Back to Journals » Clinical Interventions in Aging » Volume 10
Multicenter, prospective, comparative cohort study evaluating the efficacy and safety of alfuzosin 10 mg with regard to blood pressure in men with lower urinary tract symptoms suggestive of benign prostatic hyperplasia with or without antihypertensive med
Authors Zhang LT, Lee SW , Park K, Chung WS, Kim SW, Hyun JS, Moon DG, Yang S, Ryu JK, Yang DY, Moon KH, Min KS, Park J
Received 10 September 2014
Accepted for publication 20 October 2014
Published 17 January 2015 Volume 2015:10 Pages 277—286
DOI https://doi.org/10.2147/CIA.S74102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
Li Tao Zhang,1 Sung Won Lee,2 Kwangsung Park,3 Woo Sik Chung,4 Sae Woong Kim,5 Jae Seog Hyun,6 Doo Geon Moon,7 Sang-Kuk Yang,8 Ji Kan Ryu,9 Dae Yul Yang,10 Ki Hak Moon,11 Kweon Sik Min,12 Jong Kwan Park1
1Department of Urology, Chonbuk National University, Medical School and Biomedical Research Institute and Clinical Trial Center for Medical Devices of Chonbuk National University Hospital, Jeonju; 2Department of Urology, College of Medicine, Sungkyunkwan University, Seoul; 3Department of Urology, College of Medicine, Chonnam National University, Gwangju; 4Department of Urology, College of Medicine, Ewha Woman’s University, Seoul; 5Department of Urology, College of Medicine, Catholic University, Seoul; 6Department of Urology, College of Medicine, Kyungsang National University, Jinju; 7Department of Urology, College of Medicine, Korea University, Seoul; 8Department of Urology, Chungju Hospital, College of Medicine, Konkuk University, Chungju; 9Department of Urology, College of Medicine, Inha University, Incheon; 10Department of Urology, College of Medicine, Hallym University, Seoul; 11Department of Urology, College of Medicine, Youngnam University, Daegu; 12Department of Urology, College of Medicine, Inje University, Busan, Republic of Korea
Background: The objective of this study was to assess the efficacy and safety of alfuzosin 10 mg monotherapy or combined antihypertensive medication on blood pressure (BP) in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia (BPH/LUTS) with or without antihypertensive medication.
Methods: This was a 3-month, multicenter, randomized, open-label study in 335 patients aged ≥45 years with a clinical diagnosis of BPH/LUTS by medical history and clinical examination, a total International Prostatic Symptom Score (IPSS) ≥8 points, a maximum flow rate >5 mL/sec and ≤15 mL/sec, and a voided volume ≥120 mL. Eligible subjects were randomized to receive alfuzosin 10 mg as monotherapy (group 1) or alfuzosin 10 mg + antihypertensive combination therapy (group 2). Based on baseline BP and hypertensive history with or without antihypertensive medications at first medical examination, group 1 was divided into two subgroups of normotensive and untreated hypertensive patients, and group 2 into two subgroups of controlled hypertensive and uncontrolled hypertensive patients. The primary study outcomes were change in IPSS, BP, and heart rate from baseline. Secondary outcomes were change in IPSS-quality of life score, maximum flow rate, average flow rate, voided volume, and post-voided volume.
Results: The overall BP change was not significantly different between groups 1 and 2 (systolic BP, P=0.825; diastolic BP, P>0.999). In patients with uncontrolled or untreated hypertension, alfuzosin 10 mg alone or combined with antihypertensive therapy significantly decreased systolic and diastolic BP. The mean difference in total IPSS and IPSS-quality of life scores from baseline between groups 1 and 2 was 0.45 (95% CI: −1.26, 2.16) and 0.12 (95% CI: −0.21, 0.45), respectively (both P>0.05). Maximum flow rate, average flow rate, voided volume, and post-voided volume at endpoint were numerically, but not significantly, changed from baseline (all P>0.05).
Conclusion: This study shows that alfuzosin 10 mg is effective and well tolerated in patients with BPH/LUTS with or without antihypertensive medications. However, in patients with uncontrolled or untreated hypertension, alfuzosin 10 mg alone or in combination with antihypertensive medication appears to decrease systolic and diastolic BP, and these patients should be warned about a decrease in BP on initiation of therapy.
Keywords: alfuzosin, lower urinary tract symptoms, benign prostatic hyperplasia, antihypertensive medication, blood pressure
A Letter to the Editor has been received and published for this article.
Introduction
Benign prostatic hyperplasia (BPH) is a common urological disorder and increases in incidence with advancing age, with a prevalence of over 50% in men in their 60s and up to 90% by 85 years of age.1,2 BPH is often associated with bothersome lower urinary tract symptoms (LUTS), including frequency, urgency, nocturia, and weak urine flow.3,4 Troublesome LUTS rather than histologically evident prostatic hypertrophy typically prompt patients to seek medical care. Therefore, LUTS and BPH are closely related clinical symptoms. BPH/LUTS typically arises from increased resistance to urinary flow induced by progressive hypertrophy of the prostate.5 Left untreated, the need for surgery increases due to progressively worsening symptoms caused by BPH-related dysfunction of the urinary tract, with about 7% of affected patients developing acute urinary retention.6
The incidence of hypertension also increases with advancing age. An estimated 50% of individuals are affected by 70 years of age, and concomitant BPH and hypertension is found in an estimated 25% of men over 60 years of age.7 Noninvasive medical therapy has been increasingly recognized as the primary option for treatment. Among the treatments available, α1-adrenoceptor antagonists are preferred when treating patients with BPH/LUTS (although the first-generation α1-adrenoceptor antagonists, ie, doxazosin and terazosin, were developed for the treatment of hypertension8). However, their efficacy in the treatment of BPH/LUTS can vary considerably, especially in elderly patients and in those with hypertension.9
As such, the ratio of probable urinary events versus cardiovascular adverse events, such as postural hypotension, had been described pertaining to the concept of a uroselective α1-adrenoceptor antagonist. Recently developed novel uroselective antagonists had demonstrated appreciable cardiovascular adverse effects. Alfuzosin has demonstrated functional uroselectivity as a novel α1-adrenoceptor antagonist in BPH/LUTS.8 This dose of alfuzosin 10 mg is optimal and prevents major fluctuations in plasma drug concentrations. Recent studies have demonstrated the efficacy of alfuzosin in the treatment of distal ureteral stones10,11 and in prostatitis syndrome.12–14
Physicians have long pondered how to treat patients suffering from BPH if they are already on antihypertensive medication. The dilemma is whether such patients should be treated with alfuzosin to relieve the symptoms of BPH or not because of the potentially increased risk of hypotensive episodes.
The purpose of this prospective multicenter study was to investigate whether alfuzosin 10 mg caused a further decrease in blood pressure (BP) in patients with BPH/LUTS whose BP was already regulated by antihypertensive medications compared with BPH/LUTS patients who were not on antihypertensive or α-blocker therapy.
Patients and methods
Study design
Eleven studies conducted between February 2010 and January 2013 investigated the efficacy and safety of alfuzosin 10 mg for up to 12 weeks. This study was performed using integrated data from these randomized, open-label, multicenter studies in men with BPH/LUTS with or without antihypertensive medications. The institutional review board at each site approved this research and all subjects gave their written informed consent before undergoing any study procedure or therapy. The study inclusion criteria at screening and baseline are summarized in Table 1, and the exclusion criteria are summarized in Table 2.
  | Table 1 Study inclusion at screening and baseline |
  | Table 2 Study exclusion criteria |
Eligible subjects were randomized to receive alfuzosin 10 mg (Handok, Seoul, South Korea) as monotherapy (group 1) or matching alfuzosin 10 mg combined with antihypertensive medication (group 2). The two groups were then divided into subgroups, ie, normotensive versus untreated hypertensive subjects and controlled hypertensive versus uncontrolled hypertensive subjects, respectively, based on baseline BP and hypertensive history with or without receipt of antihypertensive medications at first medical examination. Vital signs, including systolic BP, diastolic BP, and heart rate were measured at each visit after 10 minutes resting in the supine position.
Outcome measures
The primary efficacy outcome was change in total International Prostate Symptom Score (IPSS) from baseline (randomization) to endpoint (12 weeks). Total IPSS and IPSS-QoL (quality of life) score are validated for evaluation of LUTS in clinical trials, with lower scores indicating less severe LUTS. Additional efficacy parameters were maximum flow rate, average urinary flow rate, voided volume, and post-residual urine. Uroflowmetry was performed using a standard calibrated flowmeter (B&K Medical, Herlev, Denmark). Age, body mass index, prostate-specific antigen, and mean prostatic volume were also recorded. Safety assessments consisted of treatment-emergent adverse events (TEAEs) and serious adverse events.
Statistical analysis
The statistical analysis was performed on an intent-to-treat basis for all patients who were randomized and completed treatment with the open-label study medications. Participants were excluded from the primary and secondary efficacy analyses if no post-baseline data were available. Means and proportions for baseline patient demographic and clinical characteristics were compared using the χ2 test. For demographic and efficacy parameters, the randomization visit, ie, the start of the open-label treatment period, was taken to be the baseline.
For the integrated analysis set, the baseline demographic and clinical characteristics were generalized in the open-label period for all randomized subjects by using descriptive statistics. For continuous values, efficacies in supine systolic BP and diastolic BP were calculated as the mean difference in change from baseline to endpoint between groups 1 and 2 using analysis of covariance models with terms of normotensive and untreated hypertensive in group 1, as well as controlled hypertensive and uncontrolled hypertensive in group 2.
Mean changes from baseline to endpoint in total IPSS score, IPSS-QoL score, maximum flow rate, average urinary flow rate, voided volume, and post-residual urine (12 weeks or last recorded measurement) were compared between groups 1 and 2. TEAEs were analyzed using Breslow-Day tests for homogeneity of risk ratios at a significance level of 0.10. All efficacy measures were tested at a two-sided significance level of 0.05. Unless otherwise stated, efficacy data are expressed as the mean ± standard deviation.
Results
The study included 335 subjects, of whom 276 (82.38%) were randomized to treatment (n=136 in group 1 and n=140 in group 2, Figure 1). Approximately 76.8% (n=212) of the 276 patients completed the open-label treatment period, ie, 100/136 (73.5%) in group 1 and 112/140 (80%) in group 2. Baseline demographic and clinical characteristics were similar between the two groups (Table 3). The mean age of the subjects was 64.02±7.67 years, and 54% were ≥65 years of age. At randomization, 43% of subjects had a total IPSS score >20 and 29.7% had an IPSS-QoL score <6. There was no significant difference in any baseline value between the two groups.
Changes in BP after 12 weeks of treatment
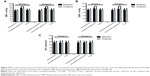
Figure 2 shows the mean changes from baseline in systolic BP, diastolic BP, and heart rate at 12 weeks stratified according to the normotensive and untreated hypertensive subgroups in group 1 and controlled and uncontrolled hypertensive subgroups in group 2.
Alfuzosin 10 mg alone or combined with antihypertensive therapy produced clinically and statistically significant decreases in systolic and diastolic BP in untreated hypertensive patients in group 1 and in patients with uncontrolled hypertension in group 2 (both P<0.001; Figure 2A and B). In contrast, there were no clinically significant mean BP changes in the normotensive subgroup in group 1 or in the subgroup with controlled hypertension in group 2; moreover, there were no statistically significant decreases in systolic BP (P=0.78 and P=0.73, respectively; Figure 2A) or diastolic BP (P=0.55 and P=0.48, respectively; Figure 2B) in either subgroup. There were no clinically or statistically significant changes in heart rate in any BP subgroup (P=0.68, P=0.30, P=0.72, and P=0.32 for the normotensive and untreated hypertensive subgroups in group 1 and the controlled hypertensive and uncontrolled hypertensive subgroups in group 2, respectively; Figure 2C).
Mean changes in BP and heart rate from baseline to endpoint are shown in Figure 3 for the four subgroups. In the subgroup with untreated hypertension from group 1, the mean 12-week decreases in systolic and diastolic BP were −11.3 mmHg and −5.1 mmHg, respectively (Figure 3A and B). In the subgroup with uncontrolled hypertension from group 2, the mean decreases in systolic and diastolic BP were −9.9 mmHg and −2.9 mmHg, respectively (Figure 3A and B). In contrast, the mean changes in systolic BP and diastolic BP in the normotensive subgroup from group 1 were 0.5 mmHg and −0.6 mmHg, and in the controlled hypertensive subgroup from group 2 were 0.7 mmHg and −0.9 mmHg, respectively (Figure 3A and B).
Compared with group 1, alfuzosin 10 mg combination therapy in group 2 decreased the mean systolic BP and diastolic BP from baseline to endpoint, albeit not in a statistically significant manner (Figure 3A and B). The mean change in systolic BP in the overall group from baseline to endpoint was higher in the alfuzosin 10 mg group (−2.3±14.3 mmHg) relative to a negative change in group 1 (−1.9±11.6 mmHg). The change in diastolic BP was similar between group 2 (−1.5±10.1 mmHg) and group 1 (−1.5±8.6 mmHg). There was a minimal decrease in supine heart rate in patients receiving alfuzosin 10 mg monotherapy in group 1 (−0.1±9.7 beats per minute) and a minimal increase in those receiving combined therapy in group 2 (0.1±9.7 beats per minute). All of these findings were minor, with none being clinically significant between group 1 and group 2 (systolic BP, P=0.825; diastolic BP, P>0.99; and heart rate, P=0.87; Figure 3C).
Efficacy after 12 weeks of treatment
Statistically significant differences in total IPSS and IPSS-QoL scores between groups 1 and 2 were observed at endpoint when compared with baseline (both P<0.001, Figure 4A and B). A significant improvement in maximum flow rate was seen only in group 2 (15.49±7.89 mL/sec at endpoint versus 13.19±6.58 mL/sec at baseline, respectively). Changes in post-residual urine between the two groups, as well as maximum flow rate in group 1 were minor, with no significant differences (all P>0.05, Figure 4D–F).
The change in total IPSS score from baseline to week 12 in group 1 failed to reach statistical significance relative to group 2 (mean difference 0.45, 95% confidence interval −1.26, 2.16; P=0.61). Further, the change in IPSS-QoL score was not significantly improved in group 1 at 12 weeks when compared with group 2 (0.12, 95% confidence interval −0.21, 0.45, P=0.47). In comparison with group 1, the mean change in maximum flow rate from baseline to endpoint in group 2 was not significant (P=0.28), as was the case for average urinary flow rate (P=0.18), voided volume ((P=0.78), and post-residual urine (P=0.08, Table 4).
Safety and tolerability
Safety was evaluated on the basis of the proportion of patients with at least one TEAE in the total study population (Table 5). Overall rates of withdrawal, discontinuation because of adverse events, and one TEAE or more, including both vasodilation-related and sexual function-related adverse events, were also summarized. Similar to previous reports, the overall proportion of subjects on alfuzosin 10 mg reporting TEAEs was relatively low. The adverse events observed were similar to those reported in previous studies of alfuzosin, with headache and dizziness being the most common. Discontinuation due to TEAEs was not common across this current study, and serious adverse events were rare.
In the overall study population, the proportion of patients with at least one TEAE (Table 5) was higher in those taking alfuzosin 10 mg in combination with antihypertensive medication than in those on alfuzosin 10 mg as monotherapy (17.85% versus 13.24%, P=0.29). The most common TEAEs in the monotherapy group and in the combination therapy group were headache (1.47% and 2.14%, respectively; P=0.67), dizziness/postural dizziness (5.88% and 4.28%, respectively; P=0.54), hypotension/postural hypotension (0.74% and 1.43%, respectively; P=0.58), and syncope (0% and 1.43%, respectively; P=0.31). The incidence of sexual function-related adverse events was also not significantly different between groups 1 and 2 (1.47% and 1.43%, respectively; P=0.97).
Withdrawals due to all causes were more common in patients receiving alfuzosin 10 mg monotherapy than in those on alfuzosin 10 mg as part of combination therapy (26.0% in group 1 and 20% in group 2; P=0.21). The rate of discontinuation because of adverse events in group 2 was similar to that in group 1 (6.43% and 5.15%, respectively; P=0.64, Table 5).
Discussion
This study demonstrates that alfuzosin 10 mg has no clinically important effects on BP when used to treat BPH/LUTS in men who are either physiologically normotensive or whose BP is regulated by other antihypertensive medications. The results provide reassurance for clinicians when prescribing an α-blocker, such as alfuzosin 10 mg, for a patient who is already on antihypertensive therapy, without the need to worry about the risk of hypotensive episodes. Further, the data suggest that alfuzosin 10 mg used as monotherapy might adequately control both BPH/LUTS and hypertension.
The incidences of BPH/LUTS and hypertension increase with age, and both are common in elderly males.15 Hence, it could be anticipated that many patients with BPH/LUTS concomitantly suffer from hypertension and vice versa. Patients with moderate or severe symptoms of BPH/LUTS should seek medical treatment.16 Due to the chronic nature of both BPH/LUTS and hypertension, safer and consistently effective medications are the best option. Current nonsurgical options favor α1-adrenoceptor antagonists, including alfuzosin, for the rapid and sustained relief of LUTS. The efficacy of alfuzosin in the treatment of BPH/LUTS has been well documented.17–21 In one of the studies, two doses of alfuzosin (10 mg and 15 mg) or tamsulosin 0.4 mg once daily were evaluated for treating symptomatic BPH/LUTS. Both medications had similar cardiovascular tolerability, and changes in BP were relatively lower with alfuzosin than with tamsulosin.21 The favorable safety profile of alfuzosin from a cardiovascular perspective might account for its preferential distribution in the human prostate.22
The current study also demonstrates that alfuzosin 10 mg was well tolerated by the cardiovascular system in the longer term. The overall discontinuation rate was 5.79% and the adverse event rate was relatively low. Adverse events related to the cardiovascular system were also few in number, with the most frequently reported events being dizziness and headache. Further, no significant change in BP or heart rate was observed in patients on alfuzosin 10 mg, including in elderly subjects with hypertension. These outcomes are consistent with those reported previously for alfuzosin 10 mg in real-life practice.18,23 This more favorable safety profile might reflect the pharmacokinetic properties of the 10 mg dose of alfuzosin. Therefore, the favorable pharmacokinetic profile of alfuzosin underlined the effectiveness on LUTS without obvious adverse events.
The improvements in total IPSS and IPSS-QoL scores with alfuzosin 10 mg at the 12-week endpoint in this study was consistent with those in several previous reports.18,19,24–26 In contrast with the findings for total IPSS and IPSS-QoL, only a numeric (rather than statistically significant) improvement in maximum flow rate was observed with alfuzosin 10 mg. The present findings did not indicate difference from previous studies for baseline demographics or clinical characteristics from Europe.18,19,24,25
The advantage of the present study is that it evaluated patients according to the presence or absence of treatment and hypertension at randomization, and assessed mean changes in BP on concomitant antihypertensive medication. Although the current study was one of standard duration for evaluating the efficacy of alfuzosin in patients with BPH/LUTS, it did not address the longer-term efficacy of alfuzosin or its impact on disease progression.
Conclusion
This short-term randomized study indicates the efficacy and safety of alfuzosin 10 mg alone or in combination with other antihypertensive medications in patients with BPH/LUTS. The changes in BP were marginal and well tolerated. However, alfuzosin 10 mg significantly decreased BP in patients with uncontrolled or untreated hypertension when compared with those who were normotensive or had controlled hypertension. Therefore, patients with uncontrolled or untreated hypertension require careful evaluation before initiation of treatment with alfuzosin 10 mg to treat BPH/LUTS. A long-term trial evaluating the efficacy of alfuzosin 10 mg in preventing the clinical progression of BPH/LUTS is warranted.
Acknowledgment
This study was sponsored by the Research Foundation of the Korean Society for Sexual Medicine and Andrology, which received funding from Handok Pharmaceuticals Co Ltd, Seoul, Republic of Korea. However, the funders had a financial role only, and had no role in the study design, data collection, or analysis, or in the decision to publish.
Author contributions
JKP: concept/design, data acquisition, and critical revision and approval of the manuscript. LTZ: statistics, data analysis/interpretation, drafting paper, and critical revision and approval of the manuscript. SWL, KP, WSC, SWK, JSH, DGM, S-KY, JKR, DYY, KHM, and KSM: concept/design, data acquisition, and critical revision and approval of the manuscript.
Disclosure
The authors certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript are as follows: LTZ, JKP, KHM, and KSM report no conflicts of interest. SWL, KP, WSC, SWK, JSH, DGM, S-KY, JKR, and DYY had presentation as a speaker at the meeting, which was hold by the Handok pharmaceutical manufacturer during the study. SWL, KP, WSC, SWK, JSH, DGM, S-KY, JKR, and DYY had nothing to disclose. Handok Pharmaceuticals Co. Ltd., Republic of Korea had no role in the design or conduct of this study, collection, management, analysis, and interpretation of the data or preparation, review, and approval of the manuscript.
References
Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. | ||
Garraway WM, Collins GN, Lee RJ. High prevalence of benign prostatic hypertrophy in the community. Lancet. 1991;338:469–471. | ||
McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793–1803. | ||
Madersbacher S, Alivizatos G, Nordling J, et al. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol. 2004;46:547–554. | ||
McVary KT. Alfuzosin for symptomatic benign prostatic hyperplasia: long-term experience. J Urol. 2006;175:35–42. | ||
Roehrborn CG, Bruskewitz R, Nickel GC, et al. Urinary retention in patients with BPH treated with finasteride or placebo over 4 years – characterization of patients and ultimate outcomes. Eur Urol. 2000;37:528–536. | ||
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. | ||
Mondaini N, Giubilei G, Ungar A, et al. Alfuzosin (10mg) does not affect blood pressure in young healthy men. Eur Urol. 2006;50:1292–1298. | ||
Kaplan SA. Uroselective alpha-blockade for benign prostatic hyperplasia: clinically significant or marketing savvy? Urology. 1999;54:776–779. | ||
Chau LH, Tai DC, Fung BT, Li JC, Fan CW, Li MK. Medical expulsive therapy using alfuzosin for patient presenting with ureteral stone less than 10 mm: a prospective randomized controlled trial. Int J Urol. 2011;18:510–514. | ||
Ahmed AF, Al-Sayed AY. Tamsulosin versus alfuzosin in the treatment of patients with distal ureteral stones: prospective, randomized, comparative study. Korean J Urol. 2010;51:193–197. | ||
Mehik A, Alas P, Nickel JC, Sarpola A, Helström PJ. Alfuzosin treatment for chronic prostatitis/chronic pelvic pain syndrome: a prospective, randomized, double-blind, placebo-controlled, pilot study. Urology. 2003;62:425–429. | ||
Basar MM, Atan A, Ozergin O, Yildiz M. The efficacy of alfuzosin treatment in patients with prostatism. Int Urol Nephrol. 2001;33:493–497. | ||
Rowe E, Smith C, Laverick L, Elkabir J, Witherow RO, Patel A. A prospective, randomized, placebo controlled, double-blind study of pelvic electromagnetic therapy for the treatment of chronic pelvic pain syndrome with 1 year of followup. J Urol. 2005;173:2044–2047. | ||
Michel MC, Heemann U, Schumacher H, Mehlburger L, Goepel M. Association of hypertension with symptoms of benign prostatic hyperplasia. J Urol. 2004;172:1390–1393. | ||
AUA Practice Guidelines Committee. AUA guideline on management of benign prostatic hyperplasia (2003). Chapter 1: diagnosis and treatment recommendation. J Urol. 2003;170(2 Pt 1):530–547. | ||
Song K, Choo MS, Lee KS, et al. The long-term effect of alfuzosin in patients with lower urinary tract symptoms suggestive of benign prostate hyperplasia: evaluation of voiding and storage function with respect to bladder outlet obstruction grade and contractility. Urology. 2011;77:1177–1182. | ||
Vallancien G, Emberton M, Alcaraz A, et al; ALF-ONE Study Group. Alfuzosin 10 mg once daily for treating benign prostatic hyperplasia: a 3-year experience in real-life practice. BJU Int. 2008;101:847–852. | ||
Roehrborn CG. Alfuzosin 10 mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: results of a 2-year placebo-controlled study. BJU Int. 2006;97:734–741. | ||
Nickel JC, Elhilali M, Emberton M, Vallancien G; Alf-One Study Group. The beneficial effect of alfuzosin 10 mg once daily in ‘real-life’ practice on lower urinary tract symptoms (LUTS), quality of life and sexual dysfunction in men with LUTS and painful ejaculation. BJU Int. 2006;97:1242–1246. | ||
Nordling J. Efficacy and safety of two doses (10 and 15 mg) of alfuzosin or tamsulosin (0.4 mg) once daily for treating symptomatic benign prostatic hyperplasia. BJU Int. 2005;95:1006–1012. | ||
Mottet N, Bressolle F, Delmas V, Robert M, Costa P. Prostatic tissual distribution of alfuzosin in patients with benign prostatic hyperplasia following repeated oral administration. Eur Urol. 2003;44:101–105. | ||
Elhilali M, Emberton M, Matzkin H, et al. Long-term efficacy and safety of alfuzosin 10 mg once daily: a 2-year experience in ‘real-life’ practice. BJU Int. 2006;97:513–519. | ||
Roehrborn C, Van Kerrebroeck P, Nordling J. Safety and efficacy of alfuzosin 10 mg once-daily in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a pooled analysis of three double-blind, placebo-controlled studies. BJU Int. 2003;92:257–261. | ||
van Kerrebroeck P, Jardin A, van Cangh P, Laval KU; ALFORTI Study Group. Long-term safety and efficacy of a once-daily formulation of alfuzosin 10 mg in patients with symptomatic benign prostatic hyperplasia: open-label extension study. Eur Urol. 2002;41:54–60. | ||
Lee SH, Park KK, Mah SY, et al. Effects of alpha-blocker ‘add on’ treatment on blood pressure in symptomatic BPH with or without concomitant hypertension. Prostate Cancer Prostatic Dis. 2010;13:333–337. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.