Back to Journals » Patient Preference and Adherence » Volume 11
mHealth intervention to support asthma self-management in adolescents: the ADAPT study
Authors Kosse RC, Bouvy ML, de Vries TW , Kaptein AA , Geers HC , van Dijk L , Koster ES
Received 13 October 2016
Accepted for publication 27 December 2016
Published 16 March 2017 Volume 2017:11 Pages 571—577
DOI https://doi.org/10.2147/PPA.S124615
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Video abstract presented by Richelle C Kosse
Views: 454
Richelle C Kosse,1 Marcel L Bouvy,1 Tjalling W de Vries,2 Ad A Kaptein,3 Harm CJ Geers,1 Liset van Dijk,4 Ellen S Koster1
1Division of Pharmacoepidemiology and Clinical Pharmacology, Utrecht Institute for Pharmaceutical Sciences, Faculty of Science, Utrecht University, Utrecht, 2Department of Paediatrics, Medical Center Leeuwarden, Leeuwarden, 3Medical Psychology, Leiden University Medical Center, Leiden, 4NIVEL, the Netherlands Institute for Health Services Research, Utrecht, the Netherlands
Purpose: Poor medication adherence in adolescents with asthma results in poorly controlled disease and increased morbidity. The aim of the ADolescent Adherence Patient Tool (ADAPT) study is to develop an mHealth intervention to support self-management and to evaluate the effectiveness in improving medication adherence and asthma control.
Intervention: The ADAPT intervention consists of an interactive smartphone application (app) connected to a desktop application for health care providers, in this study, the community pharmacist. The app contains several functions to improve adherence as follows: 1) a questionnaire function to rate asthma symptoms and monitor these over time; 2) short movie clips with medication and disease information; 3) a medication reminder; 4) a chat function with peers; and 5) a chat function with the pharmacist. The pharmacist receives data from the patient’s app through the desktop application, which enables the pharmacist to send information and feedback to the patient.
Study design: The ADAPT intervention is tested in a community pharmacy-based cluster randomized controlled trial in the Netherlands, aiming to include 352 adolescents with asthma. The main outcome is adherence, measured by patient’s self-report and refill adherence calculated from pharmacy dispensing records. In addition, asthma control, illness perceptions, medication beliefs, and asthma-related quality of life are measured.
Conclusion: This study will provide in-depth knowledge on the effectiveness of an mHealth intervention to support asthma self-management in adolescents. These insights will also be useful for adolescents with other chronic diseases.
Keywords: adherence, adolescents, asthma, intervention, mHealth, pharmacist
Introduction
The estimated adherence rates with inhaled corticosteroids (ICSs) vary between 30% and 70%.1 These low adherence rates result in uncontrolled asthma and unnecessary hospitalizations, associated with decreased quality of life and school and work absenteeism.2–4 Poor adherence is a complex problem that can be divided into intentional (consciously deciding) and unintentional (forgetting or not being able to use medicines) non-adherence behaviors.5
Adherence rates decline during adolescence; studies report adherence rates of 50% at age 12, while rates <20% are found at age 17.6,7 During this life phase, adolescents become more responsible for their own medication use, which can affect their medication intake behavior.7 As adolescents perceive low necessity toward their medication use,8 positively affecting adolescents’ beliefs toward illness and treatment might result in increased adherence rates.9–12
The Common Sense Model of Self-Regulation (CSM) describes that illness perceptions and medication beliefs can affect adherence.13 This model has been extensively used to describe health behavior related to health threats.14 It proposes cognitive and emotional representations toward the health threat, resulting in illness perceptions and medication beliefs, which affect health behavior (e.g., adherence). This is a continuous process, allowing modifications of the representations and coping mechanisms.15 Therefore, affecting cognitive or emotional representations might result in a health behavior change.
Numerous interventions have been developed to increase medication adherence; however, study results are inconsistent and show low effectiveness for most interventions.16 Interventions tailored to individual’s needs are suggested to be more effective, because every individual might need a different approach to tackle his or her specific intentional and unintentional barriers affecting adherence.17 Most existing interventions to improve asthma medication adherence are intended for adults or children, and interventions among adolescents with asthma are scarce.16
To assess the specific needs and preferences of adolescents with asthma, we conducted a focus group study. In line with previous studies, this study revealed forgetting as a major reason for ICS non-adherence.18–20 The adolescents who participated in the focus group study recommended a smartphone application (app) as an intervention to support self-management. Peer support and personalized medication reminders were suggested as important parts of this intervention.21
In the Netherlands, 96% of the adolescents have a smartphone,22 suggesting that an app might be an appropriate intervention for adolescents. Although different mobile self-management applications for asthma exist, most of these interventions are not specifically intended for adolescents.23,24 Therefore, the ADolescent Adherence Patient Tool (ADAPT) is developed. The aim of our study is to evaluate the effectiveness of the ADAPT intervention to improve adherence and asthma control in adolescents with asthma.
Methods and design
Study design
Effectiveness of the ADAPT intervention is studied in a community pharmacy-based cluster randomized controlled trial. Community pharmacies affiliated with the Utrecht Pharmacy Practice network for Education and Research (UPPER) are approached to participate in the study.25 The participating community pharmacies are randomly divided into a control (6 months usual care) and an intervention group (6 months use of the ADAPT intervention; Figure 1). In the Netherlands, asthma usual care in pharmacies is defined as “regularly repeating the inhalation instruction and signaling of excessive bronchodilator or insufficient ICS use”.26
  | Figure 1 Design of the ADAPT asthma study. |
Patients meeting the inclusion criteria, described in the following, receive a postal invitation and informed consent from their community pharmacy. For patients younger than 16 years, both parents and the patient have to sign informed consent. Upon receiving the signed informed consent, the first online questionnaire (baseline measurement) is sent via e-mail. At the end of follow-up (after 6 months), the second online questionnaire is sent. Completion of the online questionnaires is monitored, and e-mail reminders are sent when needed.
Participants
The participating pharmacies are spread across the Netherlands (Figure 2). Adolescent asthma patients (aged 12–18 years) are selected from the pharmacy information system based on medication dispensing records. Adolescents who fill two or more prescriptions for ICS or ICS in combination with a long-acting beta-agonist (ICS/LABA) during the previous 12 months are eligible for inclusion. The asthma diagnosis is verified by the patient’s general practitioner. The possession of a smartphone is an additional inclusion criterion for the ADAPT study. Patients who use ICS for indications other than asthma, have insufficient comprehension of the Dutch language, or are unable to take medication themselves are excluded.
  | Figure 2 The ADAPT study locations in the Netherlands. |
Sample size
Medication Adherence Report Scale (MARS) scores are used to measure self-reported adherence at baseline and at the end of follow-up.27 To detect a relevant difference (>1.5±4.0) in MARS scores with a power of 80%, a significance level of 95%, and accounting for 35% dropout, 151 patients per group (intervention and control) are required. As a cluster randomized design is used, a correction factor of 1.16 is needed, based on inclusion of 10 patients and variation of 0.25 per pharmacy. This results in a sample size of 175.2 patients per group; thus, a total of 352 patients have to be included. Based on previous experiences, we estimate that ~25–30 patients per pharmacy will meet the inclusion criteria; therefore, inclusion of 10 patients per pharmacy should be feasible.
Study outcomes
The primary study outcome is “medication adherence” based on patient’s self-report (measured with the MARS at baseline and at the end of follow-up) and 5-year medication refill records. The secondary study outcome is asthma control. Moreover, asthma-related quality of life, medication beliefs, and illness perception are measured.
For all participants, data collection takes place through an online questionnaire at baseline (t=0) and at the end of follow-up (t=6). This online questionnaire contains questions regarding health-related quality of life (question derived from the RAND-36; developed by the RAND Corporation),28 asthma and allergic rhinitis symptoms (Control of Allergic Rhinitis and Asthma Test [CARAT]),29 asthma-related quality of life (Pediatric Asthma Quality of Life Questionnaire),30 illness perception (Brief Illness Perception Questionnaire),31 beliefs about medicines (Beliefs about Medicines Questionnaire-Specific),32 and adherence (MARS).27 The online questionnaire also contains sociodemographic questions. Additionally, 5-year medication history of pharmacy dispensing records is extracted from the pharmacy information system to calculate ICS refill adherence and to evaluate the use of concomitant medication. A cost-effectiveness evaluation will be conducted at the end of the study.
Process evaluation
During the study, the actual use of the intervention by pharmacists and patients (filling out questionnaires and viewing movies) is monitored. When patients wish to withdraw from the study, the reason for discontinuation will be registered. Pharmacists are regularly contacted to get feedback on the implementation of the intervention. At the end of the follow-up, both patients’ and pharmacists’ experiences, barriers/facilitators for further implementation, satisfaction with the intervention, and perceived effectiveness will be evaluated with a short online questionnaire.
ADAPT intervention
The ADAPT intervention consists of a smartphone app for patients, which is securely connected to a desktop application for health care providers, in this study the community pharmacist (Figure 3). Patients can download the ADAPT smartphone app, which is freely available in the App Store and Play Store. A password – provided by the patients’ health care provider – is necessary to gain access to the full functionality of the app and to connect the app to the desktop application used by the patient’s community pharmacist. This password entry ensures that solely participants of the intervention group can make full use of the app. To ensure the patient’s privacy, data are securely sent to the database and a personally chosen password is needed to access the app. The app contains different functionalities to stimulate self-management and improve adherence behavior.
- This app helps in monitoring symptoms, with the CARAT. This questionnaire covers both allergic rhinitis and asthma symptoms (Figure 4). A total score between 0 and 30 can be obtained, where scores >24 indicate good disease control. The total score can be divided into two subscores: an allergic rhinitis subscore (items 1–4, score >8 indicates good control) and an asthma subscore (items 5–10, score ≥16 indicates good control).29 Patients are asked to complete the CARAT questionnaire at least every week, and they can generate a graphical display of their disease control over time. This may influence their emotional representation of their asthma and allergic rhinitis symptoms, which can modify illness perception and indirectly affect adherence. The pharmacist can also monitor the CARAT scores through the linked desktop application.
- Short movies about different asthma-related topics, such as medication use and asthma triggers, are available next to more lifestyle-oriented movies on how to cope with asthma in daily life, such as smoking, going to school, and performing sports. These movies have been made with active participation of asthmatic adolescents. Patients occasionally receive new movies during the study or upon request, since pharmacists have access to a movie database. Pharmacists can choose to send, for example, an inhalation instruction movie referring to the device that the patient is using. The short movies are an age-specific way to counsel patients and can affect the cognitive representation of asthma and medication use, which might result in increased adherence rates.
- Medication reminder alarm, which is adjustable to the patient’s preferences (timing and number of alerts) to prevent forgetting, i.e., unintentional non-adherence, is provided.
- This app contains a peer chat function to contact peers participating in the ADAPT study. The peer chat function gives the opportunity to share experiences and support each other; this might relieve the feelings of being different and unfairness,33 which affect their emotional representation of asthma.
- This app has a pharmacist chat function to ask questions and facilitate easy access of information and guidance for the patient. Pharmacists are in the right position to recognize medication-related problems and counsel patients on their medication use; in the Netherlands, every patient is registered at a pharmacy and counseling is obligated when medication is dispensed for the first time. Usually, there is limited contact between pharmacists and adolescents since the parents often collect prescriptions at the pharmacy.34 The pharmacist chat might bridge this gap, because it facilitates direct contact between the pharmacist and the adolescent. The patient’s general practitioner will be contacted when pharmacists notice disease-related problems, thereby adolescent’s access to health care providers will be improved.
- Two questions focusing on adherence (forgetting and intentionally skipping a dose) will appear randomly once every 2 weeks to gain insight into the adherence behavior. Hereby, pharmacists have an indication of the type of non-adherent behavior.
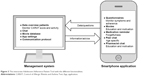  | Figure 3 The interactive Adolescent Adherence Patient Tool with the different functionalities. |
  | Figure 4 Items of the Control of Allergic Rhinitis and Asthma Test. |
Pharmacists receive a desktop application: ADAPT Asthma PRO. After permission of the patient, the pharmacist is able to receive data and send information to the patient’s app. The desktop application consists of the following elements:
- Overview of patient data, such as the patient’s app use (activity and movies seen) and the CARAT symptom scores, graphically or in a table format. Pharmacists receive e-mail alerts when a patient’s CARAT score is below the threshold. They can adjust the threshold, contact the patient, or inform the general practitioner when needed.
- Chat function to contact the patient about their CARAT score, their medication use, or their app use.
- Movie database, containing short movies covering different topics. The pharmacist has the opportunity to send specific movies to a patient, based on individual needs.
- Possibility to adjust settings of the individual patient app, such as the CARAT threshold, CARAT reminder interval, and the available movies for the patient.
- Communication protocol that supports pharmacists to interact effectively with adolescents.
All participating pharmacists receive a half-day training about asthma and medication use by adolescents, including tips for communication with this patient group. They also receive (on the spot) training and a manual on how to use the desktop application.
Ethics approval
The study is approved by the Medical Review Ethics Committee of the University Medical Centre Utrecht (NL50997.041.14) and by the Institutional Review Board of UPPER, Department of Pharmaceutical Sciences, Utrecht University. The trial is registered at the Dutch Trial Register (NTR5061). All participants have to complete informed consent before the start of the study.
Discussion
We developed the ADAPT intervention – based on results of our previous focus group study and findings from literature – to improve adherence rates and asthma control in adolescent asthma patients. Effectiveness of this intervention is studied in a community pharmacy-based cluster randomized controlled trial.
Unlike most existing interventions,35–37 the interactive and adjustable ADAPT intervention consists of more than one element, targeting several aspects of non-adherence, thereby optimally supporting asthma self-management of individual patients. Previous studies showed that educational interventions alone are insufficient and that incorporating a behavioral component might increase the efficacy of adherence-enhancing interventions.17 Therefore, the ADAPT intervention combines different components to positively affect medication intake behavior, e.g., educational movies, a medication reminder, insight in recorded symptoms, easy access to health care providers, and a peer chat function. Based on the CSM, important characteristics for a patient-centered intervention are structured, flexible, and stimulate self-regulative control,15 which are all applicable to the ADAPT intervention.
Development of the ADAPT intervention was based on needs and preferences of the target group21 and was tested by a small group of adolescents before start of the study. Moreover, the intervention is easy to implement in adolescent’s daily life as most adolescents have a smartphone and continuously carry it with them. The app enables adolescents to independently manage their medication use, without the involvement of their parents.
Effectiveness of the intervention will be studied in daily practice in order to identify patient groups who benefit most. Therefore, the intervention is designed for all adolescents with asthma, regardless of their adherence level, disease severity, or disease control. This heterogeneity might limit the effectiveness of the intervention, since there is less to achieve in well-controlled asthma or adherent patients.
We aim to stimulate a uniform implementation of the intervention through training of pharmacists and a structured communication protocol to be used in the desktop application. However, pharmacists may differ in their ability and motivation to properly use the ADAPT intervention for support and counseling of adolescents. On the other hand, these differences in counseling also represent differences in daily patient care between pharmacies and thereby increase external validity. To account for this, we will evaluate the actual delivery of the intervention.
Another limitation is the voluntary usage of the app; when patients (in the intervention group) have access to the app, we asked them to complete the CARAT questionnaire at least every week. However, it is up to the patient to use the app. To prevent nonuse, pharmacists receive the instruction to contact patients (e.g., via the chat function), when they rarely use the app. At the end of the study, we will ask patients about reasons for (not) using the app.
Although study outcomes are mostly based on patient’s self-report, validated questionnaires are used. Self-reported adherence is likely to result in an overestimation, and therefore, adherence is also calculated from 5-year medication refill records.
Conclusion
Asthma affects millions of patients worldwide, and several studies have shown insufficient adherence rates resulting in poorly controlled asthma and decreased quality of life.2–4 It is therefore important to increase adherence. The ADAPT study will show whether a tailored smartphone app for adolescents with asthma is effective in improving adherence and disease control. In addition, the study will give detailed information on the implementation of a mobile health intervention in adolescents. These insights will also be useful for the development of mobile health interventions for adolescents with other chronic diseases.
Acknowledgments
The authors thank Piet van der Wal and Pieter-Joep Huige for their input in the development of the intervention.
Liset van Dijk received funding for studies not related to this one from Pfizer, BMS, and AstraZeneca. She was an advisor for a study on asthma education adherence improvement that was partly funded by GSK and ZonMw.
For this study, funding was received from the Netherlands Organisation for Health Research and Development (ZonMw) and from Umenz Benelux BV.
Parts of the ADAPT study protocol were presented as a poster presentation at the “European Society for Patient Adherence, Compliance and Persistence” symposium 2015 and at the “Pharmaceutical Care Network Europe” (PCNE) symposium 2016. Parts of the protocol were also presented as a conference talk at the “Pharmacy Practice Research in Collaboration with Pharmacists” (PRISMA) symposium 2015. The abstract of this conference talk is published in the scientific platform of Pharmaceutisch Weekblad. 2015;9:a1545.
Author contributions
ESK and MLB drafted the initiated study design and wrote the grant proposal for the ADAPT study; they are respectively the project leader and the principal investigator of the study. ESK, MLB, LvD, HCJG, AAK, and TWdV participated in the design of the study protocol. RCK coordinated the ADAPT study (data collection) and wrote the initial manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Rand CS, Wise RA. Measuring adherence to asthma medication regimens. Am J Respir Crit Care Med. 1994;149(2 pt 2):S69–S76. | ||
Desai M, Oppenheimer JJ. Medication adherence in the asthmatic child and adolescent. Curr Allergy Asthma Rep. 2011;11(6):454–464. | ||
Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. | ||
Warschburger P, Busch S, Bauer CP, Kiosz D, Stachow R, Petermann F. Health-related quality of life in children and adolescents with asthma: results from the ESTAR study. J Asthma. 2004;41(4):463–470. | ||
Haughney J, Price D, Kaplan A, et al. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008;102(12):1681–1693. | ||
McQuaid EL, Kopel SJ, Klein RB, Fritz GK. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Pediatr Psychol. 2003;28(5):323–333. | ||
Orrell-Valente JK, Jarlsberg LG, Hill LG, Cabana MD. At what age do children start taking daily asthma medicines on their own? Pediatrics. 2008;122(6):e1186–e1192. | ||
Koster ES, Heerdink ER, De Vries TW, Bouvy ML. Attitudes towards medication use in a general population of adolescents. Eur J Pediatr. 2014;173(4):483–488. | ||
Koster ES, Philbert D, Winters NA, Bouvy ML. Adolescents’ inhaled corticosteroid adherence: the importance of treatment perceptions and medication knowledge. J Asthma. 2015;52(4):431–436. | ||
Molteni S, Giaroli G, Rossi G, Comelli M, Rajendraprasad M, Balottin U. Drug attitude in adolescents: a key factor for a comprehensive assessment. J Clin Psychopharmacol. 2014;34(1):99–108. | ||
Holley S, Morris R, Knibb R, et al. Barriers and facilitators to asthma self-management in adolescents: a systematic review of qualitative and quantitative studies. Pediatr Pulmonol. Epub 2016 Oct 7. | ||
Menckeberg TT, Bouvy ML, Bracke M, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res. 2008;64(1):47–54. | ||
Leventhal H, Nerenz D, Steele DJ. Illness representations and coping with health threats. In: Baum A, Taylor SE, Singer JE, editors. Handbook of Psychology and Health. Hillsdale: Lawrence Erlbaum Associates; 1984:219–252. | ||
Jones CJ, Smith HE, Llewellyn CD. A systematic review of the effectiveness of interventions using the Common Sense Self-Regulatory Model to improve adherence behaviours. J Health Psychol. Epub 2015 May 8. | ||
McAndrew LM, Musumeci-Szabó TJ, Mora PA, et al. Using the common sense model to design interventions for the prevention and management of chronic illness threats: from description to process. Br J Health Psychol. 2008;13(pt 2):195–204. | ||
Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;(11):CD000011. | ||
Dean AJ, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child. 2010;95(9):717–723. | ||
Naimi DR, Freedman TG, Ginsburg KR, Bogen D, Rand CS, Apter AJ. Adolescents and asthma: why bother with our meds? J Allergy Clin Immunol. 2009;123(6):1335–1341. | ||
19.Buston KM, Wood SF. Non-compliance amongst adolescents with asthma: listening to what they tell us about self-management. Fam Pract. 2000;17(2):134–138. | ||
Taddeo D, Egedy M, Frappier JY. Adherence to treatment in adolescents. Paediatr Child Health. 2008;13(1):19–24. | ||
Koster ES, Philbert D, de Vries TW, Van Dijk L, Bouvy ML. “I just forget to take it”: asthma self-management needs and preferences in adolescents. J Asthma. 2015;52(8):831–837. | ||
GfK. ‘Bijna alle jongeren bezitten een smartphone’. The Netherlands: GfK; 2015. | ||
Huckvale K, Car M, Morrison C, Car J. Apps for asthma self-management: a systematic assessment of content and tools. BMC Med. 2012;10:144. | ||
Marcano Belisario JS, Huckvale K, Greenfield G, Car J, Gunn LH. Smartphone and tablet self management apps for asthma. Cochrane Database Syst Rev. 2013;11:CD010013. | ||
Koster ES, Blom L, Philbert D, Rump W, Bouvy ML. The Utrecht Pharmacy Practice Network for Education and Research: a network of community and hospital pharmacies in the Netherlands. Int J Clin Pharm. 2014;36(4):669–674. | ||
NHG Nederlands Huisartsen Genootschap [webpage on the Internet]. NHG-Standaard Astma bij kinderen. 2014. Available from: https://www.nhg.org/standaarden/volledig/nhg-standaard-astma-bij-kinderen. Accessed October 12, 2016. | ||
Horne R, Weinman J. Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychol Heal. 2002;17(1):17–32. | ||
Hays R, Sherbourne C, Mazel R. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. | ||
Azevedo P, Correia-de-Sousa J, Bousquet J, et al. Control of allergic rhinitis and asthma test (CARAT): dissemination and applications in primary care. Prim Care Respir J. 2013;22(1):112–116. | ||
Raat H, Bueving HJ, De Jongste JC, Grol MH, Juniper EF, Van Der Wouden JC. Responsiveness, longitudinal- and cross-sectional construct validity of the pediatric asthma quality of life questionnaire (PAQLQ) in Dutch children with asthma. Qual Life Res. 2005;14(1):265–272. | ||
Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–637. | ||
Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Heal. 1999;14(1):1–24. | ||
Rhee H, Wenzel J, Steeves RH. Adolescents’ psychosocial experiences living with asthma: a focus group study. J Pediatr Health Care. 2007;21(2):99–107. | ||
Koster ES, Philbert D, Winters NA, Bouvy ML. Medication adherence in adolescents in current practice: community pharmacy staff’s opinions. Int J Pharm Pract. 2015;23(3):221–224. | ||
Rhee H, Allen J, Mammen J, Swift M. Mobile phone-based asthma self-management aid for adolescents (mASMAA): a feasibility study. Patient Prefer Adherence. 2014;8:63–72. | ||
Britto MT, Munafo JK, Schoettker PJ, Vockell AB, Wimberg JA, Yi MS. Pilot and feasibility test of adolescent-controlled text messaging reminders. Clin Pediatr (Phila). 2012;51(2):114–121. | ||
Sattoe JNT, Bal MI, Roelofs PD, Bal R, Miedema HS, van Staa A. Self-management interventions for young people with chronic conditions: a systematic overview. Patient Educ Couns. 2015;98(6):704–715. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
