Back to Journals » Clinical Ophthalmology » Volume 15
Meibomian Gland Morphology Among Patients Presenting for Refractive Surgery Evaluation
Received 19 November 2020
Accepted for publication 5 January 2021
Published 27 January 2021 Volume 2021:15 Pages 315—321
DOI https://doi.org/10.2147/OPTH.S292919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Cassandra C Brooks, Preeya K Gupta
Department of Ophthalmology, Duke University Eye Center, Durham, NC, USA
Correspondence: Preeya K Gupta
Duke University Eye Center at Page Road, 4709 Creekstone Drive, Suite 100, Durham, NC 27703, USA
Email [email protected]
Purpose: To report the prevalence of meibomian gland atrophy and gland tortuosity in patients presenting for refractive surgery evaluation.
Methods: Cross-sectional study of consecutive patients presenting for refractive surgery evaluation at the Duke Eye Center from December 2018 through January 2020. All patients underwent clinical examination and meibography imaging (Lippiview II, Johnson and Johnson Vision, CA) of the lower eyelids bilaterally. Images were graded by a masked rater using a previously validated 5-point meiboscale (0– 4) for gland atrophy and 3-point scale for gland tortuosity (0– 2). Lipid layer thickness and partial blinks were also recorded.
Results: One hundred and twenty patients (49 male) aged 21 to 62 years (mean 35.2 ± 9.2 years) were reviewed. The mean meiboscale was 1.1 ± 1.0 and the mean tortuosity score was 1.0 ± 0.7. Among all patients, 72.5% (n = 87) had any evidence of meibomian gland atrophy (meiboscale > 0) and 69.2% (n = 83) had any evidence of meibomian gland tortuosity (tortuosity grade ≥ 1). The majority of patients (n = 52) with gland atrophy had mild gland atrophy (meiboscale = 1). The mean meiboscale was 0.89 ± 0.79 and 1.38 ± 1.07 for those < 35 years and >/= 35 years old, respectively (p = 0.01). There was a moderate positive relationship between meiboscale and tortuosity (Spearman’s rho 0.3829, p < 0.001).
Conclusion: Meibomian gland atrophy is a common occurrence in patients presenting for refractive surgery evaluation. Clinicians should consider incorporating meibography as part of refractive surgery evaluation, and proactively treat meibomian gland disease given the known association between meibomian gland dysfunction, dry eye disease, and the potential for suboptimal post-operative outcomes.
Keywords: dry eye disease, meibomian gland, refractive surgery, pre-operative evaluation
Introduction
Meibomian glands are an integral component of a healthy ocular surface. Meibomian glands secrete lipids which contribute to the tear film and prevent its evaporation.1 Meibomian gland dysfunction (MGD) occurs when meibomian glands are obstructed or inflamed, leading to reduced delivery of functional lipid to the tear film. MGD results in an unstable tear film as well as ocular surface epithelial damage over time, and has been associated with approximately 86% of dry eye disease (DED).2–5 Chronic MGD can lead to gland atrophy, which can be used as one index for assessment of the overall health of the meibomian glands, correlates with ocular surface disease index (OSDI), and can be used to discriminate between normal and dry eyes.6–8 The prevalence of meibomian gland atrophy in a normal adult population has been reported as high as 72% and increases with age.9,10 Another morphological gland feature, tortuosity, has also been found to be significantly greater in patients with MGD compared to controls and correlates with lid margin scores, meiboscores, TBUT, and meibum expressibility scores, suggesting that both gland atrophy and tortuosity can be useful in assessing ocular surface health.11 Given the important role meibomian glands play in maintaining a healthy ocular surface, understanding the overall structure of the meibomian glands is important, particularly when evaluating a patient for refractive surgery.3,4,12 A recent study found a higher degree of atrophy in post-refractive surgery patients compared to controls, suggesting refractive surgery may adversely affect meibomian glands chronically and serve as a potential contributing mechanism for post-operative DED.13
Refractive surgery, though safe, effective, and largely successful with 95.4% of patients satisfied with their outcomes, is commonly adversely affected by DED.14,15 Post-operative DED is considered more common in LASIK than PRK, possibly due to corneal nerve damage.14–17 Following refractive surgery, DED symptoms are reported by approximately 94.8% at day one, 50–85.4% at week one, 40–59.4% at month one, and 20–40% at 6 months.15,18,19 Although often a transient problem, it is estimated that chronic LASIK or PRK DED lasting more than one year affects approximately 0.8% and 5% of patients, respectively.20–24 Some patient’s post-operative DED symptoms have also rarely been so severe as to negatively influence their level of satisfaction with the surgery.14,25 Beyond patient satisfaction, patients with chronic DED can also be at high risk of refractive regression following surgery, making the pre-operative of DED and its risk factors of significant importance.26
Current studies demonstrate between 15.6% and 55% of patients presenting for refractive surgery report DED symptoms and 72.7% report contact lens intolerance, likely associated with DED.15,27,28 Both of these suggest that DED is a common occurrence pre-operatively, though likely not fully recognized by all patients prior to their procedure, and potentially exacerbated by surgery. Despite the high prevalence of DED symptoms in patients presenting for refractive surgery and close association of MGD with DED, a paucity of literature exists regarding meibomian gland architecture at the time of refractive surgery evaluation. The purpose of this study is to report the prevalence of meibomian gland atrophy and tortuosity in a US-based cohort of patients presenting for refractive surgery evaluation.
Patients and Methods
Single-institution cross-sectional review of 120 patients at a single academic center. This study was approved by the Institutional Review Board, Duke University Hospital, Durham, North Carolina, USA, and was performed in accordance with the tenets of the Declaration of Helsinki and the US Health Insurance Portability and Accountability Act. A waiver of informed consent was granted due to the retrospective nature of this study. Consecutive patients were identified via a review of electronic clinical records. All patients age 18 years or greater with appointments for refractive surgery evaluation, including evaluation for laser-assisted in situ keratomileusis (LASIK), photorefractive keratectomy (PRK), or phakic intra-ocular lens (IOL) surgery from December 2018 through January 2020 had meibography (LipiView II, Johnson and Johnson Vision, CA) that was reviewed. Patients were excluded if they were being evaluated for a corneal procedure such as superficial keratectomy (SK) or phototherapeutic keratectomy (PTK). Patient’s electronic medical record was reviewed to collect relevant clinical and historical information including age, gender, race, allergies, contact lens use, ocular history (including artificial tear use and history of prior ocular surgery), and biomicroscopic slit-lamp examination. Meibomian glands of the inferior eyelids imaged the day of refractive surgery evaluation were collected for grading.
Images were graded by an experienced grader (P.K.G) who was masked to all clinical information and was not involved in obtaining the images. Grading utilized the validated meiboscale for gland atrophy.29 The scale is as follows: grade 0: no gland atrophy, grade 1: ≤25% gland atrophy, grade 2: 26% to 50% gland atrophy, grade 3: 51% to 75% gland atrophy, and grade 4: ≥75% gland atrophy. Meibomian gland tortuosity was graded using a 3-point scale developed by Arita.9 Tortuosity was defined as a >45-degree angle of the meibomian gland. The scale is as follows: grade 0: no distortion, grade 1: 1 to 4 glands distorted, and grade 2: 5 or more glands distorted. Lipid layer thickness (LLT) and partial blink data obtained with Lipiview II was recorded. The left eye of each subject was randomly selected to be included in data analysis. When the image quality of the left eye was insufficient for grading, the right eye was used.
Statistical analysis was performed with JMP Pro v 14 (SAS Institute, Cary, NC). Spearman correlations were used to evaluate the strength of association between age and meiboscale, tortuosity, LLT, and partial blink. Given that the majority of variables did not have a normal distribution, non-parametric tests were utilized. Wilcoxon rank-sum was used to evaluate the association of gender and contact lens wear with each of these factors. A p-value <0.05 was considered statistically significant.
Results
Baseline Patient Characteristics
A total of 120 patients were reviewed. Only left eyes were included in the study unless inadequate imaging occurred, in which case the right eye was included (n = 8). Mean age of patients was 35.1 years (range 21–62, SD 9.2) and 59.2% were female (n = 71). Features of the patients are summarized in Table 1. Amongst contact lens wearers (n=81), contacts were worn 13.0 ± 4.3 hours per day. Of those who reported years of wear (n=20), contacts were worn for 18.0 ± 7.0 years. Thirty-three people amongst our entire cohort of 120 reported historical or current contact lens intolerance. Tear osmolarity was 297.2 ± 9.0 (n=106).
 |
Table 1 Patient Characteristics |
Meiboscale and Meibomian Gland Tortuosity
Amongst this cohort presenting for refractive surgery evaluation, the mean meiboscale was 1.1 ±1.0 and the mean tortuosity score was 1.0 ±0.7. Of all patients, 72.5% (n = 87) had evidence of any degree of meibomian gland atrophy (meiboscale grade ≥1) and 8.3% (n = 10) had a high degree of meibomian atrophy (meiboscale grade ≥3). There were 69.2% (n = 83) of patients who had evidence of any degree of tortuosity (tortuosity grade ≥1) and 21.7% (n = 26) who had a high degree of tortuosity (tortuosity grade = 2). Meiboscale and tortuosity score distribution for the entire cohort are demonstrated in Figures 1 and 2, respectively. There was a moderate positive relationship between meiboscale and tortuosity (Spearman’s rho 0.3829, p <0.001).
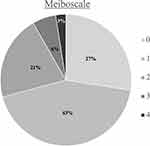 |
Figure 1 Meiboscale distribution for the entire cohort. |
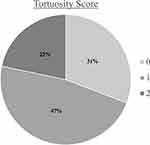 |
Figure 2 Tortuosity score distribution for the entire cohort. |
Association Between Age and Gland Structure
Subjects were separated into 2 groups by age, with those aged less than 35 years in the younger subgroup (n = 62) and 35 years or greater in the older subgroup (n = 58). The mean meiboscale was 0.89 ±0.79 and 1.38 ±1.07 for the younger and older subgroups, respectively (p = 0.01) (Figure 3). Mean tortuosity was 0.94 ±0.74 for the younger subgroup and 0.88 ±0.70 for the older subgroup (p = 0.69). The younger subgroup had 66.1% (n = 41) with a meiboscale ≥1 compared to 79.3% (n = 46) in the older group. Gland tortuosity score ≥1 was found in 69.0% (n=40) in the younger group compared to 69.4% (n = 43) in the older group. As a continuous variable, age had a statistically significant positive correlation with meiboscale (Spearman’s rho 0.2835, p= 0.0019), but not with tortuosity (Spearman’s rho 0.0124, p=0.8937). No association was found between gender or use of contact lenses and gland atrophy or tortuosity scores.
 |
Figure 3 Meiboscale by age group. |
Lipid Layer Thickness and Partial Blink
Amongst this cohort the mean LLT was 68.1 ± 19.8 nm and the mean proportion of partial blink was 53% ± 37%. There was no significant difference amongst LLT or partial blink based on sex, age, race, contact lens use, meiboscale, or tortuosity.
Discussion
In this study, we evaluated meibomian gland structure in a US-based population of patients presenting for refractive surgery evaluation. Our study suggests meibomian gland atrophy is common in patients presenting for refractive surgery evaluation, with approximately 72.5% having some evidence of meibomian gland atrophy (meiboscale ≥1). This is similar to studies of the normal adult population which report the prevalence of meibomian gland atrophy to be as high as 72%.9,10 Given the correlation of meibomian gland atrophy with MGD, OSDI, and DED, identifying patients pre-operatively with severe atrophy, in conjunction with additional factors, could guide counseling of patients with potentially increased susceptibility to MGD and DED.8 Of particular note are the 8.3% of the study population with a meiboscale grade ≥3. Patients with this degree of high-grade atrophy may warrant additional counseling on their potential future risk of DED related symptomatology when considered in conjunction with additional subjective and objective parameters. Furthermore, the presence of high grade meibomian gland atrophy may help guide refractive surgery decision making regarding whether to pursue LASIK, PRK, or an alternative method of refractive correction. Additional research to determine the significance of severe meibomian gland atrophy pre-operatively with post-operative risk of DED and its correlation with different refractive procedures would help better delineate the significance of these findings.
Consistent with existing literature, we also found that meibomian gland atrophy increases with age. Atrophy has been assumed to be the results of chronic MGD due to increased oil viscosity or hyperkeratinization, stasis, increased pressure with gland dilation and subsequent gland acini atrophy.30 A higher prevalence of atrophy with age may make the assessment of meibomian glands of potentially greater importance when considering refractive surgery in candidates with increasing age.8,10 We did not find an association with atrophy or tortuosity and gender, race, or contact lens use. Larger studies or studies with different populations focused on these potential risk factors could perhaps better elucidate contribution of these on meibomian gland architecture at the time of refractive surgery evaluation. Meibomian gland tortuosity was also common in our population, with 69.2% demonstrating some evidence of meibomian gland tortuosity (tortuosity ≥1). Data regarding gland tortuosity or distortion in the general population is more limited and difficult to compare amongst studies as a multitude of definitions have been employed. Arita et al reported mean gland tortuosity as varying between 0.091 and 0.75, with higher scores associated with a history of contact lens use and allergic conjunctivitis.9 This suggests that inflammation may contribute to the development of gland tortuosity, though no exact mechanism has been identified. Studies involving the presence of gland tortuosity or degrees of the most bent gland have suggested a correlation between tortuosity and MGD, lid margin scores, meiboscores, meibum expressibility scores, and TBUT, demonstrating the clinical importance of identifying patients with significant gland tortuosity.11
Similar to other studies, we also found a correlation between the severity of meibomian gland atrophy and tortuosity (p <0.001).8,11 Currently, the mechanism of association between gland atrophy and tortuosity is not fully understood. Additionally, as gland atrophy grades become higher there are fewer glands to assess for tortuosity. Patients with a meiboscale of 4 frequently did not have 5 glands to grade for tortuosity, which could represent a limitation of this tortuosity grading scale. Pult previously recommended observing the degree bent of the most bent gland while others have recommended adjusting for the total glands available to be assessed. Future studies comparing various methods for assessing gland distortion may help better determine the most valuable.
Other parameters we evaluated included LLT and proportion of partial blink. LLT is potentially an important factor to consider when evaluating overall ocular surface health, as prior studies have suggested a higher probability of MGD and DED in patients with a lower LLT.31,32 Studies involving control populations have found a mean LLT of approximately 67 nm (range 33–100 nm), while those with MGD have a mean LLT of 54.2 ± 17.9 nm.31,33 Finis et al suggested a LLT cutoff value of ≤60 nm for diagnosing MGD with a sensitivity and specificity of 47.9% and 90.2%, respectively.34 However, LLT can sometimes be challenging to interpret in isolation as other confounding factors such as age, ethnicity, and the presence or absence of hypersecretory MGD can all affect LLT. We found a mean LLT of 68.1 ± 19.8 nm (range 22–100 nm) amongst our cohort which is similar to previous reports amongst normal populations. However, given the large range, it may be important to consider evaluating additional DED and MGD parameters in patients with LLT amongst the lower end of the range to enhance identification of patients at risk. We also evaluated percentage of partial blinks amongst our cohort (mean percentage 53%) and found it to be similar to previously reported proportion amongst patients with DED and controls 57.4% and 59.9%, respectively.35 We did not find any statistically significant correlation amongst LLT and partial blink with age, gender, race, contact lens use, meiboscale or tortuosity, though our study was likely limited by size and demographic distribution.
A limitation of this study is that we assessed the meibomian glands at only one point in time. Studies involving pre- and post-operative imaging of meibomian glands in patients that undergo refractive surgery would allow us to better understand the impact of refractive surgery on meibomian glands. Another limitation is the study population being sampled from a single tertiary care center, which could create selection bias. Additionally, given the retrospective nature of the study, we were unable to correlate patient symptoms with their meibomian gland anatomy.
In conclusion, meibomian gland atrophy and tortuosity is common in patients presenting for refractive surgery evaluation. Our data serves to provide a foundation for our understanding of the state of meibomian glands prior to refractive surgery. We demonstrated that mild meibomian gland atrophy and tortuosity is present in a high percentage in this population, but that there is a subgroup with very prominent atrophy who may be a higher risk population when determining appropriateness for refractive surgery. Given the future implications on post-operative DED, patient satisfaction and comfort, as well as risk of regression, clinicians should consider screening and potentially addressing meibomian gland disease at the time of refractive surgery evaluation. Further prospective investigation into meibomian gland architecture over time and associated risk factors in the development of DED in refractive surgery patients as is warranted in order to better manage this patient population.
Disclosure
P. K. Gupta: consultant to Alcon, Allergan, Aurea, BioTissue, J&J Vision, NovaBay, Ocular Science, Shire, Tear Lab, Tear Science. The authors report no other conflicts of interest in this work.
References
1. Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Exp Eye Res. 1961;1:39–45. doi:10.1016/S0014-4835(61)80006-7
2. Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):1930–1937. doi:10.1167/iovs.10-6997b
3. Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Arch Ophthalmol. 1995;113(10):1266–1270. doi:10.1001/archopht.1995.01100100054027
4. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi:10.1097/ICO.0b013e318225415a
5. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100(3):347–351. doi:10.1016/S0161-6420(93)31643-X
6. Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52(4):1938–1978. doi:10.1167/iovs.10-6997c
7. Lin X, Wu Y, Chen Y, et al. Characterization of meibomian gland atrophy and the potential risk factors for middle aged to elderly patients with cataracts. Transl Vis Sci Technol. 2020;9(7):48. doi:10.1167/tvst.9.7.48
8. Pult H, Riede-Pult BH, Nichols JJ. Relation between upper and lower lids’ meibomian gland morphology, tear film, and dry eye. Optom Vis Sci. 2012;89(3):E310–E315. doi:10.1097/OPX.0b013e318244e487
9. Arita R, Itoh K, Maeda S, Maeda K, Tomidokoro A, Amano S. Association of contact lens-related allergic conjunctivitis with changes in the morphology of meibomian glands. Jpn J Ophthalmol. 2012;56(1):14–19. doi:10.1007/s10384-011-0103-6
10. Den S, Shimizu K, Ikeda T, Tsubota K, Shimmura S, Shimazaki J. Association between meibomian gland changes and aging, sex, or tear function. Cornea. 2006;25(6):651–655. doi:10.1097/01.ico.0000227889.11500.6f
11. Lin X, Fu Y, Li L, et al. A novel quantitative index of meibomian gland dysfunction, the meibomian gland tortuosity. Transl Vis Sci Technol. 2020;9(9):34. doi:10.1167/tvst.9.9.34
12. Finis D, Ackermann P, Pischel N, et al. Evaluation of meibomian gland dysfunction and local distribution of meibomian gland atrophy by non-contact infrared meibography. Curr Eye Res. 2015;40(10):982–989. doi:10.3109/02713683.2014.971929
13. Jung JW, Kim JY, Chin HS, Suh YJ, Kim TI, Seo KY. Assessment of meibomian glands and tear film in post-refractive surgery patients. Clin Experiment Ophthalmol. 2017;45(9):857–866.
14. Solomon KD, Fernandez de Castro LE, Sandoval HP, et al. LASIK world literature review: quality of life and patient satisfaction. Ophthalmology. 2009;116(4):691–701. doi:10.1016/j.ophtha.2008.12.037
15. Yu EY, Leung A, Rao S, Lam DS. Effect of laser in situ keratomileusis on tear stability. Ophthalmology. 2000;107(12):2131–2135. doi:10.1016/s0161-6420(00)00388-2
16. Shehadeh-Mashor R, Mimouni M, Shapira Y, Sela T, Munzer G, Kaiserman I. Risk factors for dry eye after refractive surgery. Cornea. 2019;38(12):1495–1499. doi:10.1097/ICO.0000000000002152
17. Lee HK, Lee KS, Kim HC, Lee SH, Kim EK. Nerve growth factor concentration and implications in photorefractive keratectomy vs laser in situ keratomileusis. Am J Ophthalmol. 2005;139(6):965–971. doi:10.1016/j.ajo.2004.12.051
18. Shoja MR, Besharati MR. Dry eye after LASIK for myopia: incidence and risk factors. Eur J Ophthalmol. 2007;17(1):1–6. doi:10.1177/112067210701700101
19. De Paiva CS, Chen Z, Koch DD, et al. The incidence and risk factors for developing dry eye after myopic LASIK. Am J Ophthalmol. 2006;141(3):438–445. doi:10.1016/j.ajo.2005.10.006
20. Siganos DS, Popescu CN, Siganos CS, Pistola G. Tear secretion following spherical and astigmatic excimer laser photorefractive keratectomy. J Cataract Refract Surg. 2000;26(11):1585–1589. doi:10.1016/S0886-3350(00)00648-9
21. Herrmann WA, Shah CP, von Mohrenfels CW, Gabler B, Hufendiek K, Lohmann CP. Tear film function and corneal sensation in the early postoperative period after LASEK for the correction of myopia. Graefes Arch Clin Exp Ophthalmol. 2005;243(9):911–916. doi:10.1007/s00417-005-1130-0
22. Mian SI, Shtein RM, Nelson A, Musch DC. Effect of hinge position on corneal sensation and dry eye after laser in situ keratomileusis using a femtosecond laser. J Cataract Refract Surg. 2007;33(7):1190–1194. doi:10.1016/j.jcrs.2007.03.031
23. Battat L, Macri A, Dursun D, Pflugfelder SC. Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology. 2001;108(7):1230–1235. doi:10.1016/S0161-6420(01)00623-6
24. Bower KS, Sia RK, Ryan DS, Mines MJ, Dartt DA. Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: manifestations, incidence, and predictive factors. J Cataract Refract Surg. 2015;41(12):2624–2634. doi:10.1016/j.jcrs.2015.06.037
25. Toda I. LASIK and the ocular surface. Cornea. 2008;27(Suppl 1):S70–S76. doi:10.1097/ICO.0b013e31817f42c0
26. Albietz JM, Lenton LM, McLennan SG. Chronic dry eye and regression after laser in situ keratomileusis for myopia. J Cataract Refract Surg. 2004;30(3):675–684. doi:10.1016/j.jcrs.2003.07.003
27. Maychuk DY. Prevalence and severity of dry eye in candidates for laser in situ keratomileusis for myopia in Russia. J Cataract Refract Surg. 2016;42(3):427–434. doi:10.1016/j.jcrs.2015.11.038
28. McGhee CN, Orr D, Kidd B, Stark C, Bryce IG, Anastas CN. Psychological aspects of excimer laser surgery for myopia: reasons for seeking treatment and patient satisfaction. Br J Ophthalmol. 1996;80(10):874–879. doi:10.1136/bjo.80.10.874
29. Pult H, Riede-Pult B. Comparison of subjective grading and objective assessment in meibography. Cont Lens Anterior Eye. 2013;36(1):22–27. doi:10.1016/j.clae.2012.10.074
30. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi:10.1167/iovs.10-6997a
31. Eom Y, Lee JS, Kang SY, Kim HM, Song JS. Correlation between quantitative measurements of tear film lipid layer thickness and meibomian gland loss in patients with obstructive meibomian gland dysfunction and normal controls. Am J Ophthalmol. 2013;155(6):1104–1110.e1102. doi:10.1016/j.ajo.2013.01.008
32. Yokoi N, Mossa F, Tiffany JM, Bron AJ. Assessment of meibomian gland function in dry eye using meibometry. Arch Ophthalmol. 1999;117(6):723–729. doi:10.1001/archopht.117.6.723
33. Jung JW, Park SY, Kim JS, Kim EK, Seo KY, Kim TI. Analysis of factors associated with the tear film lipid layer thickness in normal eyes and patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2016;57(10):4076–4083. doi:10.1167/iovs.16-19251
34. Finis D, Pischel N, Schrader S, Geerling G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea. 2013;32(12):1549–1553. doi:10.1097/ICO.0b013e3182a7f3e1
35. Ousler GW
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
