Back to Journals » Clinical Ophthalmology » Volume 9
Management of neovascular age-related macular degeneration: current state-of-the-art care for optimizing visual outcomes and therapies in development
Authors Agarwal A, Rhoades W, Hanout M, Soliman M, Sarwar S, Sadiq MA, Sepah Y, Do D, Nguyen Q
Received 25 February 2015
Accepted for publication 14 April 2015
Published 5 June 2015 Volume 2015:9 Pages 1001—1015
DOI https://doi.org/10.2147/OPTH.S74959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Scott Fraser
Aniruddha Agarwal, William R Rhoades, Mostafa Hanout, Mohamed Kamel Soliman, Salman Sarwar, Mohammad Ali Sadiq, Yasir Jamal Sepah, Diana V Do, Quan Dong Nguyen
Stanley M Truhlsen Eye Institute, University of Nebraska Medical Center, Omaha, NE, USA
Abstract: Contemporary management of neovascular age-related macular degeneration (AMD) has evolved significantly over the last few years. The goal of treatment is shifting from merely salvaging vision to maintaining a high quality of life. There have been significant breakthroughs in the identification of viable drug targets and gene therapies. Imaging tools with near-histological precision have enhanced our knowledge about pathophysiological mechanisms that play a role in vision loss due to AMD. Visual, social, and vocational rehabilitation are all important treatment goals. In this review, evidence from landmark clinical trials is summarized to elucidate the optimum modern-day management of neovascular AMD. Therapeutic strategies currently under development, such as gene therapy and personalized medicine, are also described.
Keywords: AMD, neovascular AMD, choroidal neovascular membrane, pharmacogenomics, VEGF, low-vision rehabilitation, gene therapy
Introduction
Age-related macular degeneration (AMD) is the leading cause of central visual loss and legal blindness in patients over the age of 65 years.1,2 As many as 30% of adults over the age of 75 years develop signs of senile retinal degeneration, and the prevalence of AMD is on the rise due to an aging population.3,4 The cost of current treatment regimens may not be sustainable, as the expected healthcare costs for a single patient with newly diagnosed neovascular AMD may reach up to US$250,000.5 The exudative or neovascular form of AMD, which is characterized by choroidal neovascular membrane (CNV) growth and/or serous retinal pigment epithelial (RPE) detachments, accounts for over 90% of the cases with severe visual loss.6 Complications such as subretinal hemorrhage, vitreous hemorrhage, fibrosis, and scarring are responsible for poor visual outcomes in these patients.7 The goal of therapy for many years was to salvage vision in this subset of patients with the neovascular form of the disease.
Evidence from large multicenter clinical trials in the last decade has brought about a paradigm shift with neovascular AMD.8 Increasing knowledge of the pathogenic mechanisms responsible for neovascular growth and complications in AMD has resulted in translational research targeting specific pathways that were previously unexplored.9 Treatments targeting vascular endothelial growth factor (VEGF) have been shown to improve vision in patients with neovascular AMD and now constitute the mainstay of therapy.10 Results from research on newer therapeutic strategies including gene therapy suggest that novel treatment options may be on the horizon.11 The fast pace of clinical research has led to some challenges, at times, in the optimal management of advanced AMD. Although guidelines are available from the ophthalmic community on the treatment of patients with advanced AMD,12 this review focuses on current AMD treatments and the investigations currently underway to advance the state of knowledge in the field of AMD research.
Early diagnosis and monitoring of visual function in AMD
History and physical examination
Neovascular AMD characteristically results in symptoms such as decreased vision and metamorphopsia. Unfortunately, by the time these symptoms occur, significant damage to the retinal layers and retinal pigment epithelium may have already occurred. At the time of first presentation to the ophthalmologist, more than one-third of patients may already have advanced fibrotic lesions.13 Careful attention to the status of the disease in the fellow eye is essential, as severe AMD in one eye may be associated with accelerated progression of disease in the fellow eye.14 It is estimated that the incidence of neovascular AMD in the fellow eye may be as high as 12.2% at 12 months and increased to 26.8% at 48 months.15
Regular follow-up with an ophthalmologist is imperative in ensuring early detection of anatomic and visual changes secondary to AMD.16 A comprehensive eye examination with assessment of best-corrected visual acuity (BCVA), intraocular pressure,17 slit-lamp examination, and dilated fundus examination in the clinic are the sine qua non of disease detection. The American Academy of Ophthalmology Preferred Practice Pattern® Guideline for AMD18 also recommends a thorough history be taken, including quantitative smoking history, for the diagnosis of advanced AMD.
Diagnostic imaging and ancillary tests for neovascular AMD
Various diagnostic modalities can be employed for the detection of CNV in patients with AMD, as per the Age-related Macular Degeneration: Detection of Onset of New Choroidal Neovascularization (AMD DOC) study.19 Time-domain and the newer spectral-domain optical coherence tomography (SD-OCT) and fluorescein angiography (FA) still remain the best methods to detect CNV.20 FA has been used as an initial diagnostic tool in all Phase III clinical trials of AMD.12 Once diagnosed, patients may be followed-up on SD-OCT to assess response of therapy noninvasively21–23 and FA may be reserved for cases where additional information is required.24 SD-OCT can reveal the presence of intraretinal or subretinal fluid that is not apparent on clinical examination. Indocyanine green (ICG) angiography can be used to detect various retinal and choroidal vascular abnormalities such as retinal angiomatous proliferation (also known as RAP or type 3 CNV) and polypoidal choroidal vasculopathy (PCV).25
Knowledge of CNV location, progression, and potential response to treatment is essential in the diagnosis of neovascular AMD. While SD-OCT has recently revolutionized diagnosis, newer technologies such as swept-source optical coherence tomography (OCT), which employs longer wavelengths, now allow improved imaging beyond the retinal pigment epithelium. This en face imaging system may provide a better contrast for detecting occult CNV compared to SD-OCT.26 In addition, blood flow measurements can also be assessed using Doppler OCT that can perform 3D imaging of the vasculature in PCV and other exudative macular diseases.27,28 OCT angiography can provide distinct vascular network patterns that may be otherwise obscured by subretinal hemorrhage on conventional FA. This may enable a higher diagnostic yield and quantitative assessment of vascular flow.29 In the future, new imaging tools may allow calculation of flow indices that may help in judging treatment response in neovascular AMD. As an example, assessment of photoreceptor density and perturbation in patients with AMD with adaptive optics imaging provides information with near-histological precision that may be valuable in assessing the effects of cell-based therapy in the future.30,31
Patients are instructed to use an Amsler grid for home monitoring to help identify progression of disease based on symptom recognition, and this is currently the standard of care. Daily home self-monitoring by patients using newer preferential hyperacuity perimetry-based telemonitoring devices has been recommended for earlier detection of CNV in patients with AMD. Compared to a median loss of nine letters in the standard-of-care group, patients using the self-monitoring device demonstrated a median loss of four letters from baseline at the time of CNV detection.32 It remains to be seen if digital monitoring will overtake the traditional grid as the standard of care, but it has shown promise.
Studies have shown that BCVA alone may not reflect the retinal damage secondary to AMD.33 In the Lucentis (Ranibizumab) in Diabetic Macular Edema: a Treatment Evaluation (LUCIDATE) study, visual function was assessed using microperimetry.34 Retinal microstructural changes correlate well with retinal function, as assessed by microperimetry in patients with AMD.35 Other aspects of visual function such as contrast sensitivity may be compromised in patients with AMD.36 While currently not the standard of care, these testing modalities help assess the progression of advanced AMD and may become more important as treatment goals include treatment of geographic atrophy in AMD.
Advances in prevention of advanced AMD
The role of antioxidants in the prevention of advanced AMD was established by the Age-Related Eye Disease Study (AREDS) in patients with moderate-to-severe AMD.37 AREDS2 was a multicenter randomized Phase III study, completed in 2013. Removing beta-carotene and lowering zinc did not affect the AMD progression rate. In some patients, lutein and zeaxanthin lowered the progression of AMD by 20% more than the original formulation. The current recommendation based on the AREDS2 study is vitamin supplement consisting of 500 mg vitamin C, 400 IU vitamin E, 10 mg lutein, 2 mg zeaxanthin, 80 mg zinc, and 2 mg copper.38 AREDS,39 AREDS2,38 and other large epidemiological studies such as the Blue Mountains Eye Study40 assessed the effect of dietary supplementation with omega-3 fatty acids and cessation of smoking on progression of AMD. Thus, dietary advice and smoking cessation are still considered as the mainstay for AMD treatment.41 As of 2015, there is debate among retinal specialists as to whether genetic testing should guide the recommendation of vitamin supplementation. Further research is investigating the role of diet and genetics in the development of AMD.42,43
Established therapy for treatment of neovascular AMD
Photodynamic therapy
Verteporfin photodynamic therapy (PDT) is a laser treatment that selectively generates free oxygen radicals that cause cytotoxic damage and occlusion of new vessels with regression of CNV in patients with AMD after intravenous administration of a sensitizing agent. The Treatment of AMD with Photodynamic Therapy (TAP) study assessed the safety and efficacy of PDT in patients with classic subfoveal CNV. At 24-month follow-up, the percentage of patients losing <15 letters was significantly less in the PDT group than in the control group (P<0.001). This observation was only noted with predominately classic CNV lesions and not with minimally classic lesions.44 The Verteporfin in Photodynamic Therapy (VIP) trial was a randomized, double-masked, placebo-controlled study investigating the efficacy of PDT in occult CNV. At 2 years, PDT was shown to decrease the risk of moderate and severe visual loss compared to placebo.45 However, both the trials failed to show a significant improvement in BCVA compared to baseline.
PDT is considered generally safe with few rare adverse events. In the anti-VEGF era, the use of PDT has declined due to its lower efficacy in improving BCVA. Recent evidence from the Comparison of Ranibizumab (Lucentis) And Photodynamic Therapy On Polypoidal choroidal vasculopathy (LAPTOP) study has also shown superior results with ranibizumab (RBZ) compared to PDT for PCV.46 However, it is important to note that the rates of polyp closure may be higher with PDT alone, or combined with RBZ, as compared to RBZ alone, as shown by the Visual Outcome in Patients with Symptomatic Macular PCV Treated with Either Ranibizumab as Monotherapy or Combined with Verteporfin Photodynamic Therapy (EVEREST) study.47–49 Current guidelines recommend the use of PDT in combination with anti-VEGF therapy for PCV.12,18,49 Studies have also shown the short-term efficacy of aflibercept (AFL) in the occlusion of polyps in treatment-naïve patients with PCV.50
The anti-VEGF era
The initial trials which proved the efficacy of anti-VEGF treatments, such as the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in Age-Related Macular Degeneration (ANCHOR) and Minimally Classic/Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular Age-Related Macular Degeneration (MARINA) studies, are well chronicled in a previous article.51 Prior to the VEGF era, treatments for neovascular AMD included laser photocoagulation and PDT. Anti-VEGF agents have provided an important breakthrough in the treatment of neovascular AMD. Anti-VEGF treatments are currently the standard first-line therapy for the management of neovascular AMD.18
Evolution of treatment regimens with RBZ
Historically, ANCHOR52 and MARINA53 were landmark studies that proved the efficacy of RBZ using a monthly dosing regimen. Since then, treatment strategies have focused on minimizing the frequency of treatments. The Study of rhuFab V2 (Ranibizumab) in Subjects With Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration (PIER) and Efficacy and Safety of Monthly versus Quarterly Ranibizumab Treatment in Neovascular Age-Related Macular Degeneration (EXCITE) trials tested using fixed dosing schedules to decrease the treatment burden.54,55 The Prospective OCT Study with Lucentis for Neovascular AMD (PrONTO), An Extension Study to Evaluate the Safety and Tolerability of Ranibizumab in Subjects with Choroidal Neovascularization Secondary to AMD (HORIZON), Study of Ranibizumab in Patients with Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration (SUSTAIN), and Study to Evaluate Ranibizumab in Subjects with Choroidal Neovascularization (CNV) Secondary to Age-Related Macular Degeneration (SAILOR) studies have used the pro re nata (PRN), or as needed, dosing schedule to achieve the same goal.56–59
The PIER study (n=184) was a Phase IIIb, multicenter, double-masked, sham-controlled trial of neovascular AMD patients who were randomized to intravitreal RBZ (0.3 or 0.5 mg) or sham monthly for 3 months, followed by quarterly treatments. At 1 year, patients in the 0.3 mg and 0.5 mg cohorts lost an average 1.6 and 0.2 letters from baseline compared to a loss of 16.3 letters in the sham cohort. Given that ANCHOR and MARINA showed a mean increase of 7.2 and 11.3 letters from baseline at 1 year for their 0.5 mg cohorts, this schedule has not found favor in clinical practice.54 The EXCITE study (n=353), another Phase IIIb, multicenter, active-controlled, double-masked trial demonstrated higher BCVA gain in the monthly dosing arm compared with the quarterly treatment arm of RBZ.55
The PrONTO study (n=40) was a Phase I/II prospective, open-label, 2-year investigation of a PRN approach to RBZ dosing. Patients received monthly RBZ 0.5 mg injections for 3 months followed by PRN dosing. PrONTO participants required on average 5.6 injections during the first year (2.6 injections in the last 9 months of the first year), with a mean improvement of 9.3 letters. At 2 years, the mean number of injections was 9.9, with a mean improvement of 11.1 letters. The results have been influential, as the retreatment criteria are easy to apply and the results were favorable. However, the study was very small, and many practicing physicians find the monthly visits with or without treatments to be a logistical burden for patients and clinics.56
The SAILOR study (n=4,307) was a Phase IIIb multicenter, 1-year trial of intravitreal RBZ (0.3 or 0.5 mg) using three initial monthly doses followed by PRN dosing at 3-month follow-up appointments. In the cohort of treatment-naïve patients (n=2,378), the mean BCVA improvement was 0.5 and 2.3 letters (in the 0.3 and 0.5 mg groups, respectively) and patients who had previously received treatments (n=1,929) had improvements of 1.7 and 2.3 letters (in the 0.3 and 0.5 mg cohorts, respectively).57 The SAILOR results echo the PIER results for quarterly visits, and are considered inferior to the studies involving more frequent dosing.
The HORIZON study (n=853) was a Phase III, open-label extension trial for patients who completed 2 years of the ANCHOR or MARINA trials. Treatments were PRN, no more frequent than monthly, with required visits at least every 3 months. The study participants had either had prior treatment (n=600), were prior control patients who crossed over into treatment (n=190), or were treatment naïve to RBZ (n=63). Patients who had previously had monthly injections lost a mean of 5.3 letters from study baseline, which showed a decline from their previous mean gain of 9.4 letters from the trials in which they had been enrolled. Patients in the crossover and naïve cohorts lost a mean of 2.4 and 3.1 letters, respectively, from study baseline. This study found that despite continued as-needed treatments, patients’ visual acuity declined after cessation of monthly treatments.58
The SUSTAIN study (n=513) was a Phase IIIb, multicenter, open-label, single-arm study analyzing results of PRN RBZ. The mean number of treatments over 12 months after a required set of 3-monthly injections was 2.7. At 1 year, mean BCVA increased 3.6 letters from baseline. BCVA increase was greatest at 3 months and then declined slightly at 12 months. SUSTAIN validated the outcomes of PRN dosing of anti-VEGF in AMD.59
Thus, the treatment protocols in clinical trials using intravitreal anti-VEGF therapy have evolved from a more frequent, monthly dosing to a less rigorous, as-needed approach, in order to decrease the treatment burden, with a trend toward worsening outcomes with less frequent dosing. Frequent dosing may be associated with risks of progression of geographic atrophy, as indicated by the Comparison of Age-related macular degeneration Treatments Trial (CATT) research group.60,61 In addition, with frequent injections, there may be a higher risk of stroke, endophthalmitis, retinal tears and detachments, but clinical trial sample sizes may not be sufficient to detect rare safety outcomes.10
Comparison of bevacizumab with RBZ
Recently, there has been an interest to compare the efficacy of bevacizumab (BCZ) to RBZ in order to define its role in the management of AMD. BCZ has been successfully used off-label in the management of advanced AMD.
The CATT study (n=1,208) was a multicenter, single-blind, non-inferiority trial comparing monthly and PRN BCZ and RBZ. At 2 years, the average BCVA gains were 8.8 and 7.8 letters in the monthly RBZ and BCZ groups, respectively. The corresponding PRN groups gained 6.7 and 5.0 letters, respectively. These results showed non-inferiority between the drugs, though the monthly dosing arms had outcomes slightly better than the PRN cohorts (P=0.46).62,63 The Inhibition of VEGF in Age-related Choroidal Neovascularization (IVAN) study (n=610) was a multicenter, non-inferiority randomized trial comparing monthly and PRN dosing of BCZ and RBZ in Europe. At 2 years, there was no significant difference in BCVA between the two drugs (P=0.26), and there was no significant difference between monthly or PRN dosing groups (P=0.18).64,65 The Multicenter Anti-VEGF Trial in Austria (MANTA)66 and Groupe d′Evaluation Français Avastin versus Lucentis (GEFAL)67 trials were randomized to compare PRN dosing of BCZ and RBZ. As with the CATT and IVAN trials, neither drug was found superior to the other.
The Lucentis Compared to Avastin Study (LUCAS) trial (n=441), a recent, randomized, double-blind, multicenter, non-inferiority trial, compared BCZ and RBZ using a treat-and-extend strategy. Mean BCVA increases were 7.9 letters for the BCZ cohort and 8.2 letters for the RBZ cohort (P=0.845). In the first year, the RBZ group had a mean of 8.0 injections and the BCZ group had a mean of 8.9 injections (P=0.001). The BCVA gains in the LUCAS trial were comparable to the gains in previously performed trials with monthly dosing, which validates a very commonly used treat-and-extend dosing regimen.68
Thus, BCZ is an effective treatment option comparable to RBZ. Questions regarding its safety and efficacy raised by those concerned about its preparation may not be answerable given the difficulty in running a clinical trial powered to detect a difference in side effects among anti-VEGF treatments. Treatment with BCZ results in a much lower economic burden to the health care system as compared to RBZ. Using a Markov model and data from CATT trials, the expected costs for BCZ were determined to be US$79,771 versus US$257,496 for RBZ for a hypothetical patient diagnosed with neovascular AMD over a 20-year time horizon.5 Thus, BCZ is still widely used off-label for the management of neovascular AMD despite the availability of RBZ.
AFL (or VEGF-Trap) in management of neovascular AMD
The VEGF Trap-Eye: Investigation of Efficacy and Safety in Wet AMD (VIEW)-1 and -2 trials (n=2,419) were two similarly designed, double-masked, multicenter, active-controlled, randomized, Phase III studies comparing monthly and bimonthly dosing of intravitreal AFL 0.5 mg and 2 mg to monthly RBZ 0.5 mg. Both the monthly and bimonthly AFL were non-inferior compared to monthly RBZ. The mean average BCVA gain was 8.1 and 9.4 letters in the RBZ groups, 10.9 and 7.6 letters in the monthly 2 mg AFL groups, and 7.9 and 8.9 letters in the bimonthly AFL groups. These results were heralded as a potential improvement over previously monthly, quarterly, or PRN regimens with RBZ and BCZ. However, it should be noted that there have not been any large clinical trials examining bimonthly dosing with BCZ or RBZ.69
Conbercept for neovascular AMD
Conbercept (KH 902) is a novel, recombinant, soluble VEGF receptor protein in which the binding domains of VEGF receptors 1 and 2 are combined with the Fc portion of immunoglobulin G. Thus, KH 902 is similar to AFL except for the presence of domain 4 of the VEGF receptor 2, which may enhance its association with the VEGF receptor.70 In a Phase I study, KH 902 was found to be safe with a dose of up to 3 mg.71 A Phase II trial tested 0.5 and 2.0 mg KH 902 in patients with neovascular AMD (n=122). Significant gains in BCVA were observed up to month 12.72 A recently concluded Phase III trial using KH 902 may provide evidence of beneficial effects of the drug on visual acuity.73 The results of this study are awaiting publication.
Current practice patterns with anti-VEGF agents
While most of the discussed studies have assessed monthly, quarterly, bimonthly, or PRN treatment strategies, most American retina specialists use a different dosing regimen in clinical practice. According to the 2014 American Society of Retina Specialists Preferences and Trends Survey, 78% of US retinal specialists (and 56% of international retinal specialists) treat using the treat-and-extend strategy employed in the LUCAS trial. Recently, Rayess and colleagues74 published a retrospective, interventional case series of 212 eyes showing that visual and anatomic improvements were maintained after 3 years of treatment using the treat-and-extend regimen with RBZ and BCZ.
The Seven Year Update of Macular Degeneration Patients (SEVEN-UP) study (n=65) was a multicenter, non-interventional cohort study to examine the long-term results of patients 7 years after entering the original ANCHOR/MARINA trials with monthly dosing regimens. They found 37% of eyes had maintained BCVA ≥20/70 and 37% had BCVA ≤20/200. Sixty-eight percent of study eyes had active exudative disease, and 98% of eyes had developed macular atrophy. This study helped elucidate the challenges of long-term management of exudative AMD, as these patients remain at risk of vision loss many years after treatments.75 Further studies may be necessary to understand if continued monthly dosing would prevent the visual acuity losses seen in this small cohort. However, long-term monthly therapy may also cause adverse events such as geographic atrophy.75
Table 1 summarizes the current understanding of the strategies of treatment of neovascular AMD with anti-VEGF agents.
Novel pharmacotherapeutic approaches
The current gold-standard treatment of neovascular AMD poses a significant financial burden to the health care system (Table 1). In order to overcome this challenge alternative therapeutic approaches targeting various pathways involved in the formation and progression of CNV have been explored. Among various molecular compounds in the pipeline, certain pharmacologic agents have reached Phase II/III clinical trials and may be soon incorporated into clinical practice.
Designated ankyrin repeat proteins
Designated ankyrin repeat proteins (DARPins) constitute a novel class of genetically engineered, anti-angiogenic binding proteins that demonstrate high specificity and affinity. MP0112 (now known as abicipar pegol; Allergan Inc, Irvine, CA, USA), a specific DARPin, has been designed to bind to VEGF-A resulting in longlasting inhibition. Abicipar pegol has completed Phase I/II study in the treatment of neovascular AMD.77 Following encouraging results in the preliminary studies, Phase III studies with abicipar pegol are expected to launch in 2015.
Sphingosine-1 phosphate antibody
Sphingosine-1 phosphate (S1P) antibody is a protein targeting lysosphingolipids, thereby lowering the concentration of S1P from the extracellular fluid. S1P regulates vascular and immune processes and is involved in angiogenesis, vascular stability, and trafficking of B- and T-cells.78 Sonepcizumab (LT1009/iSONEP; LPath, Inc, San Diego, CA, USA) is a monoclonal antibody that selectively binds to S1P and suppresses neovascularization in AMD when administered intravitreally.79 Sonepcizumab is currently being evaluated in Phase II clinical trials for the treatment of neovascular AMD.
Platelet-derived growth factor antibody
Fovista (E10030; Ophthotech, New York, NY, USA) is an aptamer-targeting platelet-derived growth factor (PDGF)-BB homo-dimer that binds to its receptor PDGF-B found on pericytes for its recruitment, regulation, and survival. In addition, PDGF-B has an important role in angiogenesis apart from VEGF.80 A Phase II study comparing Fovista in combination with RBZ versus RBZ alone has demonstrated superior results with combination therapy compared to monotherapy.9 Encouraged by these results, a Phase III study using Fovista in neovascular AMD is currently being conducted.9
Gene therapy
Intraocular gene therapy for the management of neovascular AMD consists of the delivery of nuclear material using viral vectors in order to permanently alter tissue function at the cellular level.
rAAV.sFLT1
Soluble fms-like tyrosine kinase-1 (sFLT1) is a soluble VEGF receptor that binds and reduces free circulating VEGF, thereby disabling vascular growth and proliferation. sFLT1 can be inserted into viral vectors for therapeutic use. When incorporated into adeno-associated virus (AAV), the product is referred to as rAAV.sFLT1 (Avalanche Biotechnologies, Inc, Menlo Park, CA, USA). After subretinal delivery, the product can potentially result in persistent blockade of VEGF actions in patients with neovascular AMD.9,81
AAV2.sFLT01
Similar to rAAV.sFLT01, this product is obtained by using the adeno-associated virus type 2 (AAV2) capsid variant for the delivery of domain 2 of the soluble FLT1. Delivered intravitreally, AAV2.sFLT01 (Genzyme Corporation, Cambridge, MA, USA) can provide lasting anti-VEGF effects in neovascular AMD in a preclinical study involving nonhuman primates.82 This treatment modality has yet to be tested in humans.
Lentivirus expressing angiostatin and endostatin
Molecules that inhibit various steps in the angiogenic pathway such as endostatin and angiostatin can be delivered using lentivirus vectors. RetinoStat (Oxford BioMedica, Oxford, UK) has been designed as a gene-based therapy for delivering these anti-angiogenic proteins to prevent ocular neovascularization. This product may have a therapeutic potential in the treatment of neovascular AMD.83
Stem cell therapy
Short-term to long-term safety evaluation of pluripotent RPE stem cells has been performed in patients with AMD in Phase I/II studies.84 The results of these studies suggest that both embryonic and induced pluripotent stem cell therapy may be a potentially safe novel therapy for patients developing advanced atrophic AMD following neovascular disease.85
Combination treatment strategies for neovascular AMD
Anti-VEGF agents and PDT
Anti-VEGF agents have been used in combination with verteporfin PDT to evaluate the benefit of combination versus monotherapy. The Verteporfin plus Ranibizumab for Choroidal Neovascularization in Age-Related Macular Degeneration (DENALI) study evaluated the effect of the combination of RBZ with PDT compared to RBZ alone. The results did not suggest clinical benefits of adding verteporfin PDT to RBZ therapy.86 Another prospective multicenter clinical trial, the Verteporfin plus Ranibizumab for Choroidal Neovascularization in Age-Related Macular Degeneration (MONT BLANC) study, did not demonstrate benefits of combining PDT with anti-VEGF agents.87 However, results of the EVEREST study47 and few uncontrolled studies88,89 suggest that combination with PDT may lead to requirement of less frequent anti-VEGF injections. Currently, combination of RBZ and PDT may be used as a second-line therapy in patients who do not respond to RBZ monotherapy.90
Intravitreal corticosteroids and PDT
Intravitreal corticosteroids, triamcinolone acetonide in particular, have been used in combination with verteporfin PDT in several randomized clinical trials.91–93 However, in the current practice, combination therapy consisting of corticosteroids is not preferred because of the risk of development of glaucoma and cataract and no definite clear benefits for this combination therapy.18
Triple therapy
In certain difficult-to-treat patients with neovascular AMD, the combination of anti-VEGF agent, intravitreal corticosteroid, and PDT has been tried.94–96 These studies have demonstrated a reduction in macular thickness and improvement in visual acuity outcomes. However, this strategy may be reserved for patients unresponsive to conventional therapy.
Combination with novel therapeutic targets
Novel therapeutic agents such as PDGF antibodies have been evaluated in combination with anti-VEGF agents for the treatment of neovascular AMD. Since both PDGF and VEGF regulate angiogenesis and supplement each other, combination therapy aims at enhancing the ability to reduce choroidal and retinal vascular proliferation. Pericytes treated with anti-PDGF may be more susceptible to the effects of anti-VEGF therapy.90 Further clinical trials are required to establish the efficacy of this combination therapy.
Advances in drug delivery to the posterior segment
Apart from development of newer drug formulations and drug delivery systems, such as nanoparticles, there has been an emphasis on designing drug-delivery devices.97 The aim of these futuristic therapeutic modalities is to provide sustainable care for patients with advanced AMD in order to reduce their hospital visits, and hence treatment burden.
Encapsulated cell technology
The technique of encapsulated cell technology (Neurotech Pharmaceuticals, Cumberland, RI, USA) is designed to deliver pharmacological agents directly to the vitreous cavity after transscleral implantation. This genetically engineered “living” device contains microspore membrane that supports RPE cells. These cells produce drug products within the eye for over 2 years. NT-503 is a VEGF antagonist that can be incorporated into the encapsulated cell technology implant.98
Refillable reservoir devices
Mini-drug pumps provide preprogrammed drug doses into the vitreous cavity. These devices (Replenish Inc, Pasadena, CA, USA) are based on micro-electromechanical system technology and can provide continuous drug delivery for up to 9 months.99 The ForSight Port Delivery System (ForSight Labs, LLC, Menlo Park, CA, USA) is another refillable device that is implanted surgically in a transscleral manner. It can be refilled in the office and can provide long-term drug delivery into the posterior chamber. It is being tested in patients with neovascular AMD.51
Colloidal drug carriers
Colloidal carriers consist of liquid suspensions of microparticles/nanoparticles or liposomes. This drug design ensures better cell membrane penetration and can be employed to deliver drugs such as verteporfin or BCZ. Various drugs can be PEGylated in order to prolong their half-life and reduce degradation. Intravitreal BCZ has been tested in hydrogel formulation in rabbit eyes and has demonstrated promising initial results.100
Suprachoroidal drug delivery
Specialized microneedles have been designed for drug delivery into the suprachoroidal space, from where the pharmacologic agents can diffuse into the vitreous cavity. This technique provides targeted access to the drug to the site of the pathology without invading the vitreous cavity.101 Clearside Biomedical Inc (Alpharetta, GA, USA) has developed suprachoroidal injection devices and drugs for evaluation in various retinal pathologies, including neovascular AMD.
Noninvasive drug-delivery techniques
Transscleral drug delivery is possible using techniques such as iontophoresis, which allows diffusion of a drug using a low voltage electric current. Transscleral delivery of BCZ and dexamethasone has been demonstrated in preclinical models.102 Topical drug-delivery formulations for AMD are also based on the concept of noninvasive drug diffusion. Pulsed high-intensity focused ultrasound has been also studied recently in order to facilitate drug delivery across the sclera noninvasively.103
Pharmacogenomics and personalized medicine in neovascular AMD
The concept of personalized medicine in AMD is fast evolving.104 There have been numerous breakthroughs in the identification of potential genetic biomarkers that could potentially guide therapeutic approaches. There is a significant heterogeneity in the treatment response and required duration of therapy with anti-VEGF agents in neovascular AMD. Thus, personalized medicine based on the pharmacogenomics principle of genotype identification may be a rational futuristic strategy.105
Previous studies have shown that certain candidate single nucleotide polymorphisms may serve as predictive markers for the progression of AMD and its response to treatment. The polymorphisms are most commonly associated with complement H factor gene (CFH) on chromosome 6,106–109 complement C3 gene on chromosome 19p,110,111 and age-related maculopathy susceptibility 2 (ARMS2)/HtrA serine peptidase 1 (HTRA1) region on chromosome 10q.112–114 The attributable risk of AMD due to mutations in the 10q26 locus of ARMS2, HTRA1, and PLEKHA1 genes may be up to 57%.115 The substitution of a histidine for tyrosine at position 402 of the CFH gene product may account for up to 50% of the attributable risk of AMD.116 In addition to predicting the progression of the disease, genetic polymorphisms may also be associated with treatment response to anti-VEGF therapy. Such an association was explored in the CATT trial.117,118 In addition, polymorphisms in the VEGF receptor genes such as VEGFR2/KDR may influence visual outcome in patients treated with anti-VEGF agents such as RBZ.119
Table 2 provides the most common genomic loci that have been associated with AMD. Prediction models have been developed using these genomic findings with greater than 80% discriminative accuracy for advanced neovascular AMD.120–124 In the future, direct-to-consumer personal genome tests may appear in outpatient clinics125,126 and may become an integral part of patient management in neovascular AMD.
  | Table 2 Common genetic variants and polymorphisms associated with progression of age-related macular degeneration or response to therapy |
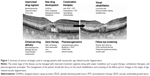
Figure 1 provides a summary of the strategies available for improving the outcomes of patients with neovascular AMD. A flowchart of a therapeutic approach in the management of advanced AMD is depicted in Figure 2.
  | Figure 2 Flowchart of the optimal management of patients with advanced age-related macular degeneration (AMD). |
Visual, vocational, and social rehabilitation
An integral component of modern-day patient management includes social and vocational rehabilitation.127 Along with other systemic comorbidities in the aged population, severe visual compromise may lead to a negative impact on the quality of life.128 Therapy consisting of possibly indefinite anti-VEGF injections in patients with AMD not only poses a significant financial but also psychological burden on patients. Promoting an integral mental health and low-vision rehabilitation (LVR) intervention together with ocular therapy can significantly reduce the burden of depression in these patients.129 As many ophthalmologists may not be able to provide comprehensive care to address every aspect of the disease impact,130 LVR is re-emerging as a necessary subspecialty in ophthalmology.131
Vision rehabilitation for patients suffering from neovascular AMD must be employed before severe visual loss sets in.51 Various strategies for improving visual performance include use of prescription glasses, low-vision aids, adaptive computer software, and modification of the patient’s environment.51,132–134 An LVR trial consisting of low-vision therapy, home visits, and assigned homework conducted by the US Department of Veterans Affairs in patients with visual acuity worse than 20/100 demonstrated the effectiveness of such a program.135 Interventions such as problem-solving therapy and supportive therapy have been shown to improve the visual function in patients with AMD, in addition to the benefits offered by the anti-VEGF therapy.130 Strategies such as eccentric viewing training can enhance the performance of activities of daily living in patients with central vision loss.136 LVR can be considered to be an integral part of the optimal management of patients with sight-threatening neovascular AMD. Table 3 summarizes various techniques of LVR.
  | Table 3 Techniques of low-vision rehabilitation for patients with severe central visual loss due to neovascular age-related macular degeneration |
Conclusion
As per the American Academy of Ophthalmology Preferred Practice Pattern® Guideline for AMD,18 major prospective clinical trials performed in patients with neovascular AMD do not provide clear guidelines for the management of all the patients encountered in clinical practice (Table 1). Thus, management strategies for AMD have been extensively reviewed in the literature periodically.12,140–142 In the last decade, there have been numerous advances and breakthroughs in the management of neovascular AMD (Figure 1). Anti-VEGF agents form the first-line therapy in the contemporary treatment of neovascular AMD. However, recognition of suboptimal response in a significant proportion of patients and awareness of the large burden of current treatments have led to the introduction of several promising therapeutic strategies. High-quality imaging and the application of pharmacogenomic principles are likely to guide future therapy. The proposed management flowchart (Figure 2) is likely to change and need revision as new discoveries are made. With a comprehensive, multi-pronged treatment and rehabilitative approach, the management of AMD will continue to advance to meet the patient needs.
Acknowledgment
The Truhlsen Eye Institute at the University of Nebraska Medical Center has received an unrestricted grant from Research to Prevent Blindness.
Disclosure
Dr Nguyen and Dr Do serve on the scientific advisory boards for Genentech, Inc. and Regeneron, Inc. Dr Nguyen also serves on the scientific advisory boards for AbbVie, Bausch and Lomb, Inc., Santen, Inc., and XOMA. The authors report no other conflicts of interest in this work.
References
Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology. 1992;99(6):933–943. | ||
Congdon N, O’Colmain B, Klaver CC, et al; Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122(4): 477–485. | ||
Friedman DS, O’Colmain BJ, Muñoz B, et al; Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. | ||
Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2): e106–e116. | ||
Stein JD, Newman-Casey PA, Mrinalini T, Lee PP, Hutton DW. Cost-effectiveness of bevacizumab and ranibizumab for newly diagnosed neovascular macular degeneration. Ophthalmology. 2014;121(4):936–945. | ||
Ferris FL 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640–1642. | ||
Rasmussen A, Sander B. Long-term longitudinal study of patients treated with ranibizumab for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2014;25(3):158–163. | ||
Nguyen QD. Introduction: Neovascular age-related macular degeneration: approaches for improving visual acuity and reducing the burden of care. Ophthalmology. 2013;120(5 Suppl):S1–S2. | ||
Tolentino MJ, Dennrick A, John E, Tolentino MS. Drugs in Phase II clinical trials for the treatment of age-related macular degeneration. Expert Opin Investig Drugs. 2014;224(2):1–17. | ||
Solomon SD, Lindsley K, Vedula SS, Krzystolik MG, Hawkins BS. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;8:CD005139. | ||
Hanout M, Ferraz D, Ansari M, et al. Therapies for neovascular age-related macular degeneration: current approaches and pharmacologic agents in development. Biomed Res Int. 2013;2013:830837. | ||
Schmidt-Erfurth U, Chong V, Loewenstein A, et al; European Society of Retina Specialists. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98(9):1144–1167. | ||
Krause L, Yousif T, Pohl K; CAPTAIN study group. An epidemiological study of neovascular age-related macular degeneration in Germany. Curr Med Res Opin. 2013;29(10):1391–1397. | ||
Gangnon RE, Lee KE, Klein BE, Iyengar SK, Sivakumaran TA, Klein R. Severity of age-related macular degeneration in 1 eye and the incidence and progression of age-related macular degeneration in the fellow eye: the Beaver Dam Eye Study. JAMA Ophthalmol. 2015;133(2):125–132. | ||
Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115(1):116–126. | ||
Loewenstein A. The significance of early detection of age-related macular degeneration: Richard & Hinda Rosenthal Foundation lecture, The Macula Society 29th annual meeting. Retina. 2007;27(7): 873–878. | ||
Bakri SJ, Moshfeghi DM, Francom S, et al. Intraocular pressure in eyes receiving monthly ranibizumab in 2 pivotal age-related macular degeneration clinical trials. Ophthalmology. 2014;121(5):1102–1108. | ||
American Academy of Ophthalmology. Age-Related Macular Degeneration. Preferred Practice Pattern® Guideline. San Francisco, CA: American Academy of Ophthalmology; 2014. Available at: http://www.aao.org/Assets/db935a77-1997-4d60-b850-71b7602f46e2/635582143853270000/age-related-macular-degeneration-ppp-pdf. Accessed May 21, 2015. | ||
Do DV, Gower EW, Cassard SD, et al. Detection of new-onset choroidal neovascularization using optical coherence tomography: the AMD DOC Study. Ophthalmology. 2012;119(4):771–778. | ||
Do DV. Detection of new-onset choroidal neovascularization. Curr Opin Ophthalmol. 2013;24(3):244–247. | ||
Malamos P, Sacu S, Georgopoulos M, Kiss C, Pruente C, Schmidt-Erfurth U. Correlation of high-definition optical coherence tomography and fluorescein angiography imaging in neovascular macular degeneration. Invest Ophthalmol Vis Sci. 2009;50(10):4926–4933. | ||
Sadda SR, Liakopoulos S, Keane PA, et al. Relationship between angiographic and optical coherence tomographic (OCT) parameters for quantifying choroidal neovascular lesions. Graefes Arch Clin Exp Ophthalmol. 2010;248(2):175–184. | ||
Keane PA, Heussen FM, Ouyang Y, et al. Assessment of differential pharmacodynamic effects using optical coherence tomography in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53(3):1152–1161. | ||
Castillo MM, Mowatt G, Elders A, et al. Optical coherence tomography for the monitoring of neovascular age-related macular degeneration: a systematic review. Ophthalmology. 2015;122(2):399–406. | ||
Yannuzzi LA. Indocyanine green angiography: a perspective on use in the clinical setting. Am J Ophthalmol. 2011;151(5):745–751.e1. | ||
de Bruin DM, Burnes DL, Loewenstein J, et al. In vivo three-dimensional imaging of neovascular age-related macular degeneration using optical frequency domain imaging at 1050 nm. Invest Ophthalmol Vis Sci. 2008;49(10):4545–4552. | ||
Miura M, Makita S, Iwasaki T, Yasuno Y. Three-dimensional visualization of ocular vascular pathology by optical coherence angiography in vivo. Invest Ophthalmol Vis Sci. 2011;52(5):2689–2695. | ||
Hong YJ, Miura M, Makita S, et al. Noninvasive investigation of deep vascular pathologies of exudative macular diseases by high-penetration optical coherence angiography. Invest Ophthalmol Vis Sci. 2013;54(5):3621–3631. | ||
Jia Y, Bailey ST, Wilson DJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435–1444. | ||
Zhang Y, Wang X, Rivero EB, et al. Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol. 2014;158(3):584–596.e1. | ||
Zarbin MA, Casaroli-Marano RP, Rosenfeld PJ. Age-related macular degeneration: clinical findings, histopathology and imaging techniques. Dev Ophthalmol. 2014;53:1–32. | ||
AREDS2-HOME Study Research Group, Chew EY, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121(2):535–544. | ||
Simader C, Ritter M, Bolz M, et al. Morphologic parameters relevant for visual outcome during anti-angiogenic therapy of neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1237–1245. | ||
Comyn O, Sivaprasad S, Peto T, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). Am J Ophthalmol. 2014;157(5):960–970. | ||
Wu Z, Ayton LN, Luu CD, Guymer RH. Relationship between retinal microstructures on optical coherence tomography and microperimetry in age-related macular degeneration. Ophthalmology. 2014;121(7):1445–1452. | ||
van Landingham SW, Massof RW, Chan E, Friedman DS, Ramulu PY. Fear of falling in age-related macular degeneration. BMC Ophthalmol. 2014;14:10. | ||
Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001; 119(10):1417–1436. | ||
Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005–2015. | ||
SanGiovanni JP, Chew EY, Agrón E, et al; Age-Related Eye Disease Study Research Group. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126(9): 1274–1279. | ||
Tan JS, Wang JJ, Flood V, Mitchell P. Dietary fatty acids and the 10-year incidence of age-related macular degeneration: the Blue Mountains Eye Study. Arch Ophthalmol. 2009;127(5):656–665. | ||
Aronow ME, Chew EY. Age-related Eye Disease Study 2: perspectives, recommendations, and unanswered questions. Curr Opin Ophthalmol. 2014;25(3):186–190. | ||
Awh CC, Lane AM, Hawken S, Zanke B, Kim IK. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology. 2013;120(11):2317–2323. | ||
Chew EY, Klein ML, Clemons TE, et al; Age-Related Eye Disease Study Research Group. No clinically significant association between CFH and ARMS2 genotypes and response to nutritional supplements: AREDS report number 38. Ophthalmology. 2014;121(11):2173–2180. | ||
Bressler NM; Treatment of Age-Related Macular Degeneration with Photodynamic Therapy (TAP) Study Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: two-year results of 2 randomized clinical trials-tap report 2. Arch Ophthalmol. 2001;119(2):198–207. | ||
Verteporfin In Photodynamic Therapy Study Group. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization – verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541–560. | ||
Oishi A, Miyamoto N, Mandai M, et al. LAPTOP study: a 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy. Ophthalmology. 2014;121(5):1151–1152. | ||
Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32(8):1453–1464. | ||
Tan CS, Ngo WK, Lim LW. Re: Oishi et al.: LAPTOP study: a 24-month trial of verteporfin versus ranibizumab for polypoidal choroidal vasculopathy (Ophthalmology. 2014;121:1151–1152). Ophthalmology. 2015;122(1):e5–e6. | ||
Koh A, Expert PCV Panel, Chen LJ, Chen SJ, et al. Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina. 2013;33(4):686–716. | ||
Ijiri S, Sugiyama K. Short-term efficacy of intravitreal aflibercept for patients with treatment-naïve polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2015;253(3):351–357. | ||
Hubschman JP, Reddy S, Schwartz SD. Age-related macular degeneration: current treatments. Clin Ophthalmol. 2009;3:155–166. | ||
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T; ANCHOR Study Group. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology. 2009;116(1):57–65.e5. | ||
Rosenfeld PJ, Brown DM, Heier JS, et al; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. | ||
Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145(2):239–248. | ||
Schmidt-Erfurth U, Eldem B, Guymer R, et al; EXCITE Study Group. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology. 2011;118(5):831–839. | ||
Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148(1):43–58.e1. | ||
Boyer DS, Heier JS, Brown DM, Francom SF, Ianchulev T, Rubio RG. A Phase IIIb study to evaluate the safety of ranibizumab in subjects with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1731–1739. | ||
Singer MA, Awh CC, Sadda S, et al. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175–1183. | ||
Holz FG, Amoaku W, Donate J, et al; SUSTAIN Study Group. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology. 2011;118(4):663–671. | ||
Grunwald JE, Daniel E, Huang J, et al; CATT Research Group. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014;121(1):150–161. | ||
Grunwald JE, Pistilli M, Ying GS, Maguire MG, Daniel E, Martin DF; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Growth of Geographic Atrophy in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology. 2015;122(4):809–816. | ||
CATT Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364(20):1897–1908. | ||
Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group, Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119(7):1388–1398. | ||
IVAN Study Investigators, Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119(7):1399–1411. | ||
Chakravarthy U, Harding SP, Rogers CA, et al; IVAN study investigators. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet. 2013;382(9900):1258–1267. | ||
Krebs I, Schmetterer L, Boltz A, et al; MANTA Research Group. A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol. 2013;97(3):266–271. | ||
Kodjikian L, Souied EH, Mimoun G, et al; GEFAL Study Group. Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 2013;120(11):2300–2309. | ||
Berg K, Pedersen TR, Sandvik L, Bragadóttir R. Comparison of ranibizumab and bevacizumab for neovascular age-related macular degeneration according to LUCAS treat-and-extend protocol. Ophthalmology. 2015;122(1):146–152. | ||
Heier JS, Brown DM, Chong V, et al; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. | ||
Wang Q, Li T, Wu Z, et al. Novel VEGF decoy receptor fusion protein conbercept targeting multiple VEGF isoforms provide remarkable anti-angiogenesis effect in vivo. PloS One. 2013;8(8):e70544. | ||
Zhang M, Zhang J, Yan M, et al; KH902 Phase 1 Study Group. A phase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2011;118(4):672–678. | ||
Li X, Xu G, Wang Y, et al; AURORA Study Group. Safety and efficacy of conbercept in neovascular age-related macular degeneration: results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121(9):1740–1747. | ||
Chengdu Kanghong Biotech Co Ltd. A Randomized, Double-masked, Multicenter, Sham-controlled, Safety and Efficacy Study of KH902 in Patients With Wet AMD (PHOENIX). In: ClinicalTrials.gov [website on the Internet]. Bethseda, MD: US National Library of Medicine; 2011 [updated March 21, 2014]. Available from: http://clinicaltrials.gov/ct2/show/NCT01436864. NLM identifier: NCT01436864. Accessed February 23, 2015. | ||
Rayess N, Houston SK 3rd, Gupta OP, Ho AC, Regillo CD. Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am J Ophthalmol. 2015;159(1):3–8.e1. | ||
Rofagha S, Bhisitkul RB, Boyer DS, Sadda SR, Zhang K; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013;120(11):2292–2299. | ||
Eldem BM, Muftuoglu G, Topbaş S, et al; the SALUTE study group. A randomized trial to compare the safety and efficacy of two ranibizumab dosing regimens in a Turkish cohort of patients with choroidal neovascularization secondary to AMD. Acta Ophthalmol. 2014. Epub August 27. | ||
Souied EH, Devin F, Mauget-Faÿsse M, et al; MP0112 Study Group. Treatment of exudative age-related macular degeneration with a designed ankyrin repeat protein that binds vascular endothelial growth factor: a phase I/II study. Am J Ophthalmol. 2014;158(4):724–732.e2. | ||
O’Brien N, Jones ST, Williams DG, et al. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res. 2009;50(11):2245–2257. | ||
Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol. 2011;162(6):1225–1238. | ||
Dong A, Seidel C, Snell D, et al. Antagonism of PDGF-BB suppresses subretinal neovascularization and enhances the effects of blocking VEGF-A. Angiogenesis. 2014;17(3):553–562. | ||
Lai CM, Estcourt MJ, Wikstrom M, et al. rAAV.sFlt-1 gene therapy achieves lasting reversal of retinal neovascularization in the absence of a strong immune response to the viral vector. Invest Ophthalmol Vis Sci. 2009;50(9):4279–4287. | ||
Lai CM, Estcourt MJ, Himbeck RP, et al. Preclinical safety evaluation of subretinal AAV2.sFlt-1 in non-human primates. Gene Ther. 2012;19(10):999–1009. | ||
Igarashi T, Miyake K, Kato K, et al. Lentivirus-mediated expression of angiostatin efficiently inhibits neovascularization in a murine proliferative retinopathy model. Gene Ther. 2003;10(3):219–226. | ||
Schwartz SD, Regillo CD, Lam BL, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385(9967):509–516. | ||
Sheridan C. Stem cell therapy clears first hurdle in AMD. Nat Biotechnol. 2014;32(12):1173–1174. | ||
Kaiser PK, Boyer DS, Cruess AF, Slakter JS, Pilz S, Weisberger A; DENALI Study Group. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month results of the DENALI study. Ophthalmology. 2012;119(5):1001–1010. | ||
Larsen M, Schmidt-Erfurth U, Lanzetta P, et al; MONT BLANC Study Group. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month MONT BLANC study results. Ophthalmology. 2012;119(5):992–1000. | ||
Hatz K, Schneider U, Henrich PB, Braun B, Sacu S, Prünte C. Ranibizumab plus verteporfin photodynamic therapy in neovascular age-related macular degeneration: 12 months of retreatment and vision outcomes from a randomized study. Ophthalmologica. 2015;233(2):66–73. | ||
Lai TY, Lee GK, Luk FO, Lam DS. Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina. 2011;31(8):1581–1588. | ||
Englander M, Kaiser PK. Combination therapy for the treatment of neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2013;24(3):233–238. | ||
Chan WM, Lai TY, Wong AL, Tong JP, Liu DT, Lam DS. Combined photodynamic therapy and intravitreal triamcinolone injection for the treatment of subfoveal choroidal neovascularisation in age related macular degeneration: a comparative study. Br J Ophthalmol. 2006;90(3): 337–341. | ||
Chaudhary V, Mao A, Hooper PL, Sheidow TG. Triamcinolone acetonide as adjunctive treatment to verteporfin in neovascular age-related macular degeneration: a prospective randomized trial. Ophthalmology. 2007;114(12):2183–2189. | ||
Arias L, Garcia-Arumi J, Ramon JM, Badia M, Rubio M, Pujol O. Photodynamic therapy with intravitreal triamcinolone in predominantly classic choroidal neovascularization: one-year results of a randomized study. Ophthalmology. 2006;113(12):2243–2250. | ||
Kovacs KD, Quirk MT, Kinoshita T, et al. A retrospective analysis of triple combination therapy with intravitreal bevacizumab, posterior sub-tenon’s triamcinolone acetonide, and low-fluence verteporfin photodynamic therapy in patients with neovascular age-related macular degeneration. Retina. 2011;31(3):446–452. | ||
Forte R, Bonavolontà P, Benayoun Y, Adenis JP, Robert PY. Intravitreal ranibizumab and bevacizumab in combination with full-fluence verteporfin therapy and dexamethasone for exudative age-related macular degeneration. Ophthalmic Res. 2011;45(3):129–134. | ||
Ehmann D, García R. Triple therapy for neovascular age-related macular degeneration (verteporfin photodynamic therapy, intravitreal dexamethasone, and intravitreal bevacizumab). Can J Ophthalmol. 2010;45(1):36–40. | ||
Schwartz SG, Scott IU, Flynn HW Jr, Stewart MW. Drug delivery techniques for treating age-related macular degeneration. Expert Opin Drug Deliv. 2014;11(1):61–68. | ||
Kauper K, Rivera M, Mills J, et al. Design considerations and performance of a next-generation encapsulated cell technology intraocular implant delivering VEGF-antagonist [meeting abstract]. Invest Ophthalmol Vis Sci. 2014;55(13):441. | ||
Saati S, Lo R, Li PY, Meng E, Varma R, Humayun MS. Mini drug pump for ophthalmic use. Trans Am Ophthalmol Soc. 2009;107:60–70. | ||
Rauck BM, Friberg TR, Medina Mendez CA, et al. Biocompatible reverse thermal gel sustains the release of intravitreal bevacizumab in vivo. Invest Ophthalmol Vis Sci. 2014;55(1):469–476. | ||
Olsen TW, Feng X, Wabner K, Csaky K, Pambuccian S, Cameron JD. Pharmacokinetics of pars plana intravitreal injections versus microcannula suprachoroidal injections of bevacizumab in a porcine model. Invest Ophthalmol Vis Sci. 2011;52(7):4749–4756. | ||
Pescina S, Ferrari G, Govoni P, et al. In-vitro permeation of bevacizumab through human sclera: effect of iontophoresis application. J Pharm Pharmacol. 2010;62(9):1189–1194. | ||
Murugappan SK, Zhou Y. Transsclera Drug Delivery by Pulsed High-Intensity Focused Ultrasound (HIFU): An Ex Vivo Study. Curr Eye Res. 2014. November 7. | ||
Souied EH, Leveziel N. Toward personalized medicine for age-related macular degeneration. Am J Ophthalmol. 2012;154(3):427–428. | ||
Heier JS. Neovascular age-related macular degeneration: individualizing therapy in the era of anti-angiogenic treatments. Ophthalmology. 2013;120(5 Suppl):S23–S25. | ||
Sundaresan P, Vashist P, Ravindran RD, et al. Polymorphisms in ARMS2/HTRA1 and complement genes and age-related macular degeneration in India: findings from the INDEYE study. Invest Ophthalmol Vis Sci. 2012;53(12):7492–7497. | ||
Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. | ||
Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720): 385–389. | ||
Baird PN, Hageman GS, Guymer RH. New era for personalized medicine: the diagnosis and management of age-related macular degeneration. Clin Experiment Ophthalmol. 2009;37(8):814–821. | ||
Yates JR, Sepp T, Matharu BK, et al; Genetic Factors in AMD Study Group. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357(6):553–561. | ||
Yanagisawa S, Kondo N, Miki A, et al. A common complement C3 variant is associated with protection against wet age-related macular degeneration in a Japanese population. PloS One. 2011;6(12): e28847. | ||
Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314(5801):992–993. | ||
Chen W, Xu W, Tao Q, et al. Meta-analysis of the association of the HTRA1 polymorphisms with the risk of age-related macular degeneration. Exp Eye Res. 2009;89(3):292–300. | ||
Deangelis MM, Ji F, Adams S, et al. Alleles in the HtrA serine peptidase 1 gene alter the risk of neovascular age-related macular degeneration. Ophthalmology. 2008;115(7):1209–1215.e7. | ||
Kanoff J, Miller J. Pharmacogenetics of the treatment response of age-related macular degeneration with ranibizumab and bevacizumab. Semin Ophthalmol. 2013;28(5–6):355–360. | ||
Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. | ||
Hagstrom SA, Ying GS, Pauer GJ, et al; Comparison of Age-Related Macular Degeneration Treatments Trials (CATT) Research Group. VEGFA and VEGFR2 gene polymorphisms and response to anti-vascular endothelial growth factor therapy: comparison of age-related macular degeneration treatments trials (CATT). JAMA Ophthalmol. 2014;132(5):521–527. | ||
Hagstrom SA, Ying GS, Pauer GJ, et al; Comparison of AMD Treatments Trials Research Group. Pharmacogenetics for genes associated with age-related macular degeneration in the Comparison of AMD Treatments Trials (CATT). Ophthalmology. 2013;120(3):593–599. | ||
Hermann MM, van Asten F, Muether PS, et al. Polymorphisms in vascular endothelial growth factor receptor 2 are associated with better response rates to ranibizumab treatment in age-related macular degeneration. Ophthalmology. 2014;121(4):905–910. | ||
Chen Y, Zeng J, Zhao C, et al. Assessing susceptibility to age-related macular degeneration with genetic markers and environmental factors. Arch Ophthalmol. 2011;129(3):344–351. | ||
Grassmann F, Fritsche LG, Keilhauer CN, Heid IM, Weber BH. Modelling the genetic risk in age-related macular degeneration. PloS One. 2012;7(5):e37979. | ||
Hageman GS, Gehrs K, Lejnine S, et al. Clinical validation of a genetic model to estimate the risk of developing choroidal neovascular age-related macular degeneration. Hum Genomics. 2011;5(5):420–440. | ||
Perlee LT, Bansal AT, Gehrs K, et al. Inclusion of genotype with fundus phenotype improves accuracy of predicting choroidal neovascularization and geographic atrophy. Ophthalmology. 2013;120(9):1880–1892. | ||
Sobrin L, Seddon JM. Nature and nurture-genes and environment- predict onset and progression of macular degeneration. Prog Retin Eye Res. 2014;40:1–15. | ||
Kalf RR, Mihaescu R, Kundu S, de Knijff P, Green RC, Janssens AC. Variations in predicted risks in personal genome testing for common complex diseases. Genet Med. 2014;16(1):85–91. | ||
Buitendijk GH, Amin N, Hofman A, van Duijn CM, Vingerling JR, Klaver CC. Direct-to-consumer personal genome testing for age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(10):6167–6174. | ||
Buitendijk GH, Rochtchina E, Myers C, et al. Prediction of age-related macular degeneration in the general population: the Three Continent AMD Consortium. Ophthalmology. 2013;120(12):2644–2655. | ||
Dev MK, Paudel N, Joshi ND, Shah DN, Subba S. Impact of visual impairment on vision-specific quality of life among older adults living in nursing home. Curr Eye Res. 2014;39(3):232–238. | ||
Rovner BW, Casten RJ, Hegel MT, et al. Low vision depression prevention trial in age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2014;121(11):2204–2211. | ||
Rovner BW, Casten RJ, Hegel MT, et al. Improving function in age-related macular degeneration: a randomized clinical trial. Ophthalmology. 2013;120(8):1649–1655. | ||
Christoforidis JB, Tecce N, Dell’Omo R, Mastropasqua R, Verolino M, Costagliola C. Age related macular degeneration and visual disability. Curr Drug Targets. 2011;12(2):221–233. | ||
Alexander MS, Lajoie K, Neima DR, Strath RA, Robinovitch SN, Marigold DS. Effect of ambient light and age-related macular degeneration on precision walking. Optom Vis Sci. 2014;91(8):990–999. | ||
Alexander MS, Lajoie K, Neima DR, Strath RA, Robinovitch SN, Marigold DS. Effects of age-related macular degeneration and ambient light on curb negotiation. Optom Vis Sci. 2014;91(8):975–989. | ||
Watson GR. Low vision in the geriatric population: rehabilitation and management. J Am Geriatr Soc. 2001;49(3):317–330. | ||
Stelmack JA, Tang XC, Reda DJ, Rinne S, Mancil RM, Massof RW; LOVIT Study Group. Outcomes of the Veterans Affairs Low Vision Intervention Trial (LOVIT). Arch Ophthalmol. 2008;126(5):608–617. | ||
Gaffney AJ, Margrain TH, Bunce CV, Binns AM. How effective is eccentric viewing training? A systematic literature review. Ophthalmic Physiol Opt. 2014;34(4):427–437. | ||
Hooper P, Jutai JW, Strong G, Russell-Minda E. Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol. 2008;43(2):180–187. | ||
Amore FM, Paliotta S, Silvestri V, Piscopo P, Turco S, Reibaldi A. Biofeedback stimulation in patients with age-related macular degeneration: comparison between 2 different methods. Can J Ophthalmol. 2013;48(5):431–437. | ||
Pijnacker J, Verstraten P, van Damme W, Vandermeulen J, Steenbergen B. Rehabilitation of reading in older individuals with macular degeneration: a review of effective training programs. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2011;18(6):708–732. | ||
Keane PA, de Salvo G, Sim DA, Goverdhan S, Agrawal R, Tufail A. Strategies for improving early detection and diagnosis of neovascular age-related macular degeneration. Clin Ophthalmol. 2015;9:353–366. | ||
Lai K, Landa G. Current choice of treatments for neovascular AMD. Expert Rev Clin Pharmacol. 2015;8(1):135–140. | ||
Singer M. Advances in the management of macular degeneration. F1000Prime Rep. 2014;6:29. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.