Back to Journals » Clinical Interventions in Aging » Volume 13
mRNA expressions of peroxisome proliferator-activated receptor gamma coactivator 1α, tumor necrosis factor-α, and interleukin-6 in paraspinal muscles of patients with lumbar kyphosis: a preliminary study
Authors Kudo D , Miyakoshi N , Hongo M , Kasukawa Y , Ishikawa Y , Fujii M, Shimada Y
Received 3 May 2018
Accepted for publication 24 July 2018
Published 7 September 2018 Volume 2018:13 Pages 1633—1638
DOI https://doi.org/10.2147/CIA.S172952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Daisuke Kudo, Naohisa Miyakoshi, Michio Hongo, Yuji Kasukawa, Yoshinori Ishikawa, Masashi Fujii, Yoichi Shimada
Department of Orthopedic Surgery, Akita University Graduate School of Medicine, Akita, Japan
Background: Kyphosis is a spine deformity that can lead to falls and reduced quality of life. Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) regulates mitochondrial biogenesis and is important for proper functioning of skeletal muscle, including the paraspinal muscles, which support and allow movement of the spine. The role of PGC-1α in paraspinal muscles in lumbar kyphosis has not been examined. We also examined the expressions of the proinflammatory cytokines tumor necrosis factor (TNF)-α and interleukin (IL)-6.
Methods: We obtained paraspinal muscle specimens from 12 patients who underwent posterior lumbar surgery. RNA was isolated from these samples, and quantitative PCR was performed to compare the expression levels of PGC-1α, TNF-α, and IL-6 mRNA between patients with decreased lumbar lordosis (LL) and normal LL patients.
Results: TNF-α and IL-6 mRNA expressions in paraspinal muscles were significantly higher in the decreased LL group than in the normal LL group (P=0.048 for both). PGC-1α mRNA expression was slightly increased in the decreased LL group, but the difference was not significant. Age was significantly positively correlated with PGC-1α mRNA expression (P=0.010). PGC-1α mRNA expression was significantly positively correlated with TNF-α mRNA expression (P=0.022). LL was significantly negatively correlated with PGC-1α, TNF-α, and IL-6 mRNA expressions (P=0.015, 0.036, and 0.010, respectively).
Conclusion: TNF-α and IL-6 mRNA expressions in paraspinal muscles were significantly higher in the decreased LL group than in the normal LL group. LL was significantly negatively correlated with PGC-1α, TNF-α, and IL-6 mRNA expressions. PGC-1α mRNA expression levels in paraspinal muscles may be affected by lumbar kyphosis.
Keywords: aging, PGC-1α, TNF-α, IL-6, lumbar kyphosis
Introduction
Spinal deformities, especially kyphosis, due to aging and its related problems, are important concerns for spine surgeons.1,2 Kyphotic deformities negatively impact quality of life (QOL) and postural balance, leading to falls in elderly or osteoporotic patients.3–5 In particular, sagittal spinal malalignment has a greater impact on QOL scores than coronal malalignment in adult patients with spine deformities.6,7 Generally, degenerative kyphosis is related to back muscle atrophy and decreased back extensor strength,8,9 the latter of which is a risk factor for falls and poor QOL.5,10 On the other hand, back muscle atrophy and fatty degeneration are also observed in patients with chronic lower back pain.11,12 Back-strengthening exercises increase both back extensor strength and QOL scores in postmenopausal women.13
Age-related loss of skeletal muscle mass and function, that is, sarcopenia, is a serious health problem. The etiology of sarcopenia is considered to be multifactorial and includes disuse, changes in endocrine function, chronic diseases, inflammation, insulin resistance, and nutritional deficiencies.14 Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) is a master regulator of mitochondrial biogenesis and a possible therapeutic target for age-related mitochondrial dysfunction in skeletal muscle. PGC-1α can protect against skeletal muscle wasting by regulating autophagy and is also required for stabilization of the neuromuscular junction program.15–17 Furthermore, chronic inflammation due to a sedentary lifestyle is linked with many chronic diseases including musculoskeletal disorders. Normally, PGC-1α expression in the skeletal muscle of older subjects is decreased, leading to loss of muscle function due to low mitochondriogenesis.18–20 Interestingly, PGC-1α plays an important antiinflammatory role.21 In fact, skeletal muscle tissue in skeletal muscle-specific PGC-1α knockout mice shows increased expressions of several proinflammatory genes such as tumor necrosis factor (TNF)-α and interleukin (IL)-6, and these mice also show elevated levels of circulating IL-6.22 In addition, chronic elevation of IL-6 and TNF-α results in skeletal muscle atrophy and inhibition of muscle regeneration.23–25 Administration of IL-6 or TNF-α to rats increases skeletal muscle protein breakdown, decreases protein synthesis and the amino acid concentration in skeletal muscle, and causes muscle wasting.26–29
To our knowledge, no studies have reported the impact of PGC-1α, TNF-α, and IL-6 expressions in paraspinal muscles on lumbar kyphosis. We hypothesized that decreased expression of PGC-1α and increased expressions of TNF-α and IL-6 in paraspinal muscles are associated with lumbar kyphosis due to muscle dysfunction. Herein, we examined the relationship among PGC-1α, TNF-α, and IL-6 expressions in paraspinal muscles and lumbar kyphosis in human subjects.
Materials and methods
Subjects
The study protocol was approved by the ethics committee of Akita University Graduate School of Medicine. Written informed consent was obtained from each patient. Twelve patients (seven males, five females; median age at the time of surgery, 61 years, range, 18–89 years) requiring posterior lumbar surgery were enrolled in this study. Preoperative lumbar lordosis (LL) was measured using X-ray in the upright position (Table 1). Five patients were diagnosed with lumbar spinal stenosis (LSS), three were diagnosed with lumbar disc herniation, and one patient each was diagnosed with lumbar vertebral fracture, lumbar degenerative kyphosis, lumbar degenerative kyphosis with LSS and an old lumbar vertebral fracture, and osteoid osteoma of the lumbar spine.
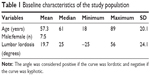  | Table 1 Baseline characteristics of the study population |
Patients were divided into a decreased LL group (n=7), which was defined as less than −2SD (33.8°) according to a previous study in the Japanese population,30 and a normal LL group (n=5).
Preparation of muscle specimens, RNA isolation, and analysis of gene expression
All patients underwent posterior decompression with/without instrumented fusion except for the patient with osteoid osteoma who underwent tumor excision. Bilateral multifidus muscles were obtained during surgery, immediately placed in sterile tubes, frozen in liquid nitrogen, and stored at −80°C until analysis. Specimens were homogenized using ISOGEN (Nippon Gene, Toyama, Japan) and then centrifuged at 12,000× g at 4°C for 10 minutes. Chloroform (200 μL) was added to the supernatant, and the mixture was centrifuged at 12,000× g at 4°C for 15 minutes. Isopropanol was added to an equivalent amount of supernatant. Then, 1 mL ethanol was added to the pellet after the supernatant was discarded, and the mixture was centrifuged at 7,500× g at 4°C for 5 minutes. The sediment was desiccated at room temperature, resuspended in 100 μL diethylpyrocarbonate-treated water, and quantified by spectrophotometry (A260/280). The RNA concentration was determined by absorbance at 260 nm using a U-2000 spectrophotometer (Hitachi Ltd., Tokyo, Japan). Isolated total RNA was treated with DNase I (Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions. Then, cDNA was synthesized using the First Strand cDNA Synthesis Kit (GE Healthcare, Buckinghamshire, UK) according to the manufacturer’s instructions. Specific primers for human PGC-1α, TNF-α, and IL-6 (QIAGEN, Tokyo, Japan) were used for quantitative polymerase chain reaction (qPCR). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified as an endogenous control gene for normalization. GAPDH primers were synthesized by Greiner Japan in Tokyo. LightCycler 480® (Roche Diagnostics, Laval, QC, Canada) and LightCycler 480 SYBR Green 1 Master (Roche Diagnostics) were used for qPCR. Reaction conditions were as follows: 5 minutes at 95°C (denaturation step); 45 cycles of 10 seconds at 95°C, 10 seconds at 58°C, and 10 seconds at 72°C; 5 seconds at 95°C; 65°C–98°C at a rate of 2.2°C/s (melting curve analysis). The relative quantification method for gene expression was performed using the E-Method,31 and gene expression was analyzed as the mean value of both sides of the paraspinal muscles. PGC-1α, TNF-α, and IL-6 mRNA expressions were normalized to GAPDH mRNA expression.
Statistical analysis
The statistical software package IBM SPSS for Mac Version 22.0 (IBM Corp., Armonk, NY, USA) was used for all data analyses. The Mann–Whitney U test was used for comparison of variables, and Fisher’s exact test was used for comparison of categorical variables between the decreased LL group and the normal group. Relationships among variables were analyzed using Spearman’s correlation coefficient. P-values <0.05 were considered statistically significant.
Results
TNF-α and IL-6 mRNA expressions in paraspinal muscle were significantly higher in the decreased LL group than in the normal LL group (P=0.048 for both; Table 2). The correlations between variables are shown in Table 3. LL was significantly negatively correlated with PGC-1α, TNF-α, and IL-6 mRNA expressions. Age was significantly positively correlated with PGC-1α mRNA expression and tended to be negatively correlated with LL. PGC-1α mRNA expression was significantly positively correlated with TNF-α mRNA expression and tended to be positively correlated with IL-6 mRNA expression.
Discussion
We predicted that age-related low levels of PGC-1α mRNA expression in paraspinal muscles and an increase in TNF-α and IL-6 mRNA expressions may be associated with back muscle dysfunction and may lead to lumbar kyphosis. Contrary to our expectation, PGC-1α mRNA expression in paraspinal muscles was not only positively correlated with age but also negatively correlated with LL. These results are different from previous studies which demonstrated that lower PGC-1α mRNA expression is related to older age. Ghosh et al19 demonstrated reduced (by 42%) basal PGC-1α mRNA levels in human vastus lateralis muscle in older (age ≥65 years) subjects compared with younger (age 18–30 years) subjects (P<0.05). Lanza et al20 also demonstrated that endurance-trained older (59–76 years old) subjects showed lower PGC-1α protein expression in the vastus lateralis muscle than younger (18–30 years old) subjects. Interestingly, PGC-1α protein expression is higher in both endurance-trained older and younger subjects than in sedentary older and younger subjects. In our study subjects, PGC-1α mRNA expression was significantly positively correlated with TNF-α mRNA expression and tended to be positively correlated with IL-6 mRNA expression. In contrast, Handschin et al32 reported that PGC-1α expression is negatively correlated with IL-6 expression (r=0.184, P=0.005) and TNF-α expression (r=0.137, P=0.016) in human vastus lateralis muscles. The reason for these discrepancies between our results and previous studies may be the different muscles examined. Paraspinal muscle activity is increased in lumbar kyphosis patients compared with age-matched LSS patients.33 Chronic increased paraspinal muscle activity in our study subjects may have accelerated PGC-1α mRNA expression in the paraspinal muscles. In fact, Pilegaard et al34 demonstrated that PGC-1α transcription and mRNA content in vastus lateralis muscles increase after exercise, especially in the trained leg compared with the untrained leg. In addition, exercise-induced upregulation of PGC-1α is not limited by aging. Ghosh et al19 demonstrated that exercise increases PGC-1α mRNA levels in older subjects by 2.6-fold compared with preexercise mRNA levels (P<0.05). Cobley et al35 also demonstrated that PGC-1α mRNA expression increases significantly (P=0.002) 3 hours after exercise in the vastus lateralis muscles of sedentary elderly males, and the increase is not influenced by age (P=0.604) or training status (P=0.992). Taken together, these observations suggest that kyphotic deformities of the spine induce upregulation of PGC-1α mRNA expression in paraspinal muscles, regardless of aging.
Increased expressions of IL-6 and TNF-α mRNA in paraspinal muscles in the decreased LL group in this study may have affected back muscle dysfunction.23–29 However, the role of IL-6 in skeletal muscle is still controversial. Serrano et al reported that IL-6 is an essential regulator of satellite cells.36 Moreover, IL-6 mRNA expression in human vastus lateralis muscle increases after exercise, but no significant increase in TNF-α mRNA expression was observed.37,38 Therefore, we cannot conclude whether the increased IL-6 mRNA expression in the decreased LL group was due to chronic inflammation or secondary changes due to increased paraspinal muscle activity.
One of our interests is a possible treatment strategy using pharmacological interventions that modulate PGC-1α expression to prevent back muscle weakness in the elderly. The efficacy of PGC-1α upregulation on muscle preservation has been suggested by several studies. Wenz et al39 reported that MCK-PGC-1α mice (PGC-1α driven by the muscle creatine kinase promoter) show preserved mitochondrial function, neuromuscular junctions, and muscle integrity during aging due to reduced apoptosis, autophagy, and proteasome degradation. Furthermore, these PGC-1α transgenic mice exhibit improved endurance with conversion to slow-twitch, fatigue-resistant, muscle fibers.15,40 Qiao et al41 reported that adiponectin, which is an adipocyte-derived hormone that regulates energy homeostasis, enhances p38 mitogen-activated protein kinase (MAPK) phosphorylation, PGC-1α expression, and mitochondrial biogenesis by suppressing MAPK phosphatase-1 expression in C2C12 myotubes. These results suggest the possibility of pharmacological treatment to enhance PGC-1α expression in skeletal muscle. Okada-Iwabu et al42 identified “AdipoRon” as an adiponectin receptor agonist and demonstrated that adiponectin/adiponectin receptor 1 signaling improves metabolism and exercise endurance by increasing mitochondrial volume and function similar to physical activity via PGC-1α upregulation. These data suggest that pharmacological interventions via modulation of adiponectin/adiponectin receptor 1 signaling may improve age-related muscle wasting.
Our study has some limitations. First, the number of participants in this study was small because this was a preliminary study. Second, to what degree paraspinal muscle activity influences mRNA expression in the decreased LL group is unclear because participants in this study did not undergo electromyography before surgery.
Conclusion
TNF-α and IL-6 mRNA expressions in paraspinal muscles were significantly higher in the decreased LL group than in the normal LL group. LL was significantly negatively correlated with PGC-1α, TNF-α, and IL-6 mRNA expressions. Age was also significantly positively correlated with PGC-1α mRNA expression in paraspinal muscles. Increased PGC-1α mRNA expression in paraspinal muscles may be affected by lumbar kyphosis.
Acknowledgment
The authors would like to thank K. Iwamoto (Bioscience Education and Research Center, Akita University Graduate School of Medicine, Akita City, Japan) for support with qPCR.
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Glassman SD, Bridwell K, Dimar JR, et al. The impact of positive sagittal balance in adult spinal deformity. Spine. 2005;30(18):2024–2029. | ||
Schwab F, Lafage V, Patel A, Farcy JP. Sagittal plane considerations and the pelvis in the adult patient. Spine. 2009;34(17):1828–1833. | ||
Miyakoshi N, Itoi E, Kobayashi M, Kodama H. Impact of postural deformities and spinal mobility on quality of life in postmenopausal osteoporosis. Osteoporos Int. 2003;14(12):1007–1012. | ||
Ishikawa Y, Miyakoshi N, Kasukawa Y, Hongo M, Shimada Y. Spinal curvature and postural balance in patients with osteoporosis. Osteoporos Int. 2009;20(12):2049–2053. | ||
Kasukawa Y, Miyakoshi N, Hongo M, et al. Relationships between falls, spinal curvature, spinal mobility and back extensor strength in elderly people. J Bone Miner Metab. 2010;28(1):82–87. | ||
Glassman SD, Berven S, Bridwell K, Horton W, Dimar JR. Correlation of radiographic parameters and clinical symptoms in adult scoliosis. Spine. 2005;30(6):682–688. | ||
Lafage V, Schwab F, Patel A, Hawkinson N, Farcy JP. Pelvic tilt and truncal inclination: two key radiographic parameters in the setting of adults with spinal deformity. Spine. 2009;34(17):E599–E606. | ||
Kasukawa Y, Miyakoshi N, Hongo M, et al. Age-related changes in muscle strength and spinal kyphosis angles in an elderly Japanese population. Clin Interv Aging. 2017;12:413–420. | ||
Sinaki M, Itoi E, Rogers JW, Bergstralh EJ, Wahner HW. Correlation of back extensor strength with thoracic kyphosis and lumbar lordosis in estrogen-deficient women. Am J Phys Med Rehabil. 1996;75(5):370–374. | ||
Miyakoshi N, Hongo M, Maekawa S, et al. Back extensor strength and lumbar spinal mobility are predictors of quality of life in patients with postmenopausal osteoporosis. Osteoporos Int. 2007;18(10):1397–1403. | ||
Chaléat-Valayer E, Mac-Thiong JM, Paquet J, et al. Sagittal spino-pelvic alignment in chronic low back pain. Eur Spine J. 2011;20(Suppl 5):634–640. | ||
Barker KL, Shamley DR, Jackson D. Changes in the cross-sectional area of multifidus and psoas in patients with unilateral back pain: the relationship to pain and disability. Spine. 2004;29(22):E515–E519. | ||
Hongo M, Itoi E, Sinaki M, et al. Effect of low-intensity back exercise on quality of life and back extensor strength in patients with osteoporosis: a randomized controlled trial. Osteoporos Int. 2007;18(10):1389–1395. | ||
Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12(4):249–256. | ||
Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. | ||
Sandri M, Lin J, Handschin C, et al. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci U S A. 2006;103(44):16260–16265. | ||
Handschin C, Kobayashi YM, Chin S, et al. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21(7):770–783. | ||
Derbré F, Gomez-Cabrera MC, Nascimento AL, et al. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1α to exercise training. Age. 2012;34(3):669–679. | ||
Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60(8):2051–2060. | ||
Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57(11):2933–2942. | ||
Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–469. | ||
Handschin C, Choi CS, Chin S, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117(11):3463–3474. | ||
Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–917. | ||
Coletti D, Moresi V, Adamo S, Molinaro M, Sassoon D. Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis. 2005;43(3):120–128. | ||
Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57(5):M326–M332. | ||
García-Martínez C, López-Soriano FJ, Argilés JM. Acute treatment with tumour necrosis factor-alpha induces changes in protein metabolism in rat skeletal muscle. Mol Cell Biochem. 1993;125(1):11–18. | ||
Goodman MN. Tumor necrosis factor induces skeletal muscle protein breakdown in rats. Am J Physiol. 1991;260(5 Pt 1):E727–E730. | ||
Goodman MN. Interleukin-6 induces skeletal muscle protein breakdown in rats. Proc Soc Exp Biol Med. 1994;205(2):182–185. | ||
Tayek JA. Effects of tumor necrosis factor alpha on skeletal muscle amino acid metabolism studied in-vivo. J Am Coll Nutr. 1996;15(2):164–168. | ||
Kanemura T, Yoshida G, Ishikawa Y, et al. Sagittal spino-pelvic alignment in an asymptomatic Japanese population: comparison of Western population. J Spine Res. 2011;2:52–58. | ||
Tellmann G. The E-Method: a highly accurate technique for gene-expression analysis. Nat Methods. 2006;3(7):i–ii. | ||
Handschin C, Chin S, Li P, et al. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282(41):30014–30021. | ||
Enomoto M, Ukegawa D, Sakaki K, et al. Increase in paravertebral muscle activity in lumbar kyphosis patients by surface electromyography compared with lumbar spinal canal stenosis patients and healthy volunteers. J Spinal Disord Tech. 2012;25(6):E167–E173. | ||
Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546(Pt 3):851–858. | ||
Cobley JN, Bartlett JD, Kayani A, et al. PGC-1α transcriptional response and mitochondrial adaptation to acute exercise is maintained in skeletal muscle of sedentary elderly males. Biogerontology. 2012;13(6):621–631. | ||
Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7(1):33–44. | ||
Steensberg A, Keller C, Starkie RL, et al. IL-6 and TNF-alpha expression in, and release from, contracting human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283(6):E1272–E1278. | ||
Pedersen BK, Fischer CP. Beneficial health effects of exercise – the role of IL-6 as a myokine. Trends Pharmacol Sci. 2007;28(4):152–156. | ||
Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106(48):20405–20410. | ||
Calvo JA, Daniels TG, Wang X, et al. Muscle-specific expression of PPARgamma coactivator-1alpha improves exercise performance and increases peak oxygen uptake. J Appl Physiol. 2008;104(5):1304–1312. | ||
Qiao L, Kinney B, Yoo HS, et al. Adiponectin increases skeletal muscle mitochondrial biogenesis by suppressing mitogen-activated protein kinase phosphatase-1. Diabetes. 2012;61(6):1463–1470. | ||
Okada-Iwabu M, Iwabu M, Ueki K, Yamauchi T, Kadowaki T. Perspective of small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Diabetes Metab J. 2015;39(5):363–372. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


