing drugs to lower the blood glucose level, TGs, and blood pressure along with improvement of the patient’s lifestyle. Treatment with common medications exerts unpleasant adverse effects such as flatulence and other related side effects in the early stage of treatment.5 This high level of adverse effects leads to a weak tolerance of the patient especially in the long-term application of the medications. In order to decrease side effects of these medications and to improve the efficiency of drug delivery for treatment of MetS, nanoformulation of natural and synthetic agents is an option. Nanosized drug carriers, consisting of phytochemicals from conventional medicines endowed with developed pharmacodynamics and pharmacokinetic characteristics, are a novel therapeutic approach. The nano-vehicles provide unique properties including enhanced bioavailability and solubility of the drug, diminished systemic adverse effects, elongated circulation time, and privileged accumulation in the precise target organ.6 Therefore, different phytochemicals including curcumin, berberine (BBR), oleoresin capsicum, naringenin, emodin, gymnemic acid, resveratrol, quercetin (QUE), scutellarin, stevioside, silybin, baicalin, and others have been used in diverse strategies for treatment and alleviation of MetS. These phytochemicals have been incorporated into diverse nanoformulation structures including polymeric-based polyesters, polyanhydride, poly (alkyl cyanoacrylate), etc, and natural ones (including cellulose, collagen, albumin, chitosan, dextran, etc). Lipid-based drug delivery systems such as micelles, liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers, and other related nanoformulations have also been included.7 Each polymeric and lipid nanostructure has precise synthesis and drug loading methods and have been described in this review.
Pathophysiology of MetS
Increasing knowledge about the exact pathophysiology of MetS is very necessary to succeed in the design of suitable medicines and pharmaceutical forms.8 Among different cellular pathways suggested, insulin resistance and excess fatty acid are the most widely accepted ones.9,10 Insulin promotes glucose uptake in the main target tissues of the liver, skeletal muscle, and adipocytes via glycoprotein receptors and inhibiting lipolysis and hepatic gluconeogenesis.11,12 Activation of the insulin receptor initiates a cascade of phosphorylation events using substrates which comprise insulin receptor substrates (IRS-1–IRS-4) as well as JAK-2, which are docking proteins that activate numerous signaling cascades.13,14 The pathways for this activating process are PI3K/PKB, known as Akt signaling. This process contributes to various metabolic activities of insulin and the MAPK signaling cascade that controls the regular function and growth of cells.2,13,14 Insulin-dependent glucose cellular uptake is activated by migration of GLUT4 from an intracellular compartment to the plasma membrane. GLUT4 is highly expressed in skeletal muscles and adipose tissues. Glucose is then phosphorylated as the substrate for glycogen synthesis or can be metabolized to adenosine triphosphate.10,15–18 Frequent stroke and heart failure propose a probable relation between this pathophysiological condition and CVDs. Changes in the levels of insulin signaling proteins like Glut4, IR-β, PI3K, IRS-1, PGC-1α, and Akt, are involved in blocking insulin-mediated glucose uptake along with other insulin functions in the cardiomyocytes.19
Insulin resistance is the most widely accepted hypothesis for underlying pathophysiology of MetS. Free fatty acids (FFAs) inhibit protein kinase activity and reduce glucose uptake in the muscle, but in the liver – with increment of protein kinase activity – result in production of atherogenic factors such as glucose, TGs, and low-density lipoprotein (LDL). In response to hyperglycemia, beta cells release more insulin to maintain euglycemia which results in hyperinsulinemia. Ultimately, failure in compensation and consequently reduction of insulin secretion occurs. FFAs can induce lipotoxicity in pancreatic beta cells, causing reduction of insulin secretion.20,21 Reduced responsiveness to normal insulin level causes type 2 diabetes and contributes to the development of hypertension because of disturbance in the vasodilator effect of insulin.2,20
Dyslipidemia
Dyslipidemia refers to unhealthy levels of one or more kinds of lipids in blood, increased levels of apo B, TGs, LDL, with reduced level of HDL, resulting from several abnormalities in structure, metabolism, and biology of atherogenic and antiatherogenic lipoproteins.22 Dyslipidemia is often due to diet and lifestyle, but prolonged elevation of insulin level can also lead to atherogenic dyslipidemia via different pathways. Firstly, insulin inhibits lipolysis, thus this disturbance in insulin signaling enhances lipolysis which can cause production of FFAs. In the hepatocytes, FFAs act as a substrate for the synthesis of TGs resulting in enhancement of very-low-density lipoprotein (VLDL) production.23 Next, insulin participates in apoB degradation and lipoprotein lipase activity, so in this case, hypertriglyceridemia could be attributed to increase in VLDL production and reduction of its clearance.23 CETP collects TGs from VLDL or LDL and exchanges them for cholesteryl esters which leads to TG-enriched HDL. Hepatic lipase can rapidly clear HDL particles rich in TGs and remove them from the circulation.23
Hypertension
Obesity is the main factor for insulin resistance with consequent hyperinsulinemia. Type 2 diabetes and a genetic predisposition with a family history of hypertension are other important risk factors for hyperinsulinemia.24 There are accumulating data indicating angiotensin II's inhibitory effect on insulin action. In fact, in vascular muscle tissues, angiotensin II interferes with insulin signaling through PI3K and its downstream Akt signaling pathway.25 This angiotensin II inhibitory effect is performed via stimulation of RhoA activity. Increased levels of RhoA activity and ROS inhibit PI3K/Akt signaling followed by a decrease in production of NO within endothelial cells and consequently increased vasoconstriction.25 Activated renin-angiotensin system may contribute to development of hypertension in MetS.26 On the other hand, in obese individuals, a probable shift from PI3K to the MAPK pathway can cause pro-atherogenic function.27,28 Hyperinsulinemia also increases re-absorption of sodium in the kidneys through activation of sympathetic nervous system, improving the cardiac output and vasoconstriction of arteries, which will all lead to hypertension.20,29
Inflammatory and oxidative mediators
Adipose tissue is a dynamic endocrine organ able to release a number of bioactive peptides including adiponectin, IL-6 (by increasing CRP production), resistin, RBP4, leptin, and TNF-α.30,31 Dysregulation of adipose tissue activity has been implicated in over-secretion of deleterious adipokines and hypo-secretion of beneficial ones.30 Leptin has a major role in the regulation of metabolism of the entire body by stimulating energy expenditure, preventing food intake, and restoring euglycemia. However, in the majority of obesity cases, resistance of leptin limits its biological efficacy. In contrast to leptin, secretion of adiponectin is often decreased in obesity. Adiponectin is directly associated with an increase of insulin sensitivity, enhancement of fatty acid oxidation, and decrease in the production of glucose by the liver.32
Natural compounds and medicinal plants for treatment of MetS
It has been shown that natural products or their derivatives are a valuable source of therapeutic agents.33 Consistent with this approach, researchers have focused on natural products in the field of prevention or treatment of MetS.34–36 MetS increases the risk of both atherosclerotic and non-atherosclerotic CVD, one of the leading causes of death in the world.37,38 Results from a meta-analysis showed that MetS increases the risk of CVD outcomes and mortality.39 Considering the morbidity and mortality caused by MetS and cardiometabolic disorders, there is a high interest in the discovery of novel compounds and pharmacological targets that might be effective in the prevention or treatment of metabolic and/or cardiovascular disorders. Many medicinal plants and natural products are considered by the public as a safe and natural alternative to synthetic drugs, of course without definite proof from randomized controlled clinical trials.40 Therefore, there is an increased interest in development of products with validated efficacy and safety.
Major pharmacological targets of natural compounds involved in prevention of MetS
Different molecular mechanisms have been reported for therapeutic activity of natural products against cardiovascular disorders and Mets. DPP-4, COX-1 and -2, AMPK, PTP1B, transcription factors NF-κB, Nrf2, PPARs, eNOS, and 5-LO are involved in anti-inflammatory activity, amelioration of blood lipid profiles, normalization of blood glucose levels, improvement of insulin sensitivity and transcriptional regulation of genes controlling lipid metabolism.41–46 Recently, PPARs and some other nuclear receptors have been under focus of scientists due to their regulatory roles in homeostasis of glucose, metabolism of lipids and lipoproteins, cellular proliferation, cellular differentiation, and cellular apoptosis.38,47 PPARs are a group of steroid/thyroid nuclear receptor superfamilies of ligand-activated transcription factors.48 PPAR subfamily includes alpha-, beta-, and gamma-subtypes which are activators of key metabolic pathways and highly expressed in tissues relevant to energy homeostasis. PPARs also possess a prominent role in differentiation of adipocyte and insulin sensitivity.49,50 PPAR-α involves fatty acid catabolism whereas PPAR-β affects homeostasis of glucose, storage of lipids, and differentiation of adipocytes. The exact function of PPAR-β is less known hitherto.51,52
There is a progressive trend in identifying potent, biologically active agents for modulating nuclear receptors such as PPARs, LXRs, and FXRs. The most active PPAR agonists and combination agonists have been examined for treatment of type 2 diabetes and MetS.53,54 For example, Bitter Chinese tree Pseudolarix kaempferi, Gordon and Korean ginseng, Panax ginseng are two plants that possess PPAR-α modulatory activity.55,56 Pseudolaric acid B isolated from the bark of P. kaempferi is an efficient compound that induces maximum luciferase response with activation of PPAR-α in H4IIEC3 cells. In the case of ginseng, ginsenosides have been shown to have an inhibitory effect on PPAR-α, and thus have hypolipidaemic effects.55,57,58
Many PPAR-γ modulators have been detected such as Saurufura A, an acyclic furanoditerpene constitute of Saururus chinensis (Saururaceae), and Genistein, a soy phyto-oestrogen compound, as PPAR-γ activators.59
Other herbal-derived transcriptional modulators are LXR, and FXR modulators.54,60 It has been reported that paxilline, secondary metabolite of Penicillium paxilli, activates LXRs.61
FXR (NR1H4), known as BAR, is a nuclear receptor involved in regulation of genes in synthesis of bile acid, metabolism of lipoprotein, transport, and absorption.62 An ethyl acetate extract of the gum resin of Commiphoramukul decreased the level of LDL cholesterol and TGs in humans with an inhibitory effect on NR1H4.63
Natural product-based nanoformulations for MetS
Here, we refer to the most relevant nanoformulation of natural products which can be applied in prevention or alleviation of MetS. The advantages of nanoformulations vs conventional formulations are their greater surface area to volume ratio, targeted drug delivery, sustained and controlled drug release, and modified bioavailability. The disadvantages of nanoformulations compared with conventional formulations are short shelf-life, unforeseeable stability, pharmacokinetics, toxicity, and also being more expensive.64 Therefore, nanoformulation methods and nanonization are offered as promising substitutions for conventional drug delivery systems. A brief report of the outcomes of administration of these natural-based nanodrugs in different models of MetS has been prepared in Table 1.
   |
Table 1 Phytochemical-based nanoformulations for management of metabolic syndrome (MetS) |
Curcumin
Curcumin, a derivative of turmeric (Curcuma longa), belongs to the curcuminoid subgroup of polyphenols with a panel of pharmacological activities such as antitumor, antioxidant, anti-inflammatory, hypolipidemic, and anticarcinogenic effects.64,66 Many researchers have demonstrated that curcumin possesses therapeutic effects regarding MetS. Curcumin has been reported to suppress PAI-1 expression, activate Nrf2, downregulate TNF-α expression, and suppress NF-κB activation. In addition, it inhibits the obesity-related Wnt/β-catenin pathway, and activates PPAR-β in hepatic stellate cells.67–70 Curcumin also interrupts leptin signaling, thus increasing adiponectin expression. Curcumin negatively affects obesity and positively affects insulin sensitization, blunt the inflammatory pathways in MetS.20 It improves obesity-associated metabolic disorders such as hyperglycemia, hyperlipidemia, and insulin resistance. It also prevents LDL cholesterol oxidation and reduces body weight gain.71 Clinical trials which have been performed for curcumin support its positive effects as an adjuvant therapy of type 2 diabetes.1,72,73 The poor water solubility of curcumin is a problem that should be resolved.74,75 Several strategies have been designed to cope with this problem, mostly including formulating this phytochemical in a nanosized structure.76 Encapsulation of curcumin in multipolymer poly (gamma-benzyl l-glutamate)-poly (ethylene glycol)-poly (gammabenzyl l-glutamate) nanoparticles (NPs) was a promising way to improve its bioactivity and water solubility. Such nanoformulation exerted a potent effect on relieving diabetic cardiomyopathy (DCM). DCM alters the systolic and/or diastolic cardiac functions which results in heart failure and myocardial ischemia. Curcumin encapsulated in multipolymer affects DCM and cross-regulates the receptors responsible for sensing calcium and endogenous CSE/hydrogen sulfide (H2S). Continuous administration of this nanoformulation relieved pathological morphological destruction of myocardium, boosted serum levels of H2S and ((Ca2+))i content in myocardial cells, and upregulated the expression of CSE, CaSR, and CaM. The presence of curcumin in the structure of the nanoformulation can be the possible reason for such advantageous effects.77
Self-emulsifying drug delivery system (SEDDS) has become important as a new approach to modify the dissolution, oral absorption, and solubility of drugs that are poorly soluble in water. SNEDDS is a branch of SEDDS which contains an isotropic mixture of drug substance, surfactant, co-surfactant, and oil, resulting in the formation of a nanoemulsion just after consumption in the gastrointestinal tract. SNEDDS based on a curcumin formulation has been developed for alleviating diabetic neuropathy and found to be an efficient tool for relieving neuroinflammation, and modifying antioxidant defense in diabetic neuropathy.78 In another study, curcumin-encapsulated NPs in Poly (lactic-co-glycolic acid)-Polyvinyl alcohol polymers were synthesized and it was studied in the streptozotocin (STZ)-induced diabetes cataract model. The average particle size was 282±5.72 nm with polydispersity index of 0.14±0.06 and nearly 56% curcumin encapsulation. The results demonstrated that curcumin-loaded Poly (lactic-co-glycolic acid) (PLGA) NPs increased efficiency and oral bioavailability in in vivo conditions.79 In another study, curcumin-loaded pluronic nanomicelles were synthesized and characterized for the treatment of diabetes. Pluronics are triblock copolymers made up from a central block which is made of poly (propylene oxide) and two poly (ethylene oxide) (PEO) chains with the general structure of HO-(C2H4O)a(C3H6O)b(C2H4O)a-H. Pluronics have pharmaceutical applications such as emulsifiers, wetting agents, surfactants, and solubilizing agents. Of course, the hydrophilic-lipophilic balance of pluronics may change with the alteration in the numbers of PPO and PEO blocks. Curcumin NPs with a size of 333±6 nm were prepared. Curcumin-loaded pluronic nanomicelles were synthesized via nanoprecipitation method with pluronics. The in vivo experiment was done in an STZ-induced diabetic model. The developed curcumin NPs had antidiabetic action because of the remarkable upregulation of both Pdx-1 and NKx6.1 genes' expression, which are significant transcription factors in insulin gene expression. This formulation was orally delivered and increased the bioavailability of the drug.80 In another study, PLGA-based NPs encapsulating curcumin and CoQ10 were developed and orally delivered to enhance therapeutic efficacy and oral bioavailability of treatment of diabetic adverse effects. This antioxidant formulation was tested in STZ-induced diabetic rats. The results demonstrated that consumption of these NPs led to a remarkable reduction of CRP, IL-6, total cholesterol, plasma TGs, and an increase in HDL cholesterol. The CoQ10 and curcumin-loaded PLGA NPs were prepared via emulsion-diffusion-evaporation technique. The particle Z-average sizes of curcumin-encapsulated NPs and CoQ10 NPs were 237±6 nm and 115±12, respectively.81 In another study, a curcumin nanoemulsion was prepared as an antihypercholestrolemic and antihypertensive agent. The potential of curcumin against hypertension was analyzed by measurement of ACE inhibition in an in vitro study. Hippuryl-L-histidyl-L-leucine is a substrate for ACE. The enzymatic process results in the generation of hippuric acid. They demonstrated to what extent curcumin is involved in ACE inhibition by measuring the quantity of hippuric acid production. The antihypercholesterolemic capability was analyzed via HMG-CoA reductase assay. The curcumin nanoemulsion was synthesized using Rasaputri method. The average diameter of curcumin nanoemulsion droplets was 42.93±29.85 nm and the polydispersity index was low and had a value of 0.36±0.04 which was uniform in size. The application of the curcumin nanoemulsion was able to inhibit HMGR along with ACE and also improved curcumin solubility in the formulation.82 In another study, curcumin-encapsulated NPs were used for treatment of diabetic peripheral neuropathy. Diabetic peripheral neuropathy is a significant and usual consequence of type 2 diabetes that leads to neuropathy. Curcumin-encapsulated NPs were used to relieve diabetic neuropathic pain via P2Y12 receptor on satellite glial cells in the dorsal root ganglia of rat. The NPs diminished the level of Cx43 mRNA in rats, which was the reason for mechanical and thermal hyperalgesia in rats. Also, the level of IL-1β and phosphorylated-AKt decreased in the dorsal root ganglia of diabetic rats after administration of NPs. Furthermore, curcumin NPs reduced the P2Y12 receptor on satellite glial cells in the dorsal root ganglia and thermal/mechanical hyperalgesia in diabetic rats.83
For wound healing purposes, one promising method to increase the bioavailability and efficacy of curcumin is the use of hydrogels. Thermos-sensitive hydrogel in the structure of gelatin microspheres containing curcumin was developed for the treatment of diabetic wounds. Curcumin NPs were enclosed in gelatin microspheres to respond to MMPs which are commonly over-expressed at diabetic wound sides. The thermo-responsive hydrogel containing curcumin NPs loaded in gelatin microspheres was used as a local delivery system in the wound site to investigate its capacity for drug release. For temperature responsiveness of hydrogels, pluronic F127 (a type of macromolecular non-ionic surfactant) was applied. F127 demonstrated temperature-dependent gel-sol and sol-gel transition. The synthesized formulation was administered to the genetically and chemically-induced diabetic rats via STZ injection. Thermo-sensitive hydrogel containing curcumin in the form of gelatin microspheres seems an ideal choice as a skin drug delivery system, not only in therapeutics, but also for pharmaceutical and cosmetic products.84 Various strategies have been developed to overcome the major barriers to the clinical translational use of curcumin. For example, nanoformulation of curcumin has shown therapeutic benefits rather than free curcumin85,86 in the management of cancer, cardiovascular, and neurodegenerative diseases. Clinical trials have shown that curcumin nanoformulations improve bioavailability of curcumin, providing a strong rationale for future medical applications after clarification of mechanistic perspectives.
Capsicum oleoresin
Oleoresin capsicum has been identified as an organic solvent extract from dried, ripe red pepper, a fruit of the Capsicum plant.87 OC has been extensively used as an additive in the food industry for taste modification and preservation of food. The butanolic and ethanolic extracts of capsicum extracts possess diverse biological benefits including antioxidant, antiobesity, anti-inflammatory, and anticancer effects.88,89 Administration of a nanoemulsion obtained from OC in obese rats decreased adipogenic gene expression and increased expression of PPAR-α, UCP2, and CPT-1α, which participate in thermogenesis and β-oxidation. Administration of this nanoformulation drastically diminished the final body weight and reduced the level of adipose tissue mass in the obese rats which received a high-fat regimen. The antiobesity effect of the nanoemulsion is due to its potential in activation of the AMPK pathway. Activation of AMPK in the rats supplemented with OC nanoemulsion was more dominant than in rats supplemented with OC. The activity of GPDH was also more restricted. However, OC caused no significant effect on the high-fat diet rats, and nanosizing of this extract was the main reason for this high antiobesity activity.90 Alginate double-layer nanoemulsion loaded with OC and single layer nanoemulsion at concentrations of 100 and 1,000 ng/mL diminished intracellular lipid content in 3T3-L1 adipocyte cells. Furthermore, the amount of FFAs and glycerol which were released into the medium increased in the cells treated with 1,000 ng/mL. Levels of mRNA of adipogenic genes including PPAR-γ and aP2, and lipolytic genes including HSL and CPT-1α, are regulated in a concentration-dependent manner. Thus, this type of nanostructured OC was found to have higher lipolytic activity, which may be an option for treatment of obesity.91
Berberine
Berberine (BBR) is an essential benzylisoquinoline alkaloid from Coptis chinensis.92 BBR has been employed for treatment of intestinal infections, congestive heart failure, hypertension, cardiac arrhythmia, cancer, hyperlipidemia, and diabetes.93,94 Studies have shown that the use of BBR in insulin-resistant animals improves TG levels, body weight, and insulin sensitivity. In fact, BBR upregulates the genes which participate in energy utilization and downregulates the genes which participate in lipogenesis.95 Its insulin-sensitizing action is similar to that of thiazolidinediones as well as metformin.96 BBR has been reported to reduce waist circumference, TG levels, and systolic blood pressure. These alterations were found to be more prominent in women suffering from MetS.97 Nanoformulated forms of BBR has shown better bioavailability. SLNs containing BBR in comparison to BBR alone have shown higher bioavailability. Oral administration of NPs (BBR-SLN) and bulk formulation of BBR remarkably reduced body weight gain, homeostasis evaluation of insulin resistance, and fasting blood glucose level.98 O-hexadecyl-dextran encapsulated BBR NPs were prepared and their cytoprotective effects on high glucose stress-induced apoptosis were evaluated in rat primary hepatocytes. Hepatocytes treated with this nanoformulation in high glucose levels of 40 mM, enhanced cells' viability in comparison to bulk BBR treated cells. The results showed a decrease in ROS production, caspase activation, oxidative stress, and prohibition of depolarization of mitochondria in cells treated with NPs.99 Oral administration of the nanoformulation prepared by PLGA-PEG-PLGA block copolymers containing BBR chloride effectively modulated PCSK-9 mRNA for treatment of high LDL cholesterol.100
Naringenin
Naringenin (5,7,4’-trihydroxyflavanone) is part of the class of flavonoids called flavanones which exist in vegetables and citrus fruits like grapefruit and oranges. It has shown antimutagenic, anti-inflammatory, antihyperglycemic, and antioxidant activities.101 Naringenin is an option for treatment of cancer, CVD, and osteoporosis. Also, it reduces levels of lipids and insulin-like characteristics. Regarding the mechanism of action of naringenin, it has been shown to absorb glucose from the intestine of diabetic rats.102 Naringenin treatment of diabetic mice showed a drastic increase in the immunological and hematological parameters of blood along with 100% survival in diabetic mice. Vasorelaxant impact of naringenin was shown in CVD and hypertension.103 The main problem of naringenin could be attributed to the fact that it is poorly dissolved in water and poorly absorbed in the intestine after oral administration due to the quick elimination by diverse enzymes located in the gut and liver.104 Therefore, for better targeting of the colon and modified absorption, nanosizing this flavanone and/or loading it onto NPs, or encapsulating it in a nanoformulation can be considered as practical methods. Core shell NPs (chitosan/alginate) impregnated with naringenin demonstrated no toxicity, with remarkable antidiabetic effects after oral administration in STZ-induced diabetic rats. This polymeric nanoformulation was a biodegradable and biocompatible vehicle for oral transfer of naringenin or other medicaments.105
Quercetin
Quercetin (QUE), a flavonoid found in various foods such as onions, citrus fruits, apples, and tea, has antioxidant and anti-inflammatory activities.106 QUE acts via mitochondrial pathways involved in adipokinesis and lipolysis.107 It has been reported that administration of QUE decreases blood pressure, insulin resistance, and cholesterol.108 QUE in combination with other polyphenols such as luteolin and apigenin can increase insulin secretion and resistance to cytotoxicity induced by internal cytokines, decreasing activation of NO synthase and NF-κB.41,109–111 QUE has been shown to improve metabolic parameters including postprandial blood glucose, waist circumference, and lipids.112
In a study, poly(ethylene glycol)-block-(poly(ethylenediaminel-glutamate)-graft-poly(ε-benzyloxycarbonyl-l-lysine)) was prepared and its in vitro and in vivo abilities in the form of nanosized complex with QUE in diabetic nephropathy was examined. The results of blood samples and left kidney analysis demonstrated modified renal function, alleviated renal oxidative stress harm, and downregulated ICAM-1 expression. Also, QUE NPs complex remarkably alleviated the mentioned effects.113 A pH sensitive formulation comprising chitosan-alginate core-shell NPs loaded with succinylated-QUE NPs demonstrated no in vivo toxicity as biocompatible and biodegradable carriers for oral administration of QUE.114 QUE (20 mg/kg) in combination with other antioxidants including rutin (20 mg/kg) and resveratrol (100 mg/kg), and QUE nanoemulsion (20 mg/kg) demonstrated positive outcomes in decreasing inflammation, pain, and apoptosis exploited by oxaliplatin, and prevented oxaliplatin-induced neuro- and hepato-toxicity in mice.115
Similar to other compounds, nanoformulations of QUE possess a higher bioavailability in comparison with formulations in larger scales. Loading QUE on PLGA via emulsion-diffusion-evaporation technique was one of the attempts to increase the bioavailability of QUE and decrease the dosage required for treatment. The oral bioavailability of this nanoformulation in rats was more than 52.3% and it caused a required drug dose because of nanosizing QUE. These results clearly highlighted the importance of oral administration of QUE NPs in a reduced dose for treatment of diabetes.116 In another study, QUE nanorods (15.4 nm) were synthesized and their effect was tested on alloxan-induced diabetic rats. Administration of this type of NPs decreased the level of fasting blood glucose in diabetic mice. QUE decreased generation of lipid peroxidation products. The activity of hexokinase, as a primary enzyme in glycolysis pathway, was diminished in diabetic samples including kidney, pancreas, and liver. The activity of G6Pase was remarkably diminished upon using QUE nanorods. Also, the activity of FBPase, an enzyme of gluconeogenesis pathway, was decreased with the application of nanorods. This nanoformulation enhanced the level of antioxidant enzyme SOD, GSH, and –thiol groups, and diminished protein oxidation and lipid peroxidation in diabetic mice. Furthermore, the kidney and liver functional markers including ALP, ALT, and AST were decreased. The rod structure was an efficient structure for treatment of diabetes, but further studies in the field are still required.117 PLGA-loaded QUE NPs with a particle size of 180 nm increased the bioavailability in STZ-induced diabetic rat model. This gives hope for the development of different QUE-based nanocarriers to improve oral delivery and for future medicine with lower carrier toxicities for diabetic patients.106
Emodin
Emodin (3-methyl-1,6,8- trihydroxyanthraquinone) is an anthra-quinone derivative, which can be found in Radix et rhizoma rhei.118 Emodin possesses anti-inflammatory, anti-nociceptive, and anticancer activities.119 Emodin has PPAR-γ-activating impacts. Intraperitoneal injections of this compound alleviated the symptoms of diabetes via regulating PPAR-γ. Emodin increased binding affinity in differentiated 3T3-L1 adipocytes via induction of enhanced glucose uptake and enhanced GLUT1 and GLUT4 mRNA expression. Furthermore, emodin is a novel activator of AMPK and had positive impact on glucose metabolism in 3T3-L1 adipocytes through enhancement of glycolysis.119 Nanomacromolecule encapsulating emodin was used for treatment of diabetic neuropathic pain. This nanoformulation had the effect on P2X3 receptor located on the dorsal root ganglia. Mechanical withdrawal threshold and thermal withdrawal latency in rats receiving emodin NPs were greater in comparison with those in diabetic rats. This nanoformulation diminished upregulation of TNF-α protein, P2X3 receptor, and the phosphorylation of ERK1/2 in the dorsal root ganglia of rats suffering from type 2 diabetes. Emodin-loaded nanomacromolecules could alleviate neuropathy via prohibiting excitatory transmission induced by P2X3 receptor in dorsal root ganglia neurons.120 Another nanoformulation called nano emodin transferosome (NET) was prepared, and mRNA expression of ATGL and G0S2 in adipose tissue of obese rats were investigated. NET could increase ATGL protein expression to apply its weight-reduction impact and decreased G0S2 protein expression in the adipose tissue of obese rats. NET diminished body weight and adipocyte size.121 Emodin has shown high first pass metabolism and great hydrophobicity associated with a limited bioavailability after oral administration. Hence, nanoemulsions and nanotransfers have been effective in better delivery and efficiency of emodin.106
Gymnemic acid
Gymnemic acid (C43H68O14) is a triterpenoid phytoconstituent of Gymnema sylvestre, possessing a strong antidiabetic effect.122 Gymnemic acid has presented diverse physiological activities such as suppressing taste sensitivity to sweetness, preventing intestinal absorption of glucose, and decreasing the level of glucose and insulin in plasma of diabetic patients.123 The poor water solubility of this phytochemical decreases its pharmacological effects.123 Thus, a modified approach is needed to enhance the bioavailability and solubility of gymnemic acid. In a study, a nanosuspension of gymnemic acid was prepared and the oral nanoformulation tested on diabetic rats. The nanoformulation demonstrated meaningful antihyperglycemic activity and generated hypoglycemia. Gymnemic acid nanoformulations could reduce blood glucose levels in diabetic rats after 3 hours. It seems that gymnemic acid nanoformulation can be an efficient option for treatment of diabetes.124
Baicalin
Baicalin (5,6-dihydroxy-2-phenyl-4H-1-benzopyran-4-one-7-O-
Scutellarin
Scutellarin (4′,5,6-trihydroxyflavone-7-O-glucuronide), one of the most active compounds of the traditional Chinese herb Erigeron breviscapus (Vant.) Hand. Mazz, has been frequently used against vascular endothelial cell dysfunction; it exerts its effect via many pathways. Experimental research and clinical observation demonstrated that scutellarin possesses a powerful effect against neovascularization and enhances vascular permeability via diminishing viscosity of blood flow and improving microcirculation.129,131 A nanoformulation based on scutellarin loaded onto amphiphilic chitosan derivatives was prepared to increase its bioavailability and efficacy for treatment of diabetic retinopathy. The synthesized particles demonstrated great capacity to be transferred from Caco-2 cells. Results indicated that administration of scutellarin diminishes retinal damage in diabetic rats. Treatment with the nanoformulation was more efficient than the scutellarin alone. Scutellarin resulted in downregulated expression of VEGFR2, VEGF, and vWF in the retina of diabetic rats. The scutellarin nanostructure enhanced the bioavailability of scutellarin and significantly improved diabetic retinopathy.132
Resveratrol
Resveratrol (3,5,4-trihydroxylstilbene) is a polyphenol found in grapes and nuts that has extensive pharmacological activities including anti-inflammatory, strong antioxidant, antiplatelet, analgesic, neuroprotective, cardioprotective, and antiaging. It remarkably modifies glucose metabolism and oxidative injury.133,134 Resveratrol has been shown to activate the sirtuin pathway. Different cellular functions, which are linked to oxidation, metabolism, and aging are regulated by the sirtuin pathway. Resveratrol affects cellular energy homeostasis, increases lipolysis, activates Nrf2, decreases adipogenesis and blood cholesterol, inhibits cyclooxygenase, and protects cardiac cells.135–139 Resveratrol has been shown to promisingly enhance insulin sensitivity, glucose tolerance, and decrease weight and body mass index in patients suffering from MetS.140 Resveratrol seems a promising adjuvant therapy for management of type 2 diabetes.141
Studies demonstrated that treatment with resveratrol exhibits antidiabetic potential in pancreatic cells via diminishing hyperglycemia, preservation of cells, enhancing insulin secretion, and antioxidant effect.142 Resveratrol-loaded nanoliposomes were prepared for alleviation of diabetes in STZ-induced diabetic animals. The liposomes were PEGylated covalently to increase plasma half-life and residence time of the nanoliposome loaded with resveratrol. The results demonstrated that liposomes enhanced the expression of ROS-inactivating enzymes such as GSH-Px and SOD with extended release of resveratrol in diabetic pancreatic β TC cells. This nanoformulation loaded with resveratrol could be an advantageous formulation for treatment of and protection against type 2 diabetes mellitus.143 Another formulation including resveratrol (resveratrol-loaded nanocapsules) was synthesized and the effect of this nanoformulation was investigated on mice with MetS. The results illustrated that the systolic and diastolic blood pressures were regulated in mice treated with nanocapsules.144 The management of diabetes with resveratrol has been challenged due to small cohorts of patients and short duration of trials, low bioavailability of RSV in humans due to rapid glucuronation, sulfation and clearance from the body. Many bioavailability studies are vague in quantifying tissue distribution and plasma bioavailability of RSV pool. Therefore, the dosage required for optimal bioavailability for sufficient tissue distribution needs to be clarified.145 New approaches to increase the bioavailability may help to actualize its potential as therapeutic agent in diabetes and related complications. The use of resveratrol or its analog could be combined with nanotechnology to treat or prevent human diabetes in the near future.
Silybin
Silybin is the main active ingredient, accounting for 34% of total mass, of silymarin. Silybins A and B are the most common diastereoisomers. The plant is mostly found in Southern Europe and Asia.145 This compound possesses low toxicity and exhibits remarkable hepatoprotective, anti-inflammatory, and anticarcinogenic effects. Silybin has been shown to be effective in obesity-induced insulin resistance related to its anti-inflammatory and fat reduction properties.146 Furthermore, the results of experiments demonstrated that silymarin remarkably diminished epididymal fat mass and body weight with unchanged food intake.146–148 In another study, PLGA polymers loaded with silybin were prepared and the effect of this nanoformulation was tested on STZ-induced rats. The loading efficiency of silybin was more than 92.11%. The results showed that this nanoformulation contributed to diabetes control and reduced hyperglycemia. Silybin is associated with powerful antioxidant characteristics, regenerative impacts on beta cells, and increased membrane permeability. This nanoformulation introduced a new approach for the control of diabetes and has been used as a coadjuvant in therapy.149
Myricitrin
Myricitrin (myricetin-3-O-α-rhamnoside) is an herbal flavonol glycoside extracted from some plants (Pouteriagender, Myrica rubra, Manilkara zapota, and Eugenia uniflora).150 Myricitrin possesses anti-nociceptive, anxiolytic, antioxidant, and anti-inflammatory effects. Due to its high antioxidant activity, it is considered as an essential supplement in medicine. Research has revealed that myricitrin prevents vein endothelial cell dysfunction caused by ROS via diminishing H2O2-induced oxidative damage, reducing malondialdehyde, and regulation of activity of antioxidant enzymes.151 The metabolism and bioavailability of flavonoids, especially flavonoid glycosides, are the essential characteristics that should be considered. Due to high polarity it is not able to cross membranes.152 SLNs containing myricitrin showed protective impact against cytotoxicity induced by STZ in β-cells. In addition, antioxidant and antidiabetic effects of SLNs containing myricitrin were reported in a STZ-nicotinamide-induced diabetic model and myotube cell of a male mouse.153
Stevioside
Stevioside can be extracted from the leaves of Stevia rebaudiana (Bertoni), known to have powerful antidiabetic activity.154 Research has demonstrated that stevioside has an impact on renal function and glucose metabolism.155 Stevioside has the ability to regulate the concentration of glucose in blood via boosting insulin utilization and insulin secretion in rats with insulin deficiency. Stevioside has been proven as one of the powerful antidiabetic agents, but it possesses less therapeutic efficiency because it is poorly absorbed in the intestine, and has scarce bioavailability. Poly-lactic acid (PLA) NPs based on Pluronic-F-68 copolymer containing stevioside were synthesized and evaluated in diabetes. The particle size of the synthesized nanoformulation was 110–130 nm with a spherical structure. Stevioside efficiently incorporated in NPs increased the bioavailability and intestinal absorption. The nanoencapsulation of stevioside in PLA showed better drug release and more absorption in the intestine compared to free stevioside.156 Due to the presence of highly bioactive molecules, S, rebaudiana is now employed in several commercial formulations.
Alpha-eleostearic acid
Bitter ground oil (BGO) contains approximately 30%–50% α-eleostearic acid, which possesses antioxidative and ROS-scavenging effects.157 Conjugated linolenic acid isomers increase immunity and behave as anti-adipogenic and anti-inflammatory agents.15 In a study, a bitter ground seed oil nanoemulsion was synthesized to enhance bioavailability of conjugated linolenic acid in an in vivo system undergoing oxidative stress. For the in vivo study, rats were divided into five groups. Group A was only administered sunflower oil; group B was orally administered sunflower oil diet and intraperitoneally alloxan; group C received alloxan intraperitoneally and sunflower oil orally: nanoemulsion (99.5: 0.5 v/v) containing 0.25% conjugated linolenic acid once a day; received alloxan intraperitoneally and BGO nanoemulsion with sunflower oil orally: nanoemulsion (99: 1 v/v) containing 0.5% conjugated linolenic acid; the diet of group E was similar to that of group C, but the conventional emulsion of BGO was used; the diet of group F was similar to that of group D, but the conventional emulsion of BGO was used. According to results, the concentration of blood glucose decreased after administration of 0.5% and 1% nanoemulsion which contained 0.25% and 0.5% α-eleostearic acid, respectively. α--eleostearic acid upregulated PPAR-γ and modified insulin sensitivity and thus glucose tolerance. The remarkable improvement was in the antioxidative enzymes including GPx, SOD, and CAT in the liver and plasma fractions. This nanoemulsion system ameliorated the stress state induced by alloxan via antioxidative defense. This research showed the possibility of application of conjugated linolenic acid as a powerful nutraceutical for further usage.158 Advanced studies on engineered nanomaterials have shown that NPs interact with the biological interfaces on a more targeted level than conventional therapeutics. Hence, it is of pivotal importance to explore the interactions at a biological interface, bringing them into practice for biomedical purposes.
Translational insights and future perspective of nanoformulations for MetS
Most studies on MetS and obesity have been conducted on the peripheral organs, while the role of the central nervous system has been poorly recognized. Improvement of the sympathetic system activity has positive roles in MetS and conditions such as obesity, hypertension, insulin resistance, dyslipidemia, and inflammation.159 For instance, leptin is responsible for increasing sympathetic renal activity and affects oxidative stress elements. Also, the role of inflammation in different determinants of MetS has been described. Adipokines are known to induce insulin resistance and atherosclerosis through specific elements such as adiponectin, TNF-α, IL-6, MCP-1, and leptin. If it is postulated that obesity is a multi-component disease, the impairment of vascular function is observed in almost all subjects.159 Therefore, further focus on the role players of MetS in the central nervous system along with vascular dysfunction and inflammatory elements is necessary. Literature review shows that natural products can affect the previously mentioned mechanisms of MetS in a positive way. Thus, good and efficient delivery to the human body is a necessity. Biodegradable NPs have been found useful for the delivery of proteins and peptides in minimum doses,160 and studies on the natural products continue. The final goal should be to carry and deliver the medicine to the target site of action with enough understanding of the mechanisms involved in MetS. Unfortunately, discovery and development of potent drugs against MetS have been greatly hampered due to lack of a suitable preclinical model. Meanwhile, several studies are still warranted to find and screen new readily available plants, isolate their active compounds, and test pharmacological activities. The safety of nanoformulations fabricated from herbal-based compounds is another concern that must be taken into account. Focusing on the “antidiabetic” activity of plants rather than their “hypoglycemic” effect would be of high priority because of further clinical implications in MetS. It seems that there is no well-developed approach to test these compounds, especially details of lipid metabolism, thus future studies will possibly consider this limitation. Nanoformulations based on natural products should be biodegradable, biocompatible, non-toxic, and in the meantime should have a great ability to enter cells and produce a rapid action, and be stable after oral administration. Risk benefit and cost effectiveness are further concerns that have to be noted. Justification of the efficacy of these novel therapies remains to be established by conducting precise clinical trials.
Conclusion
MetS is a complex of disorders including mainly impaired glucose tolerance, insulin resistance, and hyperinsulinemia. It has been shown that natural-based compounds or their derivatives are great sources for therapeutic applications, especially in diabetes and its complications and in inflammatory pathways mediated through insulin resistance in MetS. Plant-based natural products improve obesity-associated MetS such as hyperglycemia, hyperlipidemia, and insulin resistance, reduce systolic and diastolic blood pressure, and reduce body weight gain. The mechanism of effect of these phytochemicals has been schematically summarized in Figure 1. These compounds were found to suppress expression of PAI-1, downregulate ICAM-1 expression, regulate COX-1 and -2, downregulate TNF-α expression, suppress NF-κB activation, activate PPARs, and inhibit P2X3 receptor activity and the phosphorylation of ERK1/2 in the dorsal root ganglia. The low oral bioavailability of these phytochemicals is considered as an obstacle to achieve the highest therapeutic efficacy from these compounds. In order to improve the bioactivity and functionality of these products, and also for better targeting of colon and modified absorption, nanosizing or loading of NPs, or encapsulating in nanoformulation can be considered as practical methods.
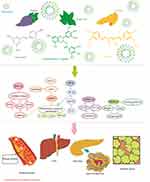 |
Figure 1 A schematic diagram of the mechanism of action of different phytochemicals against metabolic syndrome. |
Encapsulation in multipolymer NPS, nanoemulsions, and solid lipid nanoformulations, and using SEDDs, nanoliposoms, and nanosuspensions are some of the methods for enhancing solubility and bioavailability, increasing plasma half-life, and eventually improving the efficacy of these natural compounds in management of MetS.
Although the published reports have shown that these nanoformulations can be good candidates for adjunctive therapy for MetS, further clinical studies are required to explore the therapeutic benefits of nanoformulations in direct comparative studies with unformulated, approved therapeutics. Cost effectiveness studies are highly recommended, particularly for the well established nanoformulations.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tabatabaei-Malazy O, Larijani B, Abdollahi M. Targeting metabolic disorders by natural products. J Diabetes Metab Disord. 2015;14:57. doi:10.1186/s40200-015-0184-8
2. McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin Dermatol. 2018;36(1):14–20. doi:10.1016/j.clindermatol.2017.09.004
3. Swarup S, Zeltser R. Metabolic syndrome. In: StatPearls [internet]. StatPearls Publishing; 2018.
4. Deen D. Metabolic syndrome: time for action. Am Fam Physician. 2004;69:12.
5. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014. doi:10.1155/2014/943162
6. Torchilin VP. Multifunctional nanocarriers. Adv Drug Deliv Rev. 2006;58(14):1532–1555. doi:10.1016/j.addr.2006.09.009
7. Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2012;64:37–48. doi:10.1016/j.addr.2012.09.013
8. Lann D, LeRoith D. Insulin resistance as the underlying cause for the metabolic syndrome. Med Clin North Am. 2007;91(6):1063–1077, viii. doi:10.1016/j.mcna.2007.06.012
9. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet (London, England). 2005;365(9468):1415–1428. doi:10.1016/S0140-6736(05)66378-7
10. Roberts CK, Hevener AL, Barnard RJ. Metabolic syndrome and insulin resistance: underlying causes and modification by exercise training. Compr Physiol. 2013;3(1):1–58. doi:10.1002/cphy.c110062
11. Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87(2):507–520. doi:10.1152/physrev.00024.2006
12. Kim DH, Perdomo G, Zhang T, et al. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60(11):2763–2774. doi:10.2337/db11-0548
13. Meyer MM, Levin K, Grimmsmann T, Beck-Nielsen H, Klein HH. Insulin signalling in skeletal muscle of subjects with or without type II-diabetes and first degree relatives of patients with the disease. Diabetologia. 2002;45(6):813–822. doi:10.1007/s00125-002-0830-9
14. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi:10.1038/nrm1837
15. Saha SS, Ghosh M. Antioxidant and anti-inflammatory effect of conjugated linolenic acid isomers against streptozotocin-induced diabetes. Br J Nutr. 2012;108(6):974–983. doi:10.1017/S0007114511006325
16. Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi:10.1038/414799a
17. Lizcano JM, Alessi DR. The insulin signalling pathway. Curr Biol. 2002;12(7):R236–R238.
18. Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3(4):267–277. doi:10.1038/nrm782
19. Patel TP, Rawal K, Bagchi AK, et al. Insulin resistance: an additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail Rev. 2016;21(1):11–23. doi:10.1007/s10741-015-9515-6
20. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis. 2017;11(8):215–225. doi:10.1177/1753944717711379
21. Tooke JE, Hannemann MM. Adverse endothelial function and the insulin resistance syndrome. J Intern Med. 2000;247(4):425–431.
22. Halpern A, Mancini MC, Magalhaes ME, et al. Metabolic syndrome, dyslipidemia, hypertension and type 2 diabetes in youth: from diagnosis to treatment. Diabetol Metab Syndr. 2010;2:55. doi:10.1186/1758-5996-2-55
23. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi:10.1155/2014/943162
24. Bonner G. Hyperinsulinemia, insulin resistance, and hypertension. J Cardiovasc Pharmacol. 1994;24(Suppl 2):S39–S49.
25. Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286(5):H1597–H1602. doi:10.1152/ajpheart.00026.2004
26. Yanai H, Tomono Y, Ito K, Furutani N, Yoshida H, Tada N. The underlying mechanisms for development of hypertension in the metabolic syndrome. Nutr J. 2008;7:10. doi:10.1186/1475-2891-7-10
27. Ribeiro-Oliveira A
28. Hsueh WA, Quinones MJ. Role of endothelial dysfunction in insulin resistance. Am J Cardiol. 2003;92(4A):10J–17J. doi:10.1016/s0002-9149(03)00611-8
29. Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost. 2003;1(7):1575–1579.
30. Russo I. The prothrombotic tendency in metabolic syndrome: focus on the potential mechanisms involved in impaired haemostasis and fibrinolytic balance. Scientifica. 2012;2012:525374. doi:10.6064/2012/525374
31. Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19(4):972–978.
32. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi:10.1210/jc.2004-0395
33. Atanasov AG, Waltenberger B, Pferschy-Wenzig E-M, et al. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol Adv. 2015;33(8):1582–1614. doi:10.1016/j.biotechadv.2015.08.001
34. Cefalu WT, Ye J, Wang ZQ. Efficacy of dietary supplementation with botanicals on carbohydrate metabolism in humans. Endocr Metab Immune Disord Drug Targets. 2008;8(2):78–81. doi:10.2174/187153008784534376
35. Dong H, Lu FE, Zhao L. Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin J Integr Med. 2012;18(2):152–160. doi:10.1007/s11655-012-0993-2
36. Heber D. Herbs and atherosclerosis. Curr Atheroscler Rep. 2001;3(1):93–96.
37. Alzahrani T, Marrat S, Haider A. Management of dyslipidemia in primary care. Can J Cardiol. 2003;19(13):1499–1502.
38. Bays H, Stein EA. Pharmacotherapy for dyslipidaemia–current therapies and future agents. Expert Opin Pharmacother. 2003;4(11):1901–1938. doi:10.1517/14656566.4.11.1901
39. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi:10.1016/j.jacc.2010.05.034
40. Waltenberger B, Mocan A, Šmejkal K, Heiss EH, Atanasov AG. Natural products to counteract the epidemic of cardiovascular and metabolic disorders. Molecules. 2016;21:6. doi:10.3390/molecules21060807
41. Ríos JL, Francini F, Schinella GR. Natural products for the treatment of type 2 diabetes mellitus. Planta Med. 2015;81(12/13):975–994. doi:10.1055/s-0035-1546131
42. Lacroix IM, Li-Chan EC. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: a natural approach to complement pharmacotherapy in the management of diabetes. Mol Nutr Food Res. 2014;58(1):61–78. doi:10.1002/mnfr.201300223
43. Hung HY, Qian K, Morris-Natschke SL, Hsu CS, Lee KH. Recent discovery of plant-derived anti-diabetic natural products. Nat Prod Rep. 2012;29(5):580–606. doi:10.1039/c2np00074a
44. Gautam R, Jachak SM. Recent developments in anti-inflammatory natural products. Med Res Rev. 2009;29(5):767–820. doi:10.1002/med.20156
45. Hermansen K, Dinesen B, Hoie LH, Morgenstern E, Gruenwald J. Effects of soy and other natural products on LDL:HDL ratio and other lipid parameters: a literature review. Adv Ther. 2003;20(1):50–78.
46. Vasanthi HR, ShriShriMal N, Das DK. Phytochemicals from plants to combat cardiovascular disease. Curr Med Chem. 2012;19(14):2242–2251.
47. Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflammation Res. 2000;49(10):497–505. doi:10.1007/s000110050622
48. Francis GA, Annicotte JS, Auwerx J. PPAR agonists in the treatment of atherosclerosis. Curr Opin Pharmacol. 2003;3(2):186–191.
49. Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812(8):1007–1022. doi:10.1016/j.bbadis.2011.02.014
50. Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144(6):2201–2207. doi:10.1210/en.2003-0288
51. Gurnell M, Savage DB, Chatterjee VK, O’Rahilly S. The metabolic syndrome: peroxisome proliferator-activated receptor gamma and its therapeutic modulation. J Clin Endocrinol Metab. 2003;88(6):2412–2421. doi:10.1210/jc.2003-030435
52. Francis GA, Annicotte JS, Auwerx J. PPAR-alpha effects on the heart and other vascular tissues. Am J Physiol Heart Circ Physiol. 2003;285(1):H1–H9. doi:10.1152/ajpheart.01118.2002
53. Miller AR, Etgen GJ. Novel peroxisome proliferator-activated receptor ligands for Type 2 diabetes and the metabolic syndrome. Expert Opin Investig Drugs. 2003;12(9):1489–1500. doi:10.1517/13543784.12.9.1489
54. Huang TH, Kota BP, Razmovski V, Roufogalis BD. Herbal or natural medicines as modulators of peroxisome proliferator-activated receptors and related nuclear receptors for therapy of metabolic syndrome. Basic Clin Pharmacol Toxicol. 2005;96(1):3–14. doi:10.1111/j.1742-7843.2005.pto960102.x
55. Jaradat MS, Noonan DJ, Wu B, Avery MA, Feller DR. Pseudolaric acid analogs as a new class of peroxisome proliferator-activated receptor agonists. Planta Med. 2002;68(8):667–671. doi:10.1055/s-2002-33785
56. Yoon M, Lee H, Jeong S, et al. Peroxisome proliferator-activated receptor alpha is involved in the regulation of lipid metabolism by ginseng. Br J Pharmacol. 2003;138(7):1295–1302. doi:10.1038/sj.bjp.0705169
57. Yang Y, Wu T, He K, Fu ZG. Effect of aerobic exercise and ginsenosides on lipid metabolism in diet-induced hyperlipidemia mice. Zhongguo Yao Li Xue Bao. 1999;20(6):563–565.
58. Kim SH, Park KS. Effects of panax ginseng extract on lipid metabolism in humans. Pharmacological Res. 2003;48(5):511–513.
59. Dang ZC, Audinot V, Papapoulos SE, Boutin JA, Lowik CW. Peroxisome proliferator-activated receptor gamma (PPARgamma) as a molecular target for the soy phytoestrogen genistein. J Biol Chem. 2003;278(2):962–967. doi:10.1074/jbc.M209483200
60. Shi Y. Orphan nuclear receptors in drug discovery. Drug Discov Today. 2007;12(11–12):440–445. doi:10.1016/j.drudis.2007.04.006
61. Bramlett KS, Houck KA, Borchert KM, et al. A natural product ligand of the oxysterol receptor, liver X receptor. J Pharmacol Exp Ther. 2003;307(1):291–296. doi:10.1124/jpet.103.052852
62. Chiang JY. Regulation of bile acid synthesis: pathways, nuclear receptors, and mechanisms. J Hepatol. 2004;40(3):539–551. doi:10.1016/j.jhep.2003.11.006
63. Urizar NL, Moore DD. GUGULIPID: a natural cholesterol-lowering agent. Annu Rev Nutr. 2003;23:303–313. doi:10.1146/annurev.nutr.23.011702.073102
64. Davatgaran-Taghipour Y, Masoomzadeh S, Farzaei MH, et al. Polyphenol nanoformulations for cancer therapy: experimental evidence and clinical perspective. Int J Nanomedicine. 2017;12:2689. doi:10.2147/IJN.S131973
65. Rachmawati H, SORAYA I, Kurniati N, Rahma A. In vitro study on antihypertensive and antihypercholesterolemic effects of a curcumin nanoemulsion. Sci Pharm. 2016;84(1):131–140. doi:10.3797/scipharm.ISP.2015.05
66. Taghipour YD, Bahramsoltani R, Marques AM, et al. A systematic review of nano formulation of natural products for the treatment of inflammatory bowel disease: drug delivery and pharmacological targets. DARU J Pharm Sci. 2018;26(2):229–239.
67. Soetikno V, Sari FR, Veeraveedu PT, et al. Curcumin ameliorates macrophage infiltration by inhibiting NF-κB activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr Metab (Lond). 2011;8(1):35. doi:10.1186/1743-7075-8-35
68. Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr. 2010;30:173–199. doi:10.1146/annurev.nutr.012809.104755
69. Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected]. J Biol Chem. 1995;270(42):24995–25000. doi:10.1074/jbc.270.42.24995
70. Xu J, Fu Y, Chen A. Activation of peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of curcumin on rat hepatic stellate cell growth. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G20–G30. doi:10.1152/ajpgi.00474.2002
71. Gonzalez-Castejon M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: a review. Pharmacological Res. 2011;64(5):438–455.
72. Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: from kitchen to clinic. Biochem Pharmacol. 2008;75(4):787–809. doi:10.1016/j.bcp.2007.08.016
73. Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci. 2008;65(11):1631–1652. doi:10.1007/s00018-008-7452-4
74. Wahlström B, Blennow G. A study on the fate of curcumin in the rat. Acta Pharmacol Toxicol (Copenh). 1978;43(2):86–92.
75. Ernest U, Chen H-Y, Xu M-J, et al. Anti-cancerous potential of polyphenol-loaded polymeric nanotherapeutics. Molecules. 2018;23(11):2787. doi:10.3390/molecules23112787
76. Hajialyani M, Tewari D, Sobarzo-Sánchez E, Nabavi SM, Farzaei MH, Abdollahi M. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int J Nanomedicine. 2018;13:5023–5043. doi:10.2147/IJN.S174072
77. Tong F, Chai R, Jiang H, Dong B. In vitro/vivo drug release and anti-diabetic cardiomyopathy properties of curcumin/PBLG-PEG-PBLG nanoparticles. Int J Nanomedicine. 2018;13:1945. doi:10.2147/IJN.S177627
78. Joshi RP, Negi G, Kumar A, et al. SNEDDS curcumin formulation leads to enhanced protection from pain and functional deficits associated with diabetic neuropathy: an insight into its mechanism for neuroprotection. Nanomed. 2013;9(6):776–785. doi:10.1016/j.nano.2013.01.001
79. Grama CN, Suryanarayana P, Patil MA, et al. Efficacy of biodegradable curcumin nanoparticles in delaying cataract in diabetic rat model. PLoS One. 2013;8(10):e78217. doi:10.1371/journal.pone.0078217
80. El-Far YM, Zakaria MM, Gabr MM, El Gayar AM, Eissa LA, El-Sherbiny IM. Nanoformulated natural therapeutics for management of streptozotocin-induced diabetes: potential use of curcumin nanoformulation. Nanomedicine. 2017;12(14):1689–1711. doi:10.2217/nnm-2017-0106
81. Devadasu VR, Wadsworth RM, Kumar MR. Protective effects of nanoparticulate coenzyme Q 10 and curcumin on inflammatory markers and lipid metabolism in streptozotocin-induced diabetic rats: a possible remedy to diabetic complications. Drug Deliv Transl Res. 2011;1(6):448–455. doi:10.1007/s13346-011-0041-3
82. Rachmawati H, Soraya IS, Kurniati NF, Rahma A. In vitro study on antihypertensive and antihypercholesterolemic effects of a curcumin nanoemulsion. Sci Pharm. 2016;84(1):131–140. doi:10.3797/scipharm.ISP.2015.05
83. Jia T, Rao J, Zou L, et al. Nanoparticle-encapsulated curcumin inhibits diabetic neuropathic pain involving the P2Y12 receptor in the dorsal root ganglia. Front Neurosci. 2018;11:755. doi:10.3389/fnins.2017.00755
84. Liu J, Chen Z, Wang J, et al. Encapsulation of curcumin nanoparticles with MMP9-responsive and thermos-sensitive hydrogel improves diabetic wound healing. ACS Appl Mater Interfaces. 2018;10(19):16315–16326. doi:10.1021/acsami.8b03868
85. Yallapu MM, Jaggi M, Chauhan SC. Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today. 2012;17(1–2):71–80. doi:10.1016/j.drudis.2011.09.009
86. Yallapu MM, Jaggi M, Chauhan CS. Curcumin nanomedicine: a road to cancer therapeutics. Curr Pharm Des. 2013;19(11):1994–2010.
87. Melgar-Lalanne G, Hernández-Álvarez AJ, Jiménez-Fernández M, Azuara E. Oleoresins from capsicum spp.: extraction methods and bioactivity. Food Bioproc Tech. 2017;10(1):51–76. doi:10.1007/s11947-016-1793-z
88. Dwivedi V, Shrivastava R, Hussain S, Ganguly C, Bharadwaj M. Cytotoxic potential of Indian spices (extracts) against esophageal squamous carcinoma cells. Asian Pac J Cancer Prev. 2011;12(8):2069–2073.
89. Allemand A, Leonardi BF, Zimmer AR, Moreno S, Romao PRT, Gosmann G. Red pepper (Capsicum baccatum) extracts present anti-inflammatory effects in vivo and inhibit the production of TNF-α and NO in vitro. J Med Food. 2016;19(8):759–767. doi:10.1089/jmf.2015.0101
90. Kim J-Y, Lee M-S, Jung S, et al. Anti-obesity efficacy of nanoemulsion oleoresin capsicum in obese rats fed a high-fat diet. Int J Nanomedicine. 2014;9:301. doi:10.2147/IJN.S52414
91. Lee M-S, Jung S, Shin Y, et al. Lipolytic efficacy of alginate double-layer nanoemulsion containing oleoresin capsicum in differentiated 3T3-L1 adipocytes. Food Nutr Res. 2017;61(1):1339553. doi:10.1080/16546628.2017.1339553
92. Wang N, Tan H-Y, Li L, Yuen M-F, Feng Y. Berberine and coptidis rhizoma as potential anticancer agents: recent updates and future perspectives. J Ethnopharmacol. 2015;176:35–48. doi:10.1016/j.jep.2015.10.028
93. Bao J, Huang B, Zou L, et al. Hormetic effect of berberine attenuates the anticancer activity of chemotherapeutic agents. PLoS One. 2015;10(9):e0139298. doi:10.1371/journal.pone.0139298
94. Li M, Zhang M, Zhang Z-L, et al. Induction of apoptosis by berberine in hepatocellular carcinoma HepG2 cells via downregulation of NF-κB. Oncol Res Featuring Preclinical Clin Cancer Ther. 2017;25(2):233–239. doi:10.3727/096504016X14742891049073
95. Yang J, Yin J, Gao H, et al. Berberine improves insulin sensitivity by inhibiting fat store and adjusting adipokines profile in human preadipocytes and metabolic syndrome patients. Evid Based Complement Alternat Med. 2012;2012. doi:10.1155/2012/363845
96. Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55(8):2256–2264. doi:10.2337/db06-0006
97. Pérez-Rubio KG, González-Ortiz M, Martínez-Abundis E, Robles-Cervantes JA, Espinel-Bermúdez MC. Effect of berberine administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2013;11(5):366–369. doi:10.1089/met.2012.0183
98. Xue M, Yang M-X, Zhang W, et al. Characterization, pharmacokinetics, and hypoglycemic effect of berberine loaded solid lipid nanoparticles. Int J Nanomedicine. 2013;8:4677. doi:10.2147/IJN.S37465
99. Kapoor R, Singh S, Tripathi M, Bhatnagar P, Kakkar P, Gupta KC. O-hexadecyl-dextran entrapped berberine nanoparticles abrogate high glucose stress induced apoptosis in primary rat hepatocytes. PLoS One. 2014;9(2):e89124. doi:10.1371/journal.pone.0089124
100. Ochin CC, Garelnabi M. Berberine encapsulated PLGA-PEG nanoparticles modulate PCSK-9 in HepG2 cells. Cardiovasc Hematol Disord Drug Targets. 2018;18(1):61–70. doi:10.2174/1871529X18666180201130340
101. Cavia-Saiz M, Busto MD, Pilar-Izquierdo MC, Ortega N, Perez-Mateos M, Muñiz P. Antioxidant properties, radical scavenging activity and biomolecule protection capacity of flavonoid naringenin and its glycoside naringin: a comparative study. J Sci Food Agric. 2010;90(7):1238–1244. doi:10.1002/jsfa.3959
102. Ortiz-Andrade RR, Sánchez-Salgado JC, Navarrete-Vázquez G, et al. Antidiabetic and toxicological evaluations of naringenin in normoglycaemic and NIDDM rat models and its implications on extra-pancreatic glucose regulation. Diabetes Metab Syndr Obes. 2008;10(11):1097–1104. doi:10.1111/j.1463-1326.2008.00869.x
103. Prabu SM, Renugadevi J, Shagirtha K. In vivo and in vitro antioxidative efficacy of naringenin on cadmium-induced toxicity in rats. Res Rev. 2019;3(3):9–16.
104. Scalbert A, Rémésy C, Morand C, Jiménez L, Manach C. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. doi:10.1093/ajcn/79.5.727
105. Maity S, Mukhopadhyay P, Kundu PP, Chakraborti AS. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—an in vitro and in vivo approach. Carbohydr Polym. 2017;170:124–132. doi:10.1016/j.carbpol.2017.04.066
106. Hashemzaei M, Delarami Far A, Yari A, et al. Anticancer and apoptosis‑inducing effects of quercetin in vitro and in vivo. Oncol Rep. 2017;38(2):819–828. doi:10.3892/or.2017.5766
107. Leiherer A, Stoemmer K, Muendlein A, et al. Quercetin impacts expression of metabolism-and obesity-associated genes in SGBS adipocytes. Nutrients. 2016;8(5):282. doi:10.3390/nu8050282
108. Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese zucker rats. Obesity. 2008;16(9):2081–2087. doi:10.1038/oby.2008.315
109. Hasani-Ranjbar S, Larijani B, Abdollahi M. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets. 2009;8(1):2–10.
110. Mitjavila M, Moreno J. The effects of polyphenols on oxidative stress and the arachidonic acid cascade. Implications for the prevention/treatment of high prevalence diseases. Biochem Pharmacol. 2012;84(9):1113–1122. doi:10.1016/j.bcp.2012.07.017
111. Abdollahi M, Tabatabaei-Malazy O, Larijani B. A systematic review of in vitro studies conducted on effect of herbal products on secretion of insulin from Langerhans islets. J Pharm Pharm Sci. 2012;15(3):447–466. doi:10.18433/J32W29
112. Pfeuffer M, Auinger A, Bley U, et al. Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms. Nutr Metab Cardiovasc Dis. 2013;23(5):403–409. doi:10.1016/j.numecd.2011.08.010
113. Tong F, Liu S, Yan B, Li X, Ruan S, Yang S. Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. Int J Nanomedicine. 2017;12:7799. doi:10.2147/IJN.S146978
114. Mukhopadhyay P, Maity S, Mandal S, Chakraborti AS, Prajapati A, Kundu P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr Polym. 2018;182:42–51. doi:10.1016/j.carbpol.2017.10.098
115. Schwingel TE, Klein CP, Nicoletti NF, et al. Effects of the compounds resveratrol, rutin, quercetin, and quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and neurotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(9):837–848. doi:10.1007/s00210-014-0994-0
116. Chitkara D, Nikalaje SK, Mittal A, Chand M, Kumar N. Development of quercetin nanoformulation and in vivo evaluation using streptozotocin induced diabetic rat model. Drug Deliv Transl Res. 2012;2(2):112–123. doi:10.1007/s13346-012-0063-5
117. Alam MM, Abdullah K, Singh BR, Naqvi AH, Naseem I. Ameliorative effect of quercetin nanorods on diabetic mice: mechanistic and therapeutic strategies. RSC Adv. 2016;6(60):55092–55103. doi:10.1039/C6RA04821H
118. Jayasuriya H, Koonchanok NM, Geahlen RL, McLaughlin JL, Chang C-J. Emodin, a protein tyrosine kinase inhibitor from polygonum cuspidatum. J Nat Prod. 1992;55(5):696–698.
119. Dong X, Fu J, Yin X, et al. Emodin: a review of its pharmacology, toxicity and pharmacokinetics. Phytotherapy Research. 2016;30(8):1207–1218. doi:10.1002/ptr.5631
120. Li L, Sheng X, Zhao S, et al. Nanoparticle-encapsulated emodin decreases diabetic neuropathic pain probably via a mechanism involving P2X3 receptor in the dorsal root ganglia. Purinergic Signal. 2017;13(4):559–568. doi:10.1007/s11302-017-9583-2
121. Lu K, Xie S, Han S, et al. Preparation of a nano emodin transfersome and study on its anti-obesity mechanism in adipose tissue of diet-induced obese rats. J Transl Med. 2014;12(1):72. doi:10.1186/1479-5876-12-72
122. Patel K, Gadewar M, Tripathi R. Pharmacological and analytical aspects of gymnemic acid: a concise report. Asian Pac J Trop Dis. 2012;2(5):414–416. doi:10.1016/S2222-1808(12)60090-5
123. Ankit S, Chetan S, Aneja KR, Rakesh P. Gymnema sylvestre (Gurmar): a review. Der Pharmacia Lettre. 2010;2(1):275–284.
124. Ravichandran R. Studies on gymnemic acids nanoparticulate formulations against diabetes mellitus. Int J Biomed Clin Eng. 2012;1(2):1–12. doi:10.4018/IJBCE
125. Waisundara VY, Hsu A, Tan BK, Huang D. Baicalin improves antioxidant status of streptozotocin-induced diabetic wistar rats. J Agric Food Chem. 2009;57(10):4096–4102. doi:10.1021/jf8028539
126. Dinda B, Dinda S, DasSharma S, Banik R, Chakraborty A, Dinda M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68–80. doi:10.1016/j.ejmech.2017.03.004
127. Zhao L, Wei Y, Huang Y, He B, Zhou Y, Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:3769–3779. doi:10.2147/IJN.S51578
128. Shi F, Wei Z, Zhao Y, Xu X. Nanostructured lipid carriers loaded with baicalin: an efficient carrier for enhanced antidiabetic effects. Pharmacogn Mag. 2016;12(47):198. doi:10.4103/0973-1296.186347
129. Xiong F, Wang H, Cheng J, Zhu J. Determination of scutellarin in mouse plasma and different tissues by high-performance liquid chromatography. J Chromatogr B. 2006;835(1–2):114–118. doi:10.1016/j.jchromb.2006.02.041
130. Zheng C, Ou W, Shen H, Zhou Z, Wang J. Combined therapy of diabetic peripheral neuropathy with breviscapine and mecobalamin: a systematic review and a meta-analysis of Chinese studies. Biomed Res Int. 2015;2015. doi:10.1155/2015/680756
131. Zheng C, Ou W, Shen H, Zhou Z, Wang J. Combined therapy of diabetic peripheral neuropathy with breviscapine and mecobalamin: a systematic review and a meta-analysis of Chinese studies. Biomed Res Int. 2015;2015:680756. doi:10.1155/2015/680756
132. Wang J, Tan J, Luo J, et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J Nanobiotechnology. 2017;15(1):18. doi:10.1186/s12951-017-0305-2
133. Matos M, Gutiérrez G, Coca J, Pazos C. Preparation of water-in-oil-in-water (W1/O/W2) double emulsions containing trans-resveratrol. Colloids Surf A Physicochem Eng Asp. 2014;442:69–79. doi:10.1016/j.colsurfa.2013.05.065
134. Summerlin N, Soo E, Thakur S, Qu Z, Jambhrunkar S, Popat A. Resveratrol nanoformulations: challenges and opportunities. Int J Pharm. 2015;479(2):282–290. doi:10.1016/j.ijpharm.2015.01.003
135. Bremer AA. Resveratrol use in metabolic syndrome. Metab Syndr Relat Disord. 2014;12(10):493–495. doi:10.1089/met.2014.1505
136. Bhakkiyalakshmi E, Sireesh D, Rajaguru P, Paulmurugan R, Ramkumar KM. The emerging role of redox-sensitive Nrf2-Keap1 pathway in diabetes. Pharmacological Res. 2015;91:104–114. doi:10.1016/j.phrs.2014.10.004
137. Juhasz B, Mukherjee S, Das DK. Hormetic response of resveratrol against cardioprotection. Exp Clin Cardiol. 2010;15(4):e134.
138. Juhasz B, Das DK, Kertesz A, Juhasz A, Gesztelyi R, Varga B. Reduction of blood cholesterol and ischemic injury in the hypercholesteromic rabbits with modified resveratrol, longevinex.[corrected]. Mol Cell Biochem. 2011;348(1–2):199–203. doi:10.1007/s11010-010-0615-2
139. Juhasz B, Varga B, Gesztelyi R, Kemeny-Beke A, Zsuga J, Tosaki A. Resveratrol: a multifunctional cytoprotective molecule. Curr Pharm Biotechnol. 2010;11(8):810–818.
140. Mendez-del Villar M, Gonzalez-Ortiz M, Martinez-Abundis E, Perez-Rubio KG, Lizarraga-Valdez R. Effect of resveratrol administration on metabolic syndrome, insulin sensitivity, and insulin secretion. Metab Syndr Relat Disord. 2014;12(10):497–501. doi:10.1089/met.2014.0082
141. Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus–systematic review and meta-analysis. Mol Nutr Food Res. 2015;59(1):147–159. doi:10.1002/mnfr.201400173
142. Yu W, Fu YC, Wang W. Cellular and molecular effects of resveratrol in health and disease. J Cell Biochem. 2012;113(3):752–759. doi:10.1002/jcb.23431
143. Yücel Ç, Karatoprak GŞ, Aktaş Y. Nanoliposomal resveratrol as a novel approach to treatment of diabetes mellitus. J Nanosci Nanotechnol. 2018;18(6):3856–3864. doi:10.1166/jnn.2018.15247
144. Shahraki A, Bahadorikhalili S, Hashemzaei M, et al. Resveratrol nano-capsule as an efficient tool for blood pressure regulation: a study on metabolic syndrome induced mice. Biosci Biotechnol Res Commun. 2017;10(4):623–630. doi:10.21786/bbrc/10.4/4
145. Hoh CS, Boocock DJ, Marczylo TH, et al. Quantitation of silibinin, a putative cancer chemopreventive agent derived from milk thistle (Silybum marianum), in human plasma by high-performance liquid chromatography and identification of possible metabolites. J Agric Food Chem. 2007;55(7):2532–2535. doi:10.1021/jf063156c
146. Tajmohammadi A, Razavi BM, Hosseinzadeh H. Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: a review. Phytother Res. 2018;32(10):1933–1949. doi:10.1002/ptr.6153
147. Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61(14):2035–2063. doi:10.2165/00003495-200161140-00003
148. Huseini HF, Larijani B, Heshmat R, et al. The efficacy of silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res. 2006;20(12):1036–1039. doi:10.1002/ptr.1988
149. Das S, Roy P, Pal R, Auddy RG, Chakraborti AS, Mukherjee A. Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS One. 2014;9(7):e101818. doi:10.1371/journal.pone.0101818
150. Pereira M, Siba IP, Chioca LR, et al. Myricitrin, a nitric oxide and protein kinase C inhibitor, exerts antipsychotic-like effects in animal models. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(7):1636–1644. doi:10.1016/j.pnpbp.2011.06.002
151. Sun GB, Qin M, Ye JX, et al. Inhibitory effects of myricitrin on oxidative stress-induced endothelial damage and early atherosclerosis in ApoE-/- mice. Toxicol Appl Pharmacol. 2013;271(1):114–126. doi:10.1016/j.taap.2013.04.015
152. Fernandez SP, Nguyen M, Yow TT, et al. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem Res. 2009;34(10):1867–1875. doi:10.1007/s11064-009-9969-9
153. Ahangarpour A, Oroojan AA, Khorsandi L, Kouchak M, Badavi M. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid Med Cell Longev. 2018;2018. doi:10.1155/2018/7496936
154. Goyal SK, Samsher GRK, Goyal RK. Stevia (Stevia rebaudiana) a bio-sweetener: a review. Int J Food Sci Nutr. 2010;61(1):1–10. doi:10.3109/09637480903193049
155. Kujur RS, Singh V, Ram M, et al. Antidiabetic activity and phytochemical screening of crude extract of stevia rebaudiana in alloxan-induced diabetic rats. Pharmacognosy Res. 2010;2(4):258–263. doi:10.4103/0974-8490.69128
156. Barwal I, Sood A, Sharma M, Singh B, Yadav SC. Development of stevioside pluronic-F-68 copolymer based PLA-nanoparticles as an antidiabetic nanomedicine. Colloids Surf B. 2013;101:510–516. doi:10.1016/j.colsurfb.2012.07.005
157. Fang EF, Ng TB. Chapter 28 - bitter gourd (momordica charantia) oils. In: Preedy VR, editor. Essential Oils in Food Preservation, Flavor and Safety. San Diego: Academic Press; 2016:253–257.
158. Paul D, Dey TK, Mukherjee S, Ghosh M, Dhar P. Comparative prophylactic effects of α-eleostearic acid rich nano and conventional emulsions in induced diabetic rats. J Food Sci Technol. 2014;51(9):1724–1736. doi:10.1007/s13197-014-1257-2
159. Balarini CM, Braga VA. New translational insights on metabolic syndrome: obesity, hypertension, diabetes and beyond. Front Physiol. 2016;7:229. doi:10.3389/fphys.2016.00229
160. Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70(1–2):1–20.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
