Back to Journals » Clinical Ophthalmology » Volume 14
Management Strategies of Acute Retinal Necrosis: Current Perspectives
Authors Powell B, Wang D, Llop S, Rosen RB
Received 16 April 2020
Accepted for publication 17 June 2020
Published 8 July 2020 Volume 2020:14 Pages 1931—1943
DOI https://doi.org/10.2147/OPTH.S258488
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Brittany Powell, Daniel Wang, Stephanie Llop, Richard B Rosen
Department of Ophthalmology, New York Eye and Ear Infirmary of Mount Sinai, New York, NY, United States
Correspondence: Richard B Rosen
New York Eye and Ear Infirmary of Mount Sinai, 310 East 14th Street, New York, NY 10003 Tel +1 212-979-4288
Fax +1 212-979-4268
Email [email protected]
Abstract: Acute retinal necrosis is a rare yet devastating disease, with significant ocular morbidity. Over the past several decades, initial treatment regimens have shifted from intravenous antivirals requiring hospital admission to the routine use of oral antivirals with intravitreal antivirals for immediate local control. Given the rarity of this disease process and a lack of large-scale research trials, debate continues over recommended practice guidelines. In this paper, we review current diagnostic criteria and recommend a treatment algorithm based on available evidence.
Keywords: occlusive vasculitis, medical management, posterior uveitis
Introduction
Medical and surgical treatment strategies of acute retinal necrosis (ARN) have evolved considerably and mirrored advances in our understanding of the underlying pathophysiology of the disease since it was first described by Urayama and colleagues in 1971.1
This seminal case described a syndrome of acute panuveitis with retinal periarteritis progressing rapidly to diffuse necrotizing retinitis and retinal detachment nonresponsive to medical treatment in the setting of a negative infectious workup.1 Case reports of retinitis with clinical features resembling ARN in patients with systemic herpesvirus infections subsequently followed.2–4 Large case series of pathologic and electron microscopy findings from vitrectomy and enucleation specimens published in both the Japanese and English literature helped to identify an underlying etiology: an infectious trigger leading to a severe immune-mediated inflammation and obliterative vasculitis.5 These clinical and pathology reports laid the foundation for further research and solidified ARN as an infectious syndrome caused by members of the herpes virus family that can affect both immunocompetent and immunocompromised patients of any age and either gender.6,7 Varicella zoster virus (VZV) is the most common cause, followed by herpes simplex viruses (HSV-1 and HSV-2).8–12 Cytomegalovirus (CMV) and Epstein-Barr virus (EBV) have also been implicated albeit less frequently.7,12
Visual outcomes are generally grim and 48% of affected eyes have a visual acuity worse than 20/200 six months following the onset of ARN.13 Retinal detachment is the most common cause of decreased vision, reported to occur in 30% to 73% of cases in recent series, but previously in up to 85% of patients.9,14,15 Vision loss can also result from chronic vitritis, epiretinal membrane, macular ischemia, macular edema, and optic neuropathy.9,10 Bilateral ARN was first described in 1978 and has been reported in up to 70% of untreated patients.16,17 Contralateral involvement has been reported to occur anywhere from within a few months, to several years later.17,18
Although the annual incidence of ARN is low—two nationwide United Kingdom surveys estimated the incidence to be 0.5–0.63 cases per million population—the prognosis is quite poor if not treated immediately and aggressively.8,13 The use of intravenous acyclovir was first described in 1986 and resulted in the regression of retinal lesions.15 In 1991, Palay et al reported a reduction in the incidence of contralateral eye involvement from 70% to 13% with intravenous acyclovir.17 Treatment at a dose of 10mg/kg every 8 hours or 1500mg/m2 per day divided into three doses for 7–10 days followed by an oral antiviral is the most established treatment regimen.9,12,15,18 The availability of newer oral antiviral medications with greater bioavailability (valacyclovir, famciclovir) and increased use of intravitreal antivirals have led to the adoption of a new treatment algorithm of initiating treatment with oral antivirals and simultaneous intravitreal injections. This treatment algorithm is a proven success and has largely eliminated the need for a hospital admission and intravenous medication.18–20 Additional adjunctive treatment modalities have been described, including early surgical intervention with pars plana vitrectomy with or without silicone oil prior to the presence of a retinal detachment, laser retinopexy around areas of necrosis to prevent a retinal detachment, systemic or local corticosteroids, and systemic antiplatelet agents.9,18 In this paper, we review current management strategies and recommendations for the treatment of ARN.
Methods
Literature searches were last conducted in PubMed and the Cochrane Library databases on 29 May 2020 without date or language restrictions. The search used the following MeSH terms: retinal necrosis syndrome, antiviral agents, vitrectomy, light coagulation, intraocular, antiviral agents. The search used the following text terms: acute retinal necrosis, antiviral agents, antiviral therapy, acyclovir, human herpes virus, light coagulation, photocoagulation, vitrectomy, and intraocular injections.
Diagnostics
Acute retinal necrosis is a rapidly progressive disease with potentially significant ocular morbidity and involvement of the fellow eye. Early and accurate diagnosis is critical to initiating immediate antiviral therapy.
Diagnostic Criteria
In 1994, the American Uveitis Society’s Executive Committee defined ARN on the basis of the following clinical characteristics: one or more foci of retinal necrosis with discrete borders located in the peripheral retina, rapid progression in the absence of antiviral therapy, circumferential spread, evidence of occlusive vasculopathy with arterial involvement, and a prominent inflammatory reaction in both the anterior chamber and vitreous cavity.21
Although the diagnostic criteria remain unchanged, the use of polymerase chain reaction (PCR) for accurate, rapid, and precise identification of the responsible viral infection has become the standard of care. Detecting viral DNA from aqueous or vitreous humor from patients with suspected ARN has a positive predictive value of 99% and a negative predictive value of 68%.22 PCR testing may alter treatment direction through earlier diagnosis of ARN and initiation of antiviral therapy.
Slit Lamp Exam
ARN classically presents as an acute panuveitis syndrome with involvement of multiple ocular tissues. Anterior segment findings often predominate early in the course of the disease.23 The disease may affect one or both eyes with most cases beginning as unilateral disease. In one-third of cases, the fellow eye becomes involved within 1–6 weeks, but disease involvement of the second eye has been reported to occur upwards of 20 years after initial insult to the first eye.24,25
Most patients will initially present with a chief complaint of pain, redness, photophobia, floaters, and blurred vision. Careful examination of the anterior chamber will reveal a unilateral anterior uveitis with or without granulomatous or stellate appearing keratic precipitates early in the disease course. Injection of the ocular surface may result secondary to inflammation involving the sclera and adjacent structures.26,27
Fundus Examination
A dense vitritis may develop as the disease progresses and cellular immunity to the virus is stimulated. With the onset of vitritis, patients may report worsening floaters and diminished visual acuity resulting from vitreous opacification. Multiple focal, well-demarcated areas of whitening in the peripheral retina corresponding to active retinal necrosis will be present on funduscopic examination. Areas of retinal whitening and necrosis may become confluent and circumferentially progress to involve the posterior pole if treatment is not initiated in a timely manner. The macula is typically spared early in the course of the disease. Acute vasculitis and occlusive disease may be present in the form of perivascular hemorrhages, sheathing, and obliteration of arterioles. Retinal breaks often develop within the peripheral necrotic retinal lesions in 86% of patients.23 Retinal atrophy resulting from necrosis results in secondary rhegmatogenous retinal detachment in 20% to 85% of treated eyes.9,12,14,15 Final visual acuity is often limited by involvement of structures in the posterior pole including optic atrophy, cystoid macular edema, macular hole, and epiretinal membrane formation, as well as by retinal detachment.10
Fluorescein Angiography
While not diagnostic, fluorescein angiography may be helpful and provide details not readily appreciated on fundoscopic examination. The quality of the study, however, is often limited due to overlying vitritis. Angiography may demonstrate signs of occlusive arteritis and areas of capillary nonperfusion. Choroidal vasculature is often involved and angiography may demonstrate areas of early hypofluorescence and late staining consistent with ischemia-induced inflammatory changes. Intense extravasation of dye from the retinal vessels due to active vasculitis may appear as diffuse leakage. Optic nerve involvement often occurs early in the disease course and angiography will show hyperfluorescence of the optic nerve.
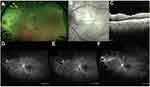
Figure 1 contains a typical fundus exam with retinal whitening and vasculitis with the corresponding optical coherence tomography which highlights the vitritis and involvement of the inner retinal layers. Angiography is notable for evidence of an occlusive arteritis, capillary non-perfusion, and involvement of the optic nerve.
Ultrasound Examination
B-scan ultrasonography may be a useful modality for assessing onset of retinal detachment, especially when visibility is limited by vitritis. Ultrasonography is able to see beneath the haze of vitritis and reveals elevation of the optic nerve head with expansion of the optic nerve sheath.
Neuroimaging
Viral meningoencephalitis has been reported in association with ARN. Patients who present with signs of neurological disease may require further investigation as appropriate.28 A lumbar puncture may be warranted. Computed tomography of the orbits can confirm optic nerve sheath enlargement with associated optic nerve edema. Magnetic resonance imaging of select cases is helpful for revealing lesions of the optic tract, chiasm and the lateral geniculate body which can occur with axonal spread.29
Laboratory and Serum Testing
Laboratory testing should always include a baseline complete blood count, liver function panel, and tests of renal function prior to initiation of antiviral therapy to monitor for drug toxicity and subsequent dosing considerations, particularly in patients with renal failure (end stage renal disease, dialysis). Additional infectious etiologies need to be ruled out and the laboratory testing should include testing for human immunodeficiency virus, tuberculosis, and syphilis. Of note, serum testing for herpesvirus antibodies does not add any value in the diagnosis of ARN and is not recommended.
Anterior Chamber and Vitreous Sample
ARN historically has been a clinicaldiagnosis, but the consequences of misdiagnoses, underdiagnoses and subsequent delays in treatment have prompted wider use of laboratory methods to aid in the diagnostic process. Serum and intraocular fluid antibody testing, retinal biopsy, viral culture, and immunocytochemistry have all been utilized, but their use has largely been limited by their poor sensitivity or specificity.6 Numerous reports have recently demonstrated the functionality of PCR testing for the diagnosis and management of ARN. Vitreous and aqueous specimens are both sensitive and specific. PCR of ocular samples for HSV and VZV has a reported sensitivity between 79% and 100% in clinically defined ARN cases.7,30-33 There is insufficient evidence of the superiority of sampling of the vitreous over the aqueous and vice versa.33 Though PCR of ocular fluids may support the clinical diagnosis of ARN, treatment should not be delayed while waiting for results.
Treatment
Early administration of antiviral medication is the cornerstone of the treatment of ARN. Advances in technology have allowed for the rapid identification of responsible viruses. Similarly, treatment options have advanced along with surgical techniques. Treatment response is determined by various parameters including the time to regression of retinitis, visual outcomes, the incidence of retinal detachment, and fellow eye involvement. We discuss the various treatment regimens available and offer our perspective based on our experience with ARN.
The body of evidence supporting the current treatment strategies is impressive, but there are significant gaps given that the existing research is entirely retrospective and consists of case series with comparative studies using historical controls. This makes it difficult to compare outcomes across studies. Tables 1 and 2 summarize the literature discussed below.
 |
Table 1 Summary of Literature Evaluating Medical Treatment |
 |
Table 2 Summary of Literature Evaluating Adjunctive Therapies |
Antiviral Therapy
The most frequently reported initial treatment of ARN includes intravenous acyclovir or oral valacyclovir. Other treatments include oral famciclovir, valganciclovir or acyclovir, and intravenous foscarnet or ganciclovir. Intravitreal foscarnet or ganciclovir may be given as adjuvant local therapy but cannot be used alone as they leave the fellow eye at risk of developing disease.
Acyclovir is an acyclic purine nucleoside analog that is converted to acyclovir monophosphate by virus-encoded thymidine kinase. Cellular enzymes catalyze the subsequent dephosphorylation and triphosphorylation steps, which results in high concentrations of acyclovir triphosphate that inhibits viral DNA synthesis through competitive inhibition of viral DNA polymerase. As a result, acyclovir is highly specific for herpes-infected cells and non-toxic to uninfected cells. Acyclovir can be given both orally and intravenously.
Valacyclovir is an orally administered prodrug that is converted to acyclovir during first-pass metabolism. It has a much higher bioavailability of 54–60% compared to oral acyclovir, which has a bioavailability of 15–30%.34,35
Famciclovir is an orally given prodrug that is converted to penciclovir in the liver. It has a bioavailability of 77%.36 Penciclovir resembles acyclovir in chemical nature, mechanism of action, and spectrum of antiviral activity, but is poorly absorbed, which is why famciclovir is used clinically. This medication should be considered in cases of acyclovir-resistant ARN.
Foscarnet is an organic analog of inorganic pyrophosphate that selectively inhibits the pyrophosphate binding sites on viral DNA polymerases at concentrations that do not affect human DNA polymerases. Foscarnet is an effective alternative treatment in acyclovir-resistant HSV strains.37 It may be locally administered through an intravitreal injection or intravenously. Foscarnet does not require compounding and the dose requires no dilution from the commercially available intravenous solution.
Ganciclovir is an inhibitor of viral DNA polymerase that has activity against CMV and HSV. It can be administered intravenously, orally, or intravitreally. In ARN, it has been studied as an additional intravitreal medication which has been shown to be effective in treating herpetic infections. However, it requires pharmacy compounding and is therefore not as widely used.
Acyclovir-resistant HSV strains have been reported to occur in less than 1% of immunocompetent patients and up to 14% of immunocompromised patients.37 Acyclovir-resistant VZV strains are far less common and have only been reported in a small case series.37 Foscarnet can be used successfully in acyclovir-resistant strains because it does not require activation by thymidine kinase.38–41
The traditional treatment regimen for ARN since the 1980s has been induction therapy with intravenous acyclovir followed by oral antiviral medications.15,17 In recent years, oral antiviral therapy with intravitreal foscarnet has emerged as a more popular treatment option, since it may avoid the need for hospital admission.14,18,42,43 We will review the various treatment strategies along with the available evidence assessing their success and outline our treatment algorithm for this disease with high ocular morbidity.
Adverse Effects of Antivirals
Whether given intravenously or orally, systemic antivirals have known adverse effects for which patients require routine monitoring. While there is limited systemic absorption when administered via intravitreal injection, there still exists a risk for such adverse effects. Acyclovir, famciclovir, ganciclovir, and valacyclovir all require dose adjustments in geriatric and renal patients and careful attention must be placed on whether the patient is taking other nephrotoxic medications.
Acyclovir and valacyclovir are well tolerated in the oral form, but intravenously administered can cause neurotoxicity and renal toxicity due to a crystalline nephropathy.36 Patients can commonly experience a headache, rash, and gastrointestinal symptoms. Immunocompromised patients taking valacyclovir are at a higher risk for nephrotoxicity and thrombocytopenia and need to be regularly monitored; these patients are specifically at risk for thrombotic thrombocytopenic purpura/hemolytic uremic syndrome (TTP/HUS).36
Famciclovir can be similarly associated with a rash, headaches, and gastrointestinal symptoms.
Ganciclovir, when given intravenously, is commonly associated with bone marrow suppression resulting in anemia, granulocytopenia, and thrombocytopenia and renal toxicity.36 Intravitreal injections of this medication carry the additional risk of endophthalmitis, vitreous hemorrhage, and retinal detachment. Valganciclovir has a similar adverse effect profile as ganciclovir as it is a prodrug for ganciclovir.
Foscarnet can result in headaches and gastrointestinal symptoms and less likely can result in nephrotoxicity, hypocalcemia, and neurotoxicity.36 Intravitreal injections of this medication carry the additional risk of endophthalmitis, vitreous hemorrhage, and retinal detachment.
While these medications are often given in conjunction with an infectious disease specialist, it is important to understand the potential risks in this particularly susceptible population.
Systemic Therapy: Intravenous Antivirals
Intravenous antiviral therapy was historically the standard of care and is still applicable in specific cases. Blumenkranz et al first described their experience with intravenous acyclovir in 13 eyes of 12 patients with ARN who were treated with acyclovir 1500 mg/m2/day for a mean of 10.9 days. Patients were treated with oral aspirin or warfarin and 9 of the 12 patients were treated with oral corticosteroids. While progression of the lesions was noted during the first 48 hours following initiation of therapy, regression of the lesions was first noted at 3.9 days following therapy but required 32.5 days for complete resolution. The incidence of a retinal detachment was 86.6%, which was higher than for untreated historical controls. Three of the 11 patients with unilateral disease developed fellow eye involvement in a time period ranging from 1 to 5 years later.15 Subsequent reports featuring treatment with high dose intravenous acyclovir followed this report and noted lower rates of retinal detachments when compared to earlier case series.44,45
Palay et al conducted the first retrospective comparative study of ARN patients treated with intravenous acyclovir compared to observation. This case series had a total of 54 immunocompetent patients with unilateral disease; 31 were treated with intravenous acyclovir 1500 mg/m2/day for 7–10 days and then oral acyclovir for 2–4 weeks (unknown dose and frequency) and the remaining 23 were observed. Of the treated patients, 87% remained disease-free in the contralateral eye versus 30% of the untreated patients.17
Admission to a hospital is costly and often ARN patients are admitted for an extended amount of time, putting them at risk for developing hospital-related diseases. There is a role for hospitalization with intravenous administration of antivirals in patients who have substantial barriers to treatment/care (eg homeless), those who cannot reliably take oral medication, and elderly patients requiring extensive care. Oral antivirals provide us with additional treatment options, but the role of intravenous antivirals is particularly important for these specific groups of patients.
Systemic Therapy: Oral Antivirals
Oral antivirals have become increasingly popular when administered with adjunct intravitreal antivirals. Oral treatment allows for the outpatient treatment of ARN which is cost-saving and avoids exposure of the patient to hospital-acquired diseases. Huynh et al demonstrated that oral valacyclovir can reach concentrations in the vitreous and achieve inhibitory ranges of HSV-1, HSV-2 and VZV.42 Of note, there are no studies directly comparing oral and intravenous therapy. Comparisons across studies are difficult given the retrospective nature of the studies, with differences in the reported baseline parameters and outcome measurements.
Tibbetts et al studied oral antivirals with historical controls treated with intravenous antivirals. This group conducted a retrospective multicenter study examining 58 patients with unilateral disease. Patients were divided into the acyclovir-only era (36 eyes from 1981 to 1997) and the new antiviral era (22 eyes from 1998 to 2008). The patients in the acyclovir-only era received intravenous acyclovir for 7–10 days. Half of these patients continued their antiviral treatment with six weeks of oral acyclovir while the other half did not. In the new antiviral era group, 15 eyes were initially treated intravenous acyclovir and 6 of these patients also received intravitreal antiviral therapy. Seven of the patients in this new antiviral group were initially managed with oral therapy with or without concomitant intravitreal therapy. Two of these patients were started on oral antivirals and then switched to intravenous therapy, but the clinical course and the reasoning for this decision were not explained. Initial antiviral treatment management was at the discretion of the ophthalmologist and baseline characteristics of these patients were not described. The choice of initial oral or intravenous antiviral therapy did not have an effect on the final visual acuity outcome or development of a retinal detachment.18
Additional case series by Emerson et al and Taylor et al assessed the use of oral valacyclovir without intravitreal therapy in patients with similar outcomes.20,46 Emerson et al reviewed 6 eyes of 4 patients who were treated with oral valacyclovir 1000mg three times daily or famciclovir 500mg three times daily for a total duration of 5 weeks to 3 months.46 Symptoms and visual acuity improved within 2 to 4 weeks in 3 of the 4 patients.46 Neither eye with initial unilateral involvement developed findings in the fellow eye.46 Taylor et al reviewed 10 eyes of 9 patients who were treated with oral valacyclovir. Observed retinitis resolved in 100% of affected eyes with a median time to complete resolution of 21 days.20 None of the patients experienced either disease reactivation or second eye involvement over the course of the study.20 The current body of evidence suggests that oral and intravenous therapy have comparable outcomes and that either is effective for induction therapy; however, future studies should directly compare oral versus intravenous antiviral therapy in the initial management of ARN.
Intravitreal Therapy
In recent years, adjuvant therapy with intravitreal antivirals has become increasingly popular. There are several retrospective case series which include data from patients treated with intravitreal therapy, but there are two studies which purposely address the use of intravitreal antivirals in patients being treated with systemic antivirals.11,47 Additionally, a number of case reports and series support the use of monotherapy of intravitreal foscarnet and ganciclovir27,48–50 or even simultaneous use of both agents intravitreally.14,51
Wong et al describe outcomes from 104 eyes that received intravenous acyclovir for 7–10 days followed by oral antiviral therapy at two study sites. All of the patients at one study site (64 eyes) received an intravitreal injection of foscarnet within 3 days of presentation while none of the patients at the second study site (40 eyes) were given intravitreal antiviral. The combination treatment was associated with a reduced risk of retinal detachment (35% vs 60%) when compared to systemic treatment alone. Visual acuity data were not presented and follow-up differed between the two sites, but this was the first study to evaluate an outcome using intravitreal antivirals.11
The other comparative study was completed by Yeh et al. The systemic only group was treated with two weeks of intravenous antivirals followed by oral antivirals. The systemic plus intravitreal injection group was treated with either intravenous acyclovir or oral valacyclovir in combination with serial foscarnet injections every 3–4 days until there was no evidence of active disease. There was no difference between the groups in the presenting visual acuity. Follow-up differed greatly between the groups with the combination group having a shorter follow-up (27 months vs 64 months). The patients receiving combination therapy were more likely to gain 2 lines of vision and showed a significant decrease in the incidence of retinal detachment. Additionally, the incidence of severe vision loss to 20/200 or worse was reduced in the combination group.47
There is a clear benefit to using combination systemic and intravitreal antiviral therapy to reduce significant vision loss and reduce the incidence of retinal detachment.10,47,52 The foscarnet dose of 2.4mg in 0.1mL in an adult eye provides excellent local control of the virus given that the injected concentration is 20- to 30-fold higher than following intravenous administration, which far exceeds the reported inhibitory concentration levels.33
Corticosteroids, Aspirin, Heparin, and Warfarin
Acute decreases in vision resulting from ischemic optic neuropathy have led to trials looking into the effect of anticoagulants such as aspirin, along with high-dose oral steroids after initiation of antiviral therapy. Corticosteroids can be employed both topically and orally to decrease the severe inflammatory response associated with ARN. Some advocate the addition of oral corticosteroids 24 to 48 hours after initiating antiviral treatment.53 Intravitreal corticosteroid injection must be used with caution as it may potentiate the rapid progression of retinitis. Additionally, others have supported local corticosteroid in the setting of cystoid macular edema after the resolution of active retinitis; however, potential for retinitis recurrence should be considered and carefully monitored.54
Choudhury et al described an interventional case series of four patients treated with valacyclovir, oral corticosteroids, and supplemental intravitreal triamcinolone, which resulted in decreased vitritis and improved final VA of 20/40 in three patients.54 Aizman and colleagues published the only case series to assess the use of oral steroids in addition to oral antivirals. Sixteen eyes were managed with oral valacyclovir or famciclovir and oral steroids were started when disease regression was observed. Initial response to treatment was noted at an average of 6.3 days with complete resolution at an average of 17 days. None of the patients with unilateral disease developed fellow eye involvement, but follow-up ranged from 7 to 72 weeks.19 Systemic corticosteroid treatment does not appear to increase the risk of development of retinal detachment.12 Other reports are unable to conclusively show an improvement in visual outcomes and clinically appreciated inflammation with the use of corticosteroids.18
Given the extensive retinal arteritis and retinal vascular occlusions that occur in ARN, advocates have suggested using adjunctive aspirin and warfarin for treatment. Hyperaggregation of platelets has been reported in seven patients with bilateral ARN, as determined by adenosine 5-diphosphate aggregation testing and partial prothrombin times.55 Strong evidence, however, does not currently exist for the use of anticoagulation. The safety of these medications should be carefully considered in conjunction with the patient’s systemic and physical health before being utilized.
Adjunctive Therapies
Prophylactic Laser to Prevent Retinal Detachment
There are multiple case series assessing the utility of prophylactic laser retinopexy to decrease the risk of retinal detachment in those patients at a particularly high risk for this complication.12–14,18,43,45 However, there is no evidence to suggest that prophylactic laser reduces the rate of retinal detachment given limitations in the interpretations of the studies.
Tibbets et al reviewed the largest case series of 58 eyes in which 58% of the patients with laser retinopexy developed a retinal detachment. This case series did not present the selection criteria to decide which patients were to be observed versus those who were to undergo laser retinopexy.18 As is the case in much of the research on this topic, the visual acuity in those not treated was often worse than those treated. Laser can only be applied when the media is clear enough to allow for the procedure and the retinitis is limited in its retinal real estate. This confounding factor is the major limitation in the studies that found the rate of retinal detachment to be reduced in those patients prophylactically treated with laser retinopexy.12–14 The inherent selection bias and limited evidence do not advocate for the use of prophylactic laser retinopexy at this time. Added to this, laser retinopexy results in increased inflammation in an eye already plagued with an inflammatory response to an infectious toxin, and this uptick in inflammatory markers can only be detrimental.
Early Vitrectomy Before Retinal Detachment
Rather than increase the inflammatory mediators with prophylactic laser retinopexy, why not remove the damaging inflammatory mediators and vitreoretinal traction, and apply laser demarcation around necrotic retina to protect the posterior pole? This question has led some to advocate for early vitrectomy before the retina detaches in an effort to prevent retinal detachment and protect the macula.
Several studies have evaluated the visual and anatomic outcomes of early vitrectomy.9,56–58 The largest was conducted by Iwahashi-Shima et al, who retrospectively reviewed 104 eyes, 48 of which underwent early vitrectomy. The selection criteria for which eyes underwent early vitrectomy were not described, but all patients had a minimum of one-year follow-up. Baseline and final visual acuity measurements did not differ between the groups. The early vitrectomy group had a 58% final retinal attachment rate versus 75% of the eyes in the observation group. This is the strongest study to date and found no anatomic or visual benefit to early vitrectomy.56
Other comparative studies found that early vitrectomy resulted in significantly better visual acuity outcomes and significantly decreased rates of retinal detachment.9,58 However, these studies are severely limited given different baseline characteristics, unclear methods specifically as related to use of silicone oil, variable follow-up time, and no evidence of a visual benefit, and therefore cannot allow us to conclude definitively that there is a benefit to early surgical intervention.9,57,58
While unproven as a prophylactic measure, silicone oil permits consistent, long-term tamponade after retinal detachment from viral retinitis. However, no direct comparative studies have been conducted assessing silicone oil versus other tamponade techniques.
Conclusion
Acute retinal necrosis, while rare, can result in severe ocular morbidity if not accurately diagnosed and immediately treated. Treatment should begin immediately with either oral or intravenous antivirals with concurrent intravitreal therapy to treat active disease and prevent fellow eye involvement. Polymerase chain reaction testing of aqueous or vitreous can reliably and safely confirm cases of suspected ARN, but treatment should not be delayed while awaiting results.
Induction therapy can consist of intravenous dosing of acyclovir 10mg/kg three times a day for 7–10 days followed by the long-term use of oral valacyclovir 2000mg by mouth three times per day with the dose slowly decreased over time as described in our treatment algorithm (Figure 2). This particular oral dose has been shown to provide plasma drug levels of acyclovir comparable to when acyclovir 10mg/kg three times a day is administered.33,38 Notably, smaller doses of oral valacyclovir have been successfully used.19,20,35
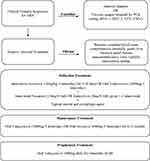 |
Figure 2 Recommended treatment algorithm of acute retinal necrosis. |
Research supports the use of intravitreal foscarnet as part of the induction regimen in order to attain immediate vitreous drug levels. The use of intravitreal antiviral reduces risk of severe vision loss and incidence of retinal detachment, but does not prevent fellow eye involvement and should always be used as an adjunct to systemic therapy. We recommend giving intravitreal foscarnet 2.4mg/0.1mL or ganciclovir 2mg/0.1mL repeated 1–2 times a week until there is disease regression. There does not seem to be evidence that giving both intravitreal foscarnet and intravitreal ganciclovir provides additional benefit.
Once induction therapy has been completed with systemic and intravitreal antivirals, long-term maintenance therapy should continue for a minimum of 6 months and consist of oral valacyclovir 1000mg three times daily. Patients are typically continued on oral valacyclovir 1000mg for the remainder of their life to prevent recurrence or fellow eye involvement. While there are no studies addressing lifelong administration specifically, this is evidence that maintenance therapy prevents fellow eye involvement which has been reported to occur 20 years after initial insult to the first eye.25
With advances in laboratory diagnostics and elucidation of the viral etiology of ARN, our understanding of the disease process and associated clinical outcomes have improved. Several retrospective studies have shown that early treatment with high-dose systemic antiviral therapy combined with intravitreal antiviral injections leads to improved clinical outcomes. PCR testing of aqueous and vitreous samples may be utilized to confirm clinical suspicion of ARN. However, treatment should always be prompt given the severe ocular and visual consequences of delays in initiating treatment. Literature supporting prophylactic laser and early vitrectomy is not conclusive at this time. Additionally, many questions remain about ARN and its treatment, specifically regarding long-term prophylaxis antiviral treatment and adjunctive therapies.
Future Research
This is a rare disease and a true randomized prospective clinical study would prove difficult but could help standardize the treatment regimen of this disease. It would be particularly helpful to assess whether specific etiologies could benefit from early surgical intervention, combined antiviral intravitreal injections, or different intravenous or oral antivirals. There is growing evidence that different viral etiologies present with varying degrees of severity, and it has been shown that those cases due to VZV are more severe than HSV.10,42 As our diagnostic techniques become more advanced and our armamentarium of medications continues to evolve, particularly with regard to resistant viral strains, therapy may need to become more tailored in the future. Surgical techniques will also continue to evolve and the utility of early surgical intervention will need to be re-assessed in the light of these newer surgical techniques. As research in treatments progresses, hopefully so will our understanding of the immune system and why certain individuals are more prone than others to ARN, so that we can explore preventive strategies.
Copyright Statement
One of the authors is a military service member or employee of the US Government. This work was prepared as part of their official duties. Title 17, USC, § 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17, USC§ 101 defines a US Government work as a work prepared by a military service member or employee of the US Government as part of that person’s official duties.
Disclosure
Dr. Powell, Dr. Wang, and Dr. Llop have no financial disclosures. Dr. Rosen has the following financial disclosures: Commercial Relationship(s): Boehringer-Ingelheim:Code C (Consultant); Astellas:Code C (Consultant); Regeneron:Code C (Consultant); Bayer:Code C (Consultant); OptoVue:Code C (Consultant); OptoVue:Code P (Patent); Genentech-Roche:Code C (Consultant); NanoRetina:Code C (Consultant); OD-OS:Code C (Consultant); Opticology:Code I (Personal Financial Interest); Guardion:Code I (Personal Financial Interest); Teva:Code C (Consultant);CellView: Code C (Consultant). The authors report no other conflicts of interest in this work.
References
1. Urayama A. Unilateral acute uveitis with retinal periarteritis and detachment. Jpn J Clin Ophthalmol. 1971;25:607–619.
2. Brown RM, Mendis U. Retinal arteritis complicating herpes zoster ophthalmicus. Br J Ophthalmol. 1973;57(5):344–346. doi:doi:10.1136/bjo.57.5.344
3. Cibis GW. Neonatal herpes simplex retinitis. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1975;196(1):39–47. doi:doi:10.1007/bf00410025
4. Culbertson WW, Blumenkranz MS, Haines H, Gass DM, Mitchell KB, Norton EW. The acute retinal necrosis syndrome. Part 2: histopathology and etiology. Ophthalmology. 1982;89(12):1317–1325. doi:doi:10.1016/s0161-6420(82)34638-2
5. Hayasaka S, Asano T, Yabata K, Ide A. Acute retinal necrosis. Br J Ophthalmol. 1983;67(7):455–460. doi:doi:10.1136/bjo.67.7.455
6. Duker JS, Blumenkranz MS. Diagnosis and management of the acute retinal necrosis (ARN) syndrome. Surv Ophthalmol. 1991;35(5):327–343. doi:doi:10.1016/0039-6257(91)90183-g
7. Ganatra JB, Chandler D, Santos C, Kuppermann B, Margolis TP. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129(2):166–172. doi:doi:10.1016/s0002-9394(99)00316-5
8. Muthiah MN, Michaelides M, Child CS, Mitchell SM. Acute retinal necrosis: a national population-based study to assess the incidence, methods of diagnosis, treatment strategies and outcomes in the UK. Br J Ophthalmol. 2007;91(11):1452–1455. doi:doi:10.1136/bjo.2007.114884
9. Hillenkamp J, Nölle B, Bruns C, Rautenberg P, Fickenscher H, Roider J. Acute retinal necrosis: clinical features, early vitrectomy, and outcomes. Ophthalmology. 2009;116(10):1971–1975.e2. doi:doi:10.1016/j.ophtha.2009.03.029
10. Wong RW, Jumper JM, McDonald HR, et al. Emerging concepts in the management of acute retinal necrosis. Br J Ophthalmol. 2013;97(5):545–552. doi:doi:10.1136/bjophthalmol-2012-301983
11. Wong R, Pavesio CE, Laidlaw DAH, Williamson TH, Graham EM, Stanford MR. Acute retinal necrosis: the effects of intravitreal foscarnet and virus type on outcome. Ophthalmology. 2010;117(3):556–560. doi:doi:10.1016/j.ophtha.2009.08.003
12. Lau CH, Missotten T, Salzmann J, Lightman SL. Acute retinal necrosis features, management, and outcomes. Ophthalmology. 2007;114(4):756–762. doi:doi:10.1016/j.ophtha.2006.08.037
13. Cochrane TF, Silvestri G, McDowell C, Foot B, McAvoy CE. Acute retinal necrosis in the United Kingdom: results of a prospective surveillance study. Eye (Lond). 2012;26(3):
14. Meghpara B, Sulkowski G, Kesen MR, Tessler HH, Goldstein DA. Long-term follow-up of acute retinal necrosis. Retina (Philadelphia, Pa). 2010;30(5):795–800. doi:doi:10.1097/IAE.0b013e3181c7013c
15. Blumenkranz MS, Culbertson WW, Clarkson JG, Dix R. Treatment of the acute retinal necrosis syndrome with intravenous acyclovir. Ophthalmology. 1986;93(3):296–300. doi:doi:10.1016/s0161-6420(86)33740-0
16. Young NJ, Bird AC. Bilateral acute retinal necrosis. Br J Ophthalmol. 1978;62(9):581–590. doi:doi:10.1136/bjo.62.9.581
17. Palay DA, Sternberg P, Davis J, et al. Decrease in the risk of bilateral acute retinal necrosis by acyclovir therapy. Am J Ophthalmol. 1991;112(3):250–255. doi:doi:10.1016/s0002-9394(14)76725-x
18. Tibbetts MD, Shah CP, Young LH, Duker JS, Maguire JI, Morley MG. Treatment of acute retinal necrosis. Ophthalmology. 2010;117(4):818–824. doi:doi:10.1016/j.ophtha.2009.09.001
19. Aizman A, Johnson MW, Elner SG. Treatment of acute retinal necrosis syndrome with oral antiviral medications. Ophthalmology. 2007;114(2):307–312. doi:doi:10.1016/j.ophtha.2006.06.058
20. Taylor SR, Hamilton R, Hooper CY, et al. Valacyclovir in the treatment of acute retinal necrosis. BMC Ophthalmol. 2012;12:48. doi:doi:10.1186/1471-2415-12-48
21. Holland GN. Standard diagnostic criteria for the acute retinal necrosis syndrome. Executive Committee of the American Uveitis Society. Am J Ophthalmol. 1994;117(5):663–667. doi:doi:10.1016/s0002-9394(14)70075-3
22. Harper TW, Miller D, Schiffman JC, Davis JL. Polymerase chain reaction analysis of aqueous and vitreous specimens in the diagnosis of posterior segment infectious uveitis. Am J Ophthalmol. 2009;147(1):140–147.e2. doi:doi:10.1016/j.ajo.2008.07.043
23. Fisher JP, Lewis ML, Blumenkranz M, et al. The acute retinal necrosis syndrome. I. Clinical manifestations. Ophthalmology. 1982;89:1309–1316. doi:10.1016/S0161-6420(82)34628-X
24. Saari KM, Boke W, Manthey KF, et al. Bilateral acute retinal necrosis. Am J Ophthalmol. 1982;93:403–411. doi:10.1016/0002-9394(82)90128-3
25. Schlingemann RO, Bruinenberg M, Wertheim-van Dillen P, et al. Twenty years’ delay of fellow eye involvement in herpes simplex virus type 2-associated bilateral acute retinal necrosis syndrome. Am J Ophthalmol. 1996;122:891–892. doi:10.1016/S0002-9394(14)70390-3
26. Whitcup SM. Chapter 12: acute retinal necrosis and progressive outer retinal necrosis. In: Nussenblatt RB, Whitcup SM, editors. Uveitis: Fundamentals and Clinical Practice. Mosby/Elsevier; 2010:
27. Ramsay A, Cunningham E, Pavesio C. Acute retinal necrosis presenting with scleritis and raised intraocular pressure. Br J Ophthalmol. 2000;84(10):1208–1209. doi:doi:10.1136/bjo.84.10.1203h
28. Cardine S, Chaze PA, Bourcier F, et al. Bilateral acute retinal necrosis syndrome associated with meningoencephalitis caused by herpes simplex virus. A case report. J Fr Ophtalmol. 2004;27(7):795–800. doi:10.1016/S0181-5512(04)96216-X
29. Farrell TA, Wolf MD, Folk JC, et al. Magnetic resonance imaging in a patient with herpes zoster keratouveitis and contralateral acute retinal necrosis. Am J Ophthalmol. 1991;112(6):735–736. doi:10.1016/S0002-9394(14)77289-7
30. Tran THC, Rozenberg F, Cassoux N, Rao NA, LeHoang P, Bodaghi B. Polymerase chain reaction analysis of aqueous humour samples in necrotising retinitis. Br J Ophthalmol. 2003;87(1):79–83. doi:doi:10.1136/bjo.87.1.79
31. de Boer JH, Verhagen C, Bruinenberg M, et al. Serologic and polymerase chain reaction analysis of intraocular fluids in the diagnosis of infectious uveitis. Am J Ophthalmol. 1996;121:650–658. doi:10.1016/S0002-9394(14)70631-2
32. Abe T, Tsuchida K, Tamai M. A comparative study of the polymerase chain reaction and local antibody production in acute retinal necrosis syndrome and cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1996;234:419–424. doi:10.1007/BF02539407
33. Schoenberger SD, Kim SJ, Thorne JE, et al. Diagnosis and treatment of acute retinal necrosis: a report by the American academy of ophthalmology. Ophthalmology. 2017;124(3):382–392. doi:doi:10.1016/j.ophtha.2016.11.007
34. Soul-Lawton J, Seaber E, On N, Wootton R, Rolan P, Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39(12):2759–2764. doi:doi:10.1128/aac.39.12.2759
35. Höglund M, Ljungman P, Weller S. Comparable aciclovir exposures produced by oral valaciclovir and intravenous aciclovir in immunocompromised cancer patients. J Antimicrob Chemother. 2001;47(6):855–861. doi:doi:10.1093/jac/47.6.855
36. Tam PMK, Hooper CY, Lightman S. Antiviral selection in the management of acute retinal necrosis. Clin Ophthalmol. 2010;4:11–20.
37. Gilbert C, Bestman-Smith J, Boivin G. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist Updat. 2002;5(2):88–114. doi:doi:10.1016/s1368-7646(02)00021-3
38. Lee MY, Kim KS, Lee WK. Intravitreal foscarnet for the treatment of acyclovir-resistant acute retinal necrosis caused by varicella zoster virus. Ocul Immunol Inflamm. 2011;19(3):212–213. doi:doi:10.3109/09273948.2010.544857
39. Hatchette T, Tipples GA, Peters G, Alsuwaidi A, Zhou J, Mailman TL. Foscarnet salvage therapy for acyclovir-resistant varicella zoster: report of a novel thymidine kinase mutation and review of the literature. Pediatr Infect Dis J. 2008;27(1):75–77. doi:doi:10.1097/INF.0b013e3181598315
40. Breton G, Fillet AM, Katlama C, Bricaire F, Caumes E. Acyclovir-resistant herpes zoster in human immunodeficiency virus-infected patients: results of foscarnet therapy. Clin Infect Dis. 1998;27(6):1525–1527. doi:doi:10.1086/515045
41. Balfour HH, Benson C, Braun J, et al. Management of acyclovir-resistant herpes simplex and varicella-zoster virus infections. J Acquir Immune Defic Syndr. 1994;7(3):254–260.
42. Huynh TH, Johnson MW, Comer GM, Fish DN. Vitreous penetration of orally administered valacyclovir. Am J Ophthalmol. 2008;145(4):682–686. doi:10.1016/j.ajo.2007.11.016
43. Sims JL, Yeoh J, Stawell RJ. Acute retinal necrosis: a case series with clinical features and treatment outcomes. Clin Experiment Ophthalmol. 2009;37(5):473–477. doi:doi:10.1111/j.1442-9071.2009.02083.x
44. Hirst LW, Beyer TL, Waters D, Fleischman J. Successful management of acute retinal necrosis with intravenous acyclovir. Ann Ophthalmol. 1987;19(12):445–448.
45. Crapotta JA, Freeman WR, Feldman RM, et al. Visual outcome in acute retinal necrosis. Retina (Philadelphia, Pa). 1993;13(3):208–213. doi:doi:10.1097/00006982-199313030-00004
46. Emerson GG, Smith JR, Wilson DJ, Rosenbaum JT, Flaxel CJ. Primary treatment of acute retinal necrosis with oral antiviral therapy. Ophthalmology. 2006;113(12):2259–2261. doi:doi:10.1016/j.ophtha.2006.05.063
47. Yeh S, Suhler EB, Smith JR, et al. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):399–407. doi:doi:10.3928/23258160-20140908-02
48. Guo LB, Sun D, Ye JJ, Geng S, Xu HY, Zhang MF. Intravitreal injection of Ganciclovir in the treatment of acute retinal necrosis. Chin j Ophthalmol. 2007;43(7):631–637.
49. Kishore K, Jain S, Zarbin MA. Intravitreal ganciclovir and dexamethasone as adjunctive therapy in the management of acute retinal necrosis caused by varicella zoster virus. Ophthalmic Surg Lasers Imaging. 2011;42:e87–90. doi:10.3928/15428877-20110901-06
50. Luu KK, Scott IU, Chaudhry NA, Verm A, Davis JL. Intravitreal antiviral injections as adjunctive therapy in the management of immunocompetent patients with necrotizing herpetic retinopathy. Am J Ophthalmol. 2000;129(6):811–813. doi:10.1016/S0002-9394(00)00462-1
51. Kauffman B, Rescigno R, Zarbin MA, Bhagat N. High–dose intravitreal ganciclovir and foscarnet for the treatment of acute retinal necrosis and progressive outer retinal necrosis. Invest Ophthalmol Vis Sci. 2006;47(13):3049.
52. Flaxel CJ, Yeh S, Lauer AK. Combination systemic and intravitreal antiviral therapy in the management of acute retinal necrosis syndrome (an American ophthalmological society thesis). Trans Am Ophthalmol Soc. 2013;111:133–144.
53. Shantha JG, Weissman HM, Debiec MR, Albini TAYS, Yeh S. Advances in the management of acute retinal necrosis. Int Ophthalmol Clin. 2015;55(3):1–13. doi:10.1097/IIO.0000000000000077
54. Choudhury H, Jindal A, Mithal K, Bawdekar AC, Pathengay A. Intravitreal triamcinolone acetonide as an adjuvant in the management of acute retinal necrosis. Can J Ophthalmol. 2014;49(3):279–282. doi:10.1016/j.jcjo.2014.03.010
55. Ando F, Kato M, Goto S, et al. Platelet function in bilateral acute retinal necrosis. Am J Ophthalmol. 1983;96:27–32. doi:10.1016/0002-9394(83)90451-8
56. Iwahashi-Shima C, Azumi A, Ohguro N, et al. Acute retinal necrosis: factors associated with anatomic and visual outcomes. Jpn J Ophthalmol. 2013;57(1):98–103. doi:doi:10.1007/s10384-012-0211-y
57. Ishida T, Sugamoto Y, Sugita S, Mochizuki M. Prophylactic vitrectomy for acute retinal necrosis. Jpn J Ophthalmol. 2009;53(5):486–489. doi:doi:10.1007/s10384-009-0698-z
58. Luo Y-H, Duan X-C, Chen B-H, Tang L-S, Guo X-J. Efficacy and necessity of prophylactic vitrectomy for acute retinal necrosis syndrome. Int J Ophthalmol. 2012;5(4):482–487. doi:doi:10.3980/j.issn.2222-3959.2012.04.15
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.