Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Management of Complex Cryptoglandular Anal Fistula: Challenges and Solutions
Authors Garg P , Sodhi SS, Garg N
Received 27 September 2020
Accepted for publication 15 October 2020
Published 11 November 2020 Volume 2020:13 Pages 555—567
DOI https://doi.org/10.2147/CEG.S198796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Everson L.A. Artifon
Pankaj Garg,1 Sohail Singh Sodhi,2 Navdeep Garg3
1Department of Colorectal Surgery, Garg Fistula Research Institute, Panchkula, Haryana, India; 2Department of General Surgery, Dayanand Medical College and Hospital, Ludhiana, Punjab, India; 3Department of General Surgery, Government Medical College and Hospital, Chandigarh, India
Correspondence: Pankaj Garg
Department of Colorectal Surgery, Garg Fistula Research Institute, 1042, Sector 15, Panchkula, Haryana 134113, India
Fax +91-172-2594556
Email [email protected]
Abstract: Anal fistulae can be a very difficult disease to manage. The management of complex fistulae is even more challenging. The risk to the fecal continence mechanism due to damage to the anal sphincters and refractoriness to the treatment (high recurrence rate) pose the two biggest challenges in the management of this disease. Apart from these, there are several other challenges in the treatment of complex fistulae. The intriguing and uphill task is that satisfactory solutions to most of these challenges are still not known, and there is hardly any consensus on whatever treatment solutions are available. To summarize, there is no gold-standard treatment available for treating complex anal fistulae, and the search for a satisfactory treatment option is still on. In this review, the endeavor has been to discuss and highlight recent path-breaking updates in the management of complex anal fistulae.
Keywords: anal fistula, recurrence, incontinence, classification, fistulotomy, sphincter
Introduction
Anal fistula is a common disease that has been troubling mankind for the last few centuries. Despite several advancements in this field, gold-standard treatment of anal fistula still eludes us. There are several challenges that make the management of complex anal fistulae quite difficult. In this review, major challenges are first discussed, followed by discussion of solutions (Table 1).
Definition of Complex Anal Fistulae
It is pertinent to define complex anal fistulae. From a practical point of view, a fistula that is difficult to manage, has a higher risk of recurrence and poses a greater threat to continence is classified as a complex fistula.1 Fistulotomy is the oldest, simplest, and most widely used procedure for anal fistulae and has a very high success rate (95%–98%) when patient selection is done appropriately.2 In other words, the fistulae that can be safely (without risk of any incontinence) managed by fistulotomy are classified as simple fistulae. Fistulae that cannot be managed safely and successfully by fistulotomy are categorized as complex fistulae.3 This categorization seems logical and practical. The various classifications are meant to make this categorization clear, but they fail to do so, as shall be discussed later.3
Challenges in Managing Complex Anal Fistulae
There are several challenges in managing complex anal fistulae, which are broadly divided in six categories (Table 1).
- High fistulae (transsphincteric infralevator, supralevator, suprasphincteric, and extrasphincteric fistulae)
High fistulae can be high transsphincteric (infralevator), suprasphincteric, supralevator, or extrasphincteric fistulae. The real danger in these fistulae is the risk of incontinence if a significant part of the external sphincter is cut or damaged inadvertently. It will be pertinent to discuss differences among these fistulae.
These fistulae involve a significant proportion of the external sphincter (at least a third of the external sphincter, Figure 1).
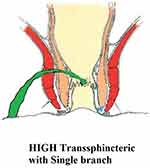 |
Figure 1 A high transsphincteric fistula. |
These fistulae go higher up and cross the levator muscle to reach the supralevator space. They do not cut across the levator muscle, but ascend in the intersphincteric plane into the supralevator space (Figure 2).4
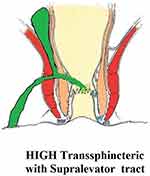 |
Figure 2 A high transsphincteric fistula with supralevator extension. |
These fistulae do not enter the supralevator space, but still involve almost completely the external sphincter. They ascend superiorly from the internal opening into the intersphincteric space and pierce the junction between the puborectalis (uppermost point of the external sphincter) and the levator muscle to enter the ischiorectal fossa (Figure 3). As such, these U-shaped fistulae curve upward to involve almost 100% of the external sphincter (Figure 3). These fistulae are difficult to manage, as they ascend quite high, due to which it becomes difficult at times to reach the top of the fistula. Second, the risk of incontinence is quite high if these fistulae are managed by a sphincter-cutting procedure like fistulotomy or a cutting seton.4 Third, it is not easy to accurately assess and diagnose these fistulae.
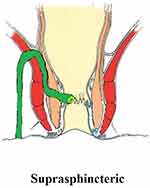 |
Figure 3 A suprasphincteric fistula. |
These fistulae rupture/pierce the levator muscle to enter the supralevator space (Figure 4). As shall be discussed later, they either do not exist or are extremely rare.4
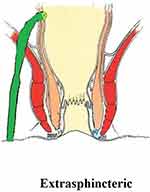 |
Figure 4 An extrasphincteric fistula. |
Challenge: These high fistulae are very difficult to evaluate (delineate accurately) preoperatively and pose a grave challenge in management, as any procedure that cuts the external sphincter, such as fistulotomy or a cutting seton, poses a serious risk to the continence mechanism.
These are fistulae with multiple tracts, as the fistula can spread in different directions. They have more symptoms and are cumbersome to the patient.
Challenge: These fistulae are difficult to evaluate preoperatively, as all the tracts need to be delineated accurately. Also, thesy are difficult to manage, as the risk of recurrence is high: even a single missed tract will lead to recurrence.5
A good proportion of anal fistulae can have an associated abscess. This happens in 15%–40% of cases.6,7 The incidence of associated abscess has been shown to be higher for complex fistulae.8 Alternatively, an anal fistula can present as an anorectal or ischiorectal abscess. Not commonly, the patient may present with septicemia.
Challenge: The evaluation of patients with anal fistulae with associated abscess can be difficult, especially in patients with isolated intersphincteric abscesses. There can be a few challenges in management. First, there can be associated inflammation in the sphincter complex, due to the acute abscess. This increases the risk of sphincter damage if definitive surgery is performed. Second, it is debated and there is lack of consensus as to whether this condition should be managed as a single stage or in multiple stages (first, the abscess is drained, and the fistula is managed afterward).
This happens in 10%–22% of patients operated for anal fistula.5,9,10 There will be fistulae in which it is not possible to localize the internal opening, even after a thorough clinical examination and assessment by advanced radiological modalities (magnetic resonance imaging [MRI]/transrectal ultrasound [TRUS]). It has been found that the inability to accurately locate the internal opening is one of the prime reasons for recurrence of anal fistulae.5,10,11 It has also been found that of all the risk factors associated with recurrence of anal fistulae, nondetection of the internal opening was associated with the highest risk.11
Challenge: These fistulae in which the internal opening cannot be localized pose a great challenge in management. Almost all the procedures performed to treat anal fistulae require accurate identification of the internal opening. A few procedures, such as an anal fistula plug,1,12 video-assisted anal fistula treatment,13,14 and an over-the-scope clip,15 involve direct closure of the internal opening. Therefore, the management of such cases is an uphill task and poses a great therapeutic challenge.
There are fistulae that keep recurring despite undergoing several operations. Every fistula surgeon comes across such a patient occasionally, and it is frustrating to manage such cases. A recent large meta-analysis found that the main reasons for recurrence of fistulae, in decreasing order of risk, were internal opening not found (RR 8.54), high transsphincteric fistula (RR 4.77), multiple tracts (RR 4.77), horseshoe extension (RR 1.92) and recurrent fistula (RR 1.52).11 These recurrences can be early or delayed.
Early recurrences: these include fistulae that do not heal at all or recur within 1 year of complete healing.
Delayed recurrence: these are fistulae that recur after 1 year of complete healing (cessation of pus discharge from all external openings and the anus).
Challenge: the main challenge in these fistulae lies in knowing the exact cause of recurrence and tackling that. However, at times the reason for recurrence can be a lack of technical expertise. Some highly complex fistulae, such as suprasphincteric and supralevator, need higher surgical expertise for proper management.4
A cryptoglandular anal fistula can have associated TB or other secondary diseases.
Challenge: Inability to diagnose and manage these diseases can lead to nonhealing or delayed recurrence of the fistula.
Solutions in the Management of Complex Anal Fistulae
- Use of appropriate imaging modalities
Imaging plays a pivotal role in managing complex anal fistulae. The advent of MRI and TRUS revolutionized the way anal fistulae can be imaged. They enhanced the understanding of anatomy and pathophysiology in a remarkable way.
There are a few salient features about fistula imaging that need to be highlighted.
- Assessing the extent of sphincter involvement: MRI or TRUS gives objective, accurate information about the amount of sphincter involvement by the fistula. Therefore, whenever a high fistula is suspected on clinical examination, MRI/TRUS should be done. However, the importance of clinical examination should not be underestimated.
Clinical assessment of anal sphincter involvement Clinical assessment of the amount of anal sphincter involved can be done by performing a gentle per rectal examination in the surgeon’s office. The location of the internal opening and height of the intersphincteric tract can be assessed by feeling the induration inside the rectum. If the induration is palpable quite high up, then there is a good possibility that it is a high fistula (and more sphincter is involved). This information can be supplemented by examination under anesthesia before performing surgery. A metal probe is inserted into the fistula tract, and then the amount of sphincter can be palpated between the probe and the finger inserted in the rectum. This also gives a fair idea about the amount of sphincter involved by the fistula.
Radiological assessment of anal sphincter involvement MRI and TRUS are the best modalities available to assess the amount of sphincter involvement.6,7,16,17 The importance of this evaluation cannot be overemphasized, as this plays a major role in deciding the proper management of fistulae and is the prime point in grading fistulae by the latest classifications.3,18 It is not difficult to understand that evaluation of sphincter involvement especially of the external sphincter, is not possible by clinical examination in all cases.3,7 This is especially true for high fistulae. Inaccurate assessment of sphincter involvement can lead to serious consequences. Underestimation of sphincter involvement can lead to erroneous selection of a sphincter-cutting procedure like fistulotomy. This increases the risk of incontinence.18 On the other hand, overestimation of sphincter involvement by the fistulae can lead to erroneous selection of a sphincter-saving procedure, which increases the risk of recurrence. This is so because unlike fistulotomy, which can be safely done in low fistulae with a success rate of 90%–98%,18 most sphincter-saving procedures have a success rate of 30%–68%.1,15,19–27 Therefore, overestimation of sphincter involvement can lead to selection of a surgical procedure with a lower success rate.
Status of Extrasphincteric Fistulae
There is emerging evidence that extrasphincteric fistulae do not exist or are extremely rare4 (Figure 4). Several studies including a large series of >1,300 MRI scans did not find even a single extrasphincteric fistula in the cohort.6 There could be three reasons for this:
- It is possible that extrasphincteric fistulae were perhaps overdiagnosed, as good imaging modalities like MRI and TRUS were not available when this fistula was first described.29 During those times, high fistula-in-ano were assessed and delineated only by clinical examination, operative findings, and fistulography. Understandably, it must have been difficult to diagnose extrasphincteric fistulae based only on operative findings and/or fistulography. It is quite possible that many high transsphincteric or supralevator fistulae were erroneously labeled as extrasphincteric fistulae.
- The second reason could be that extrasphincteric fistulae are mainly caused iatrogenically.29 It is possible that this cause (iatrogenic) was much more common six decades back, when there was no MRI or TRUS. As per Parks et al, extrasphincteric fistulae used to occur when a high translevator extension of transsphincteric fistula (Parks grade IIb) was drained into the rectum.29,30 With the advent of advanced radiological modalities (MRI and TRUS) and more understanding of anorectal anatomy over the last several years, iatrogenically caused extrasphincteric fistulae have perhaps reduced drastically.
- The third reason for the rarity/nonexistence of extrasphincteric fistulae could be that they are unlikely to occur from a pathophysiological point of view. Whenever there is a collection in the ischiorectal fossa, it is extremely difficult for the pus present there to rupture into the supralevator space.4 This is because there is a barrier in the form of the strong levator plate (muscle). Second, when there is ample space available for the infection to spread into the soft compressible tissue (fat) in the ischiorectal fossa, it would require a large amount of pus to generate enough pressure for the abscess to rupture or perforate through the levator muscle.4 Even if such high pressure is generated, then by that time the abscess has invariably ruptured through the perianal skin, rather than rupturing through the levator plate. Therefore, extrasphincteric fistulae rarely occur spontaneously.4,30
Due to these factors, extrasphincteric fistulae perhaps do not exist or are very rare. Therefore, these fistulae should be diagnosed only when there is strong evidence on MRI confirming their presence.
These findings highlight the point that an MRI/TRUS should perhaps be done for every fistula patient. Conventionally, it is recommended that only recurrent fistulae should undergo MRI assessment.17,31 As such, going by the findings of the aforementioned study, at least a third of primary (nonrecurrent) simple fistulae would actually be complex, and if managed like a simple fistula would have a very high risk of recurrence.7 Therefore, MRI/TRUS should perhaps be done in every fistula patient.7
Though an MRI in every patient would cost more, this needs to be assessed against potential recurrence. In a nutshell, MRI can be costly, but is cheaper than a recurrence.
It happens not infrequently that the fistula looks completely healed on clinical examination, with cessation of all pus discharge and closure of all external openings, but the patient suffers from delayed recurrence after a few months or a couple of years. One of the prime reasons for this is that though the external opening has closed, the internal opening and/or the tract in the intersphincteric space did not heal and led to the recurrence. The nonhealing of the internal opening and intersphincteric tract or abscess may not be detected on clinical examination in all patients, but can be easily picked up on MRI/TRUS (Figure 5). Therefore, what seems a delayed recurrence may be a fistula that never actually healed. MRI plays a pivotal role in identifying such cases.
It is important to understand the role of classification in the management of anal fistulae. The purpose of any classification is twofold: first to grade the disease as per its severity, and second to guide management of the disease. Incidentally, the two commonly used classifications for anal fistula, Parks and St James’s University Hospital (SJUH), were not serving either purpose.29,32 A low transsphincteric fistula of 1 cm involving 5% of the external sphincter (Parks grade II and SJUH grade III) is simpler and easier to manage than a high intersphincteric fistula reaching up to the levator muscle (Parks grade I and SJUH grade I). Moreover, these two classifications do not provide any guidance regarding the management of anal fistulae. This shortcoming was improved upon by another classification, proposed recently in 2017, known as Garg classification (Table 2).3,18 This classification categorized fistulae in five grades. The first two grades, grades I and II are low fistulae (involving less than a third of the external sphincter) and classified as simple fistulae. Fistulotomy can be undertaken in these fistulae safely without any risk of incontinence. Grades III–V are high fistulae (involving more than a third of the external sphincter) and are classified as complex fistulae3 (Table 2). Fistulotomy is contraindicated, and a sphincter-saving procedure should be performed in these patients.3
 |
Table 2 Anal Fistula Classification |
Most surgeons cannot independently interpret MRI of their patients and are dependent on the radiologist’s report for the management of the fistula. Due to this, the radiologist’s report needs to contain all the relevant information about the MRI that will be useful to the surgeon. Therefore, use of an appropriate classification (Garg classification) by radiologists will guide surgeons precisely regarding the complexity of the fistulae, whereby fistulae can be safely managed by fistulotomy at even a primary health–care level, ie, which fistulae need a sphincter-sparing procedure and which fistulae should be referred to a fistula expert (Table 2).3,18
The significance of the intersphincteric space in the pathogenesis and management of anal fistulae was first highlighted by Eisenhammer in 1958.33 There are two concepts regarding management of the intersphincteric tract that need discussion. First, the intersphincteric tract, being bound by the internal and the external sphincter sphincters on both sides, is like a sepsis/abscess in a closed space. The intersphincteric component is present in most complex anal fistulae. Recent studies have shown that if sepsis in the intersphincteric space is not eradicated, then there are high chances of nonhealing or delayed recurrence of the fistula.2, 28 The second relevant point is that a single drainage does not help, as the intersphincteric abscess/sepsis needs continuous drainage for a few days to achieve complete healing.2 This is not difficult to understand. An ordinary abscess in the skin, anywhere in the body, is not adequately cured by a single aspiration. Rather, the abscess needs continuous drainage (deroofing) till the wound heals completely. Similarly, sepsis in the intersphincteric space needs continuous drainage (deroofing) till complete healing has happened.2
Once these two concepts — intersphincteric tract is like an abscess in a closed space (ISTAC) and draining all pus and ensuring continuous drainage (DRAPED) — are understood (Garg’s cardinal principles), then it becomes easy to understand the success rate of various procedures while treating complex anal fistulae.2 Procedures like an anal fistula plug,34 fibrin glue,35 video-assisted anal fistula treatment,36 advancement flap,37 over-the-scope clip,38 fistula tract laser closure, and stem-cell therapy, which do not address the intersphincteric tract/sepsis at all, have low success rates in complex anal fistulae.2 These procedures have an overall success rate of 20%–75% while treating anal fistulae.1,13 On closer scrutiny, it can be observed that most fistulae addressed with these procedures were actually simple fistulae.2 Therefore, the success rate of these procedures in exclusive complex fistulae is expected to be even lower.2 On the other hand, ligation of the intersphincteric tract (LIFT) takes care of the first step (ISTAC), but ignores the second step (DRAPED).2 Though the intersphincteric tract/sepsis is debrided and cleaned in LIFT, the intersphincteric space is closed and not kept open till complete healing has happened. Therefore, LIFT has a moderate success rate (40%–60%) in complex anal fistulae.39–41
The only procedures that take care of both steps are fistulotomy,2 fistulectomy with primary sphincter repair (FPR),42,43 and transanal opening of the intersphincteric space (TROPIS).28 In all these three procedures, the intersphincteric part of the fistula is either opened up and healing occurs as a secondary intention (fistulotomy and TROPIS) or the intersphincteric tract is completely excised (FPR). Therefore, these procedures (TROPIS and FPR) have high success rates — 90%–98% — in complex fistulae.28,43 Fistulotomy can be performed only in low fistulae (those involving less than a third of the external sphincter), as in high fistulae it can increase the risk of incontinence. However, in low fistulae with an intersphincteric component, fistulotomy has high healing rates (95%–99%).2
TROPIS is relatively a new procedure (2017).28 In this procedure, the intersphincteric space is laid open with the aim of deroofing the intersphincteric tract of the fistula into the anal canal.4,28 After careful assessment of MRI, the course of the intersphincteric tract from the internal opening is noted. A curved artery forceps is inserted into this intersphincteric tract from the internal opening.8,28 The tissue over the artery forceps (mucosa and the internal sphincter) is cut with an electrocautery to lay open the intersphincteric tract into the anal canal. The direction of the cut is circular or oblique, depending on the direction and course of the intersphincteric tract from the internal opening. If a branch of the intersphincteric tract courses superiorly, as in supralevator or suprasphincteric fistulae, then this superior branch is also laid open.4,28
As discussed, the role of sepsis in the intersphincteric plane in the pathophysiology of complex anal fistulae has been known since 1958,33 but for the next six decades the transanal laying open of the intersphincteric space was performed only for intersphincteric abscesses and “pure” intersphincteric fistulae.44,45 These constitute only approximately 10% of complex anal fistulae.8 Extension of the TROPIS concept to all complex fistulae was done for the first time in 2017.28 The procedure has shown promising results in complex fistulae, with an overall success rate of 85%–92%.8,28 The reason for this could be that by deroofing the intersphincteric space into the rectum, the TROPIS procedure takes care of both the ISTAC and the DRAPED concepts.2,28
There is no conclusive evidence if a simple drainage or a sphincter-cutting procedure is better in the treatment of anorectal abscess–fistula.46 However, there are certain recommendations that are common in most guidelines. A 2018 Association of Coloproctology of Great Britain and Ireland position statement47 recommended that in anal fistula patients with acute anorectal abscess, there is a higher risk of incontinence if definitive surgery is carried out. Simple incision and drainage is a safer alternative, but is associated with a higher rate of recurrence. Immediate fistulotomy, although associated with a lower recurrence rate than simple incision and drainage, carries a risk of misjudgment of the depth of the fistula.47 In a large meta-analysis, Quah et al identified lower recurrence rates of anorectal sepsis (abscess or fistula) with immediate fistula treatment.46 However, there was a tendency for a higher risk of incontinence to flatus and soiling when a primary sphincter-cutting procedure was performed. The 2017 German S348 guidelines suggest that superficial fistulae, which involve only small parts of the anal sphincter, should be treated with primary fistulotomy, provided the surgery is performed by an experienced surgeon. An experienced surgeon is defined as one who has operated on at least 60 fistulae.48 Despite this, every division of the anal sphincter bears the risk of fecal incontinence. In cases of unclear findings or high fistulae, definitive procedures should be performed later.48
However, a recent study has shown that even in high fistulae with acute anorectal abscess, definitive fistula surgery (a sphincter-saving procedure) can be done with excellent results, provided proper preoperative evaluation is done.8 In this large prospective study comprising only high fistulae, the TROPIS sphincter-saving procedure,was done as the primary definitive procedure in fistulae with acute anorectal abscess (n=115, study group). The same procedure was done in chronic fistulae with no acute sepsis/abscess (n=191, control group).8 Overall healing rates in the acute anorectal abscess and the chronic fistula groups were 87% (100 of 115) and 88% (168 of 191), respectively, and were not significantly different (p=0.85).8 Differences between preoperative and postoperative continence levels, measured by objective Vaizey’s continence scores, between the acute anorectal abscess and the chronic fistula groups were 0.057±0.47 and 0.014±0.39, respectively, and were not significantly different either (p=0.77 on Mann–Whitney U test).8 As such, a trend is emerging that fistulae with acute anorectal sepsis, including high fistulae, can be treated with definitive surgery in the first operation, provided the surgeon is experienced and well versed in the operative procedure being performed. However, more data are needed before any definite guidelines can be given on this.
Management is very difficult in anal fistulae in which the internal opening cannot be localized after examination (in the clinic and under anesthesia) and detailed MRI assessment.5,10 The recurrence rate is also very high in such fistulae.5,10 As mentioned before, this factor is associated with the highest risk of fistula recurrence.11 There are few data available in the literature regarding the management of anal fistulae in which the internal opening cannot be found. However, in a large study, a protocol was proposed recently (Garg protocol), which has been shown to be effective in managing fistulae in which the internal opening is not localizable.9 In this study,9 the internal opening was categorized as “nonlocalizable” when these four steps failed to find the position of the internal opening:
- preoperative clinical examination: per-rectal examination in the clinic to feel the point of maximum induration
- intraoperative examination under anesthesia on the operating table: per-rectal examination to feel the point of maximum induration and visual inspection of the anal canal with a bivalve rectal speculum to identify the internal opening
- intraoperative injection of a colored solution through the external opening to observe its egress into the anal canal through the internal opening
- radiological assessment with MRI: MRI is able to accurately pinpoint the location of internal opening in most cases.
Management Protocol
In an aforementioned study, fistulae in which the internal opening could not be located by utilizing the four steps just mentioned were categorized as “fistulae with internal opening not found” and managed by a two-step protocol, as follows.9
- The MRI scan was reassessed in the operating room. The point was noted where the fistula tract was reaching up to the sphincter complex. It was assumed that the internal opening was present at that position only, and the fistula was managed accordingly with a definitive procedure. For example, if the fistula tract was reaching up to the sphincter complex in the posterior midline, but not seen reaching up to the anal mucosa (internal opening location not certain), then it was assumed that the internal opening was present in the posterior midline, and the fistula was managed accordingly. The same assumption was followed for fistulae in other locations.
- When the internal opening was not localizable in horseshoe fistulae, step 1 was not helpful, as horseshoe fistulae did not reach (touch) the sphincter complex at one place. Rather, the horseshoe fistulae were reaching the sphincter complex in a circumferential manner in the intersphincteric or transsphincteric plane. As per this protocol, in horseshoe fistulae, the internal opening was assumed to be in the midline in almost all cases (posterior midline in posterior horseshoe and anterior midline in anterior horseshoe fistulae), and the fistulae were managed accordingly with a definitive procedure.
In this study, 700 patients were operated on and followed for 3 years (median).9 Internal opening was found in 78% (n=546) and could not be localized in 22% (n=154) of patients.9 The protocol was followed for fistulae in which the internal opening was not found. These fistulae in which the internal opening was not found were managed by the same procedures as those for which the internal opening was found (fistulotomy for low fistulae, and a sphincter-saving procedure [TROPIS] for high fistulae). Fistulae healed completely in 89% (486 of 546) of the “internal-opening found” group and in 90.9% (140/156) in the “internal opening not found” group (p=1.01). Changes in continence scores after surgery were similar in the two groups.9 The results of this study in a large cohort with a long follow-up highlighted the efficacy of the suggested protocol (Garg protocol) in this subset of complex fistulae, which are so difficult to manage. Further studies will corroborate the usefulness of this protocol.
As discussed, one of the main reasons for delayed recurrence is non healing of the intersphincteric tract and non closure of the internal opening. Clinical healing is defined as cessation of pus from all external openings or through the anus, and all external openings have closed. Not uncommonly, this can happen with the persistence of sepsis in the intersphincteric space and/or patent internal opening (Figure 5). The latter can cause relapse of symptoms (pus discharge or abscess formation) months or even years later. MRI is quite sensitive in detecting persistent sepsis (residual tract) in the intersphincteric space and the patent internal opening.7 It has been shown that radiological healing of the intersphincteric tract and closure of the internal opening on MRI correlate very well with long-term healing rates of complex fistulae.6 Radiological healing is defined as complete healing of the intersphincteric space, as well as closure of the internal opening on MRI/TRUS, along with complete healing of all fistula tracts in the ischiorectal fossa.6,7,17 Therefore, it is prudent to corroborate clinical healing with radiological healing in all complex anal fistulae.6 This decreases the incidence of delayed recurrences in complex anal fistulae substantially.6
Though there can be many diseases associated with cryptoglandular fistulae, the disease causing maximum morbidity in anal fistulae is Mycobacterium tuberculosis (TB).49 The main challenge in TB lies in its timely detection. If a diagnosis of TB is missed, then this increases the risk of recurrence.49 The latest studies done on large numbers of patients have shown that conventional tests (histopathology and acid-fast bacilli smear) are not very sensitive in detecting TB.49 PCR has been shown to be much more sensitive for this purpose. In one large cohort, the detection rate of histopathology (tissue sample) for diagnosing TB was 1.1% (two of 181), while the detection rate of PCR (tissue or pus samples) was 10.3% (47 of 456; p<0.0001).49 Thus, PCR was far more sensitive than histopathology in detecting TB. Moreover, pus sample were more sensitive for detecting TB than testing of tissue from the fistula tract with the same test (PCR). Pus testing on PCR detected TB in 16.5% (19 of 115), while tissue testing on PCR detected TB in 8.2% (28 of 341; p<0.0009).49 Also, it has been shown that TB is more common in complex fistulae compared to simple fistulae.49
Based on these findings, it is recommended that PCR be used routinely to detect TB in fistula patients, especially in endemic areas.49 As PCR cannot distinguish dead from viable mycobacteria, it is prudent that any positive PCR report be correlated with the clinical picture. A PCR test positive for TB along with a background of nonhealing of fistulae, development of newer tracts/abscess, or delayed recurrence (after 3–6 months of healing of initial fistula) would make a strong case for starting antitubercular therapy (ATT).49 Multiple samples may be required to detect TB. A negative initial sample does not exclude the presence of TB. In suspected patients, repeated samples should be sent.49 Also, it has been shown that the cure rate is excellent when TB is detected and ATT started before surgery or within 6 weeks of surgery.49 However, if ATT is started after six weeks following surgery, the chances of recurrence are high.49 Therefore, the timely detection and prompt treatment of associated TB is helpful in preventing non healing fistulae or delayed recurrence.49
Conclusion
Complex anal fistulae pose a plethora of challenges in their management. These challenges and their solutions are quite overlapping and interlinked. Therefore, it is important to understand all the challenges together and then analyze the solutions to each challenge (Table 1). Recent developments in the last decade have significantly enhanced understanding of the pathophysiology and management of the complex anal fistulae. Due to this, it is possible to achieve healing rates of up to 80%–95% in patients with complex cryptoglandular anal fistulae. However, more studies, preferably randomized controlled trials, are needed to corroborate the efficacy of these new concepts and treatments.
 |
Table 1 Overview of Challenges in Managing Complex anal Fistulae and their Solutions |
Abbreviations
MRI, magnetic resonance imaging; TRUS, transrectal ultrasound; TROPIS, transanal opening of intersphincteric space; LIFT, ligation of intersphincteric fistula tract; ISTAC, intersphincteric tract is an abscess in a closed space; DRAPED, draining all pus and ensuring continuous drainage.
Author Contributions
All authors (PG, SSS, and NG) made a significant contribution to the work reported, whether in conception, study design, execution, acquisition of data, analysis, and interpretation, took part in drafting, revising, or critically reviewing the article, gave final approval to the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
The authors declare that no funding was received.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Jayne DG, Scholefield J, Tolan D, et al. A multicenter randomized controlled trial comparing safety, efficacy, and cost-effectiveness of the surgisis anal fistula plug versus surgeon’s preference for transsphincteric fistula-in-ano: the FIAT trial. Ann Surg. 2020. doi:10.1097/SLA.0000000000003981
2. Garg P. A new understanding of the principles in the management of complex anal fistula. Med Hypotheses. 2019;132:109329. doi:10.1016/j.mehy.2019.109329
3. Garg P. Garg classification for anal fistulas: is it better than existing classifications?- A review. Indian J Surg. 2018;80(6):606–608. doi:10.1007/s12262-018-1788-2
4. Garg P. Understanding and treating supralevator fistula-in-ano: MRI analysis of 51 cases and a review of literature. Dis Colon Rectum. 2018;61(5):612–621. doi:10.1097/DCR.0000000000001051
5. Garcia-Aguilar J, Belmonte C, Wong WD, et al. Anal fistula surgery. Factors associated with recurrence and incontinence. Dis Colon Rectum. 1996;39(7):723–729. doi:10.1007/BF02054434
6. Garg P. Comparison of Preoperative and postoperative MRI after fistula-in-ano surgery: lessons learnt from an audit of 1323 mri at a single centre. World J Surg. 2019;43(6):1612–1622. doi:10.1007/s00268-019-04926-y
7. Garg P, Singh P, Kaur B. Magnetic resonance imaging (MRI): operative findings correlation in 229 fistula-in-ano patients. World J Surg. 2017;41(6):1618–1624. doi:10.1007/s00268-017-3886-x
8. Garg P. Transanal opening of the Intersphincteric Space (TROPIS) procedure for high complex anal fistulas: long-term follow-up of 325 patients. Colorectal Dis. 2020. (Under submission).
9. Garg P. A simple protocol to effectively manage anal fistulas with no obvious internal opening: an audit of 757 operated cases. Tech Coloproctol. 2020. (under submission).
10. Sygut A, Mik M, Trzcinski R, et al. How the location of the internal opening of anal fistulas affect the treatment results of primary transsphincteric fistulas. Langenbecks Arch Surg. 2010;395(8):1055–1059. doi:10.1007/s00423-009-0562-0
11. Mei Z, Wang Q, Zhang Y, et al. Risk Factors for Recurrence after anal fistula surgery: a meta-analysis. Int J Surg. 2019;69:153–164.
12. Adamina M, Ross T, Guenin MO, et al. Anal fistula plug: a prospective evaluation of success, continence and quality of life in the treatment of complex fistulae. Colorectal Dis. 2014;16(7):547–554. doi:10.1111/codi.12594
13. Regusci L, Fasolini F, Meinero P, et al. Video-assisted anal fistula treatment (VAAFT) for complex anorectal fistula: efficacy and risk factors for failure at 3-year follow-up. Tech Coloproctol. 2020;24(7):741–746. doi:10.1007/s10151-020-02213-w
14. Zelic M, Karlovic D, Krsul D, et al. Video-assisted anal fistula treatment for treatment of complex cryptoglandular anal fistulas with 2 years follow-up period: our experience. J Laparoendosc Adv Surg Tech A. 2020.
15. Prosst RL, Joos AK, Ehni W, et al. Prospective pilot study of anorectal fistula closure with the OTSC proctology. Colorectal Dis. 2015;17(1):81–86. doi:10.1111/codi.12762
16. Erden A. MRI of anal canal: common anal and perianal disorders beyond fistulas: part 2. Abdom Radiol (NY). 2018;43(6):1353–1367. doi:10.1007/s00261-017-1306-1
17. Halligan S, Tolan D, Amitai MM, et al. ESGAR consensus statement on the imaging of fistula-in-ano and other causes of anal sepsis. Eur Radiol. 2020;30(9):4734–4740. doi:10.1007/s00330-020-06826-5
18. Garg P. Assessing validity of existing fistula-in-ano classifications in a cohort of 848 operated and MRI assessed anal fistula patients – cohort study. Ann Med Surg (Lond). 2020;59:122–126. doi:10.1016/j.amsu.2020.09.022
19. Emile SH, Khan SM, Adejumo A, et al. Ligation of intersphincteric fistula tract (LIFT) in treatment of anal fistula: an updated systematic review, meta-analysis, and meta-regression of the predictors of failure. Surgery. 2020;167(2):484–492. doi:10.1016/j.surg.2019.09.012
20. Hong KD, Kang S, Kalaskar S, et al. Ligation of intersphincteric fistula tract (LIFT) to treat anal fistula: systematic review and meta-analysis. Tech Coloproctol. 2014;18(8):685–691. doi:10.1007/s10151-014-1183-3
21. de Bonnechose G, Lefevre JH, Aubert M, et al. Laser ablation of fistula tract (LAFT) and complex fistula-in-ano: “the ideal indication” is becoming clearer. Tech Coloproctol. 2020;24(7):695–701. doi:10.1007/s10151-020-02203-y
22. Adegbola SO, Sahnan K, Pellino G, et al. Short-term efficacy and safety of three novel sphincter-sparing techniques for anal fistulae: a systematic review. Tech Coloproctol. 2017;21(10):775–782. doi:10.1007/s10151-017-1699-4
23. Clark SK. Video-assisted anal fistula treatment for complex anal fistula: a long-term follow-up study, Giarratano et al. Colorectal Dis. 2020;22(8):856. doi:10.1111/codi.15222
24. Zhao B, Wang Z, Han J, et al. Long-term outcomes of ligation of the inter-sphincteric fistula tract plus bioprosthetic anal fistula plug (LIFT-plug) in the treatment of trans-sphincteric perianal fistula. Med Sci Monit. 2019;25:1350–1354. doi:10.12659/MSM.914925
25. Herreros MD, Garcia-Olmo D, Guadalajara H, et al. Stem cell therapy: a compassionate use program in perianal fistula. Stem Cells Int. 2019;2019:6132340. doi:10.1155/2019/6132340
26. Lightner AL, Wang Z, Zubair AC, et al. A systematic review and meta-analysis of mesenchymal stem cell injections for the treatment of perianal Crohn’s disease: progress made and future directions. Dis Colon Rectum. 2018;61(5):629–640. doi:10.1097/DCR.0000000000001093
27. Wilhelm A, Fiebig A, Krawczak M. Five years of experience with the FiLaC laser for fistula-in-ano management: long-term follow-up from a single institution. Tech Coloproctol. 2017;21(4):269–276. doi:10.1007/s10151-017-1599-7
28. Garg P. Transanal opening of intersphincteric space (TROPIS) - A new procedure to treat high complex anal fistula. Int J Surg. 2017;40:130–134. doi:10.1016/j.ijsu.2017.02.095
29. Parks AG, Gordon PH, Hardcastle JD. A classification of fistula-in-ano. Br J Surg. 1976;63(1):1–12. doi:10.1002/bjs.1800630102
30. Ferguson EF
31. Erden A. MRI of anal canal: normal anatomy, imaging protocol, and perianal fistulas: part 1. Abdom Radiol (NY). 2018;43(6):1334–1352.
32. Morris J, Spencer JA, Ambrose NS. MR imaging classification of perianal fistulas and its implications for patient management. Radiographics. 2000;20(3):
33. Eisenhammer S. A new approach to the anorectal fistulous abscess based on the high intermuscular lesion. Surg Gynecol Obstet. 1958;106(5):595–599.
34. Safar B, Jobanputra S, Sands D, et al. Anal fistula plug: initial experience and outcomes. Dis Colon Rectum. 2009;52(2):248–252. doi:10.1007/DCR.0b013e31819c96ac
35. van Koperen PJ, Wind J, Bemelman WA, et al. Fibrin glue and transanal rectal advancement flap for high transsphincteric perianal fistulas; is there any advantage? Int J Colorectal Dis. 2008;23(7):697–701. doi:10.1007/s00384-008-0460-x
36. Walega P, Romaniszyn M, Nowak W. VAAFT: a new minimally invasive method in the diagnostics and treatment of anal fistulas–initial results. Pol Przegl Chir. 2014;86(1):7–10. doi:10.2478/pjs-2014-0002
37. Dubsky PC, Stift A, Friedl J, et al. Endorectal advancement flaps in the treatment of high anal fistula of cryptoglandular origin: full-thickness vs. mucosal-rectum flaps. Dis Colon Rectum. 2008;51(6):852–857.
38. Mennigen R, Laukotter M, Senninger N, et al. The OTSC((R)) proctology clip system for the closure of refractory anal fistulas. Tech Coloproctol. 2015;19(4):241–246. doi:10.1007/s10151-015-1284-7
39. Jayne DG, Scholefield J, Tolan D, et al. Anal fistula plug versus surgeon’s preference for surgery for trans-sphincteric anal fistula: the FIAT RCT. Health Technol Assess. 2019;23(21):1–76. doi:10.3310/hta23210
40. Osterkamp J, Gocht-Jensen P, Hougaard K, et al. Long-term outcomes in patients after ligation of the intersphincteric fistula tract. Dan Med J. 2019;66(4).
41. Stellingwerf ME, van Praag EM, Tozer PJ, et al. Systematic review and meta-analysis of endorectal advancement flap and ligation of the intersphincteric fistula tract for cryptoglandular and Crohn’s high perianal fistulas. BJS Open. 2019;3(3):231–241. doi:10.1002/bjs5.50129
42. Seyfried S, Bussen D, Joos A, et al. Fistulectomy with primary sphincter reconstruction. Int J Colorectal Dis. 2018;33(7):911–918. doi:10.1007/s00384-018-3042-6
43. Ratto C, Litta F, Donisi L, et al. Fistulotomy or fistulectomy and primary sphincteroplasty for anal fistula (FIPS): a systematic review. Tech Coloproctol. 2015;19(7):391–400. doi:10.1007/s10151-015-1323-4
44. Toyonaga T, Matsushima M, Kiriu T, et al. Factors affecting continence after fistulotomy for intersphincteric fistula-in-ano. Int J Colorectal Dis. 2007;22(9):1071–1075. doi:10.1007/s00384-007-0277-z
45. Athanasiadis S, Kohler A, Nafe M. Treatment of high anal fistulae by primary occlusion of the internal ostium, drainage of the intersphincteric space, and mucosal advancement flap. Int J Colorectal Dis. 1994;9(3):153–157. doi:10.1007/BF00290193
46. Quah HM, Tang CL, Eu KW, et al. Meta-analysis of randomized clinical trials comparing drainage alone vs primary sphincter-cutting procedures for anorectal abscess-fistula. Int J Colorectal Dis. 2006;21(6):602–609. doi:10.1007/s00384-005-0060-y
47. Williams G, Williams A, Tozer P, et al. The treatment of anal fistula: second ACPGBI position statement - 2018. Colorectal Dis. 2018;20(Suppl 3):5–31.
48. Ommer A, Herold A, Berg E, et al. German S3 guidelines: anal abscess and fistula (second revised version). Langenbecks Arch Surg. 2017;402(2):191–201. doi:10.1007/s00423-017-1563-z
49. Garg P, Garg M, Das BR, et al. Perianal tuberculosis: lessons learned in 57 patients from 743 samples of histopathology and polymerase chain reaction and a systematic review of literature. Dis Colon Rectum. 2019;62(11):1390–1400. doi:10.1097/DCR.0000000000001493
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

