Back to Journals » Vascular Health and Risk Management » Volume 16
Lumivascular Optical Coherence Tomography–Guided Atherectomy in Recurrent Femoropopliteal Occlusive Diseases Associated with In-Stent Restenosis: Case-Series Report
Authors Chan YC, Cheung GC, Cheng SW
Received 11 May 2020
Accepted for publication 8 July 2020
Published 29 July 2020 Volume 2020:16 Pages 325—329
DOI https://doi.org/10.2147/VHRM.S260190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Harry Struijker-Boudier
Yiu Che Chan, Grace C Cheung, Stephen W Cheng
Division of Vascular and Endovascular Surgery, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, Hong Kong, People’s Republic of China
Correspondence: Yiu Che Chan
Division of Vascular and Endovascular Surgery, Department of Surgery, University of Hong Kong Medical Centre, Queen Mary Hospital, South Wing, 14th Floor, K Block, Pokfulam Road, Hong Kong, People’s Republic of China
Tel +852-2255-4969
Fax +852-2255-4967
Email [email protected]
Abstract: Lumivascular optical coherence tomography (OCT) is a novel adjunct in the field of medicine. It offers clear real-time imaging of artery walls before and during endovascular intervention. This study reports our initial experience on the use of lumivascular OCT-guided atherectomy in the management of two patients with recurrent restenosis in their femoropopliteal arteries associated with in-stent restenosis. Endovascular procedures were successful with a Pantheris atherectomy device (Avinger, Redwood City, CA, USA) and drug-eluting balloons. The OCT images clearly distinguished normal anatomy from plaque pathology, were of great advantage in both the accurate diagnosis and treatment of target lesions, and may reduce radiation during the endovascular procedure. However, the price of the device and its need for contrast infusion limit its routine clinical use.
Keywords: optical coherence tomography, peripheral arterial disease, vascular, endovascular, diagnosis, intervention, atherectomy
Introduction
Contrast arteriography is currently the gold standard of vascular imaging during endovascular procedures. However, it carries risks of contrast nephropathy and irradiation exposure for the operator and patient. Lumivascular technologies with optical coherence tomography (OCT) use light-wave reflectivity to provide real-time intraoperative high-resolution cross-sectional images of the vessel wall.1 This acts as quality control during the endovascular procedure, assessing the depth and adequacy of atherectomy without the use of any contrast arteriography.
One of the means of application of OCT in peripheral arterial disease is the Pantheris atherectomy device (Avinger, Redwood City, CA, USA) with real-time lumivascular assessment of vessel-lumen and target-lesion characteristics. We have been using this technology-guided atherectomy in our institution since August 2019. This project has the HAMSINP reference F19/46 and local IRB-approval reference UW 19–477. The aim of this paper is to report our initial successful experience in OCT-guided atherectomy in two patients with symptomatic recurrent femoropopliteal occlusive disease associated with in-stent restenosis. Written informed consent was provided by the patients to have their case details and any accompanying images published.
Cases
Our first patient was a 93-year-old woman presenting with a 2-month history of recurrent painful nonhealing ulcer of the right toe. Her medical history was significant for diabetes mellitus, hypertension, and atrial fibrillation on rivaroxaban. She had initially presented to us with a toe ulcer 6 years previously, and received distal superficial femoral artery (SFA) angioplasty with a 6×60 mm angioplasty balloon and Supera 5×60 mm stent insertion (IDEV Technologies/Abbott Laboratories, Webster, TX, USA). There was 50% stenosis of the mid-SFA and popliteal artery on follow-up, the ulcer had recurred, and she underwent reangioplasty with a 5.5×80 mm balloon of the mid-SFA, then angioplasty and stenting with a 5.5×80 mm angioplasty balloon and 5×80 mm Supera stent 3 years later. Unfortunately, the right-toe ulcer was slow to heal, and she developed moderate–severe restenosis in the entire mid- to distal SFA to the below-knee popliteal arteries 6 months later, with severe in-stent restenosis in the popliteal stent, necessitating mid-SFA to below-knee popliteal angioplasty (predilation with 4×150 mm balloon, then drug-eluting balloons of 4×120 mm, 5×120 mm, and 5×80 mm (In.Pact Admiral; Medtronic, Galway, Dublin). She remained well for 2 years, with regular surveillance, and the toe ulcer healed finally.
She returned 2 years later with debilitating short-distance right-calf claudication, and duplex and intraoperative angiography (Figure 1A and B) found distal SFA to above-knee popliteal artery 50%–70% restenosis, total of about 8 cm (Tosaka class II2), and 50% restenosis of the below-knee popliteal artery. Using an ipsilateral antegrade femoral approach with a 6 Fr sheath, the target lesion was crossed with 0.018 Nitrex wire (Medtronic) and changed to 0.014 Nitrex wire with a 5 Fr Quick-Cross catheter (Spectranetics, Würzburg, Germany). An Emboshield filter (Abbott Laboratories) was placed in the distal below-knee popliteal artery. The below-knee popliteal stenosis received angioplasty with a 4×80 mm balloon, and the distal SFA to above-knee popliteal stenosis was treated with OCT-guided atherectomy (Figure 1C). Neointimal hyperplasia and atheroma material was removed (Figure 1D). The lesions received angioplasty with drug-eluting balloons (5×80 mm, LegFlow; Cardionovum, Bonn, Germany; Figure 1E). Her claudication resolved, and has recovered well since. She remained well with patent artery up to last follow-up at 6 months.
Our second patient was a 71-year-old lady presenting with a 3-month history of debilitating right-leg claudication. Her medical history was significant for diabetes mellitus, hypertension, and coronary artery–bypass grafts. Preoperative duplex showed 80% stenosis in the proximal right SFA with 50% stenosis in the above-knee popliteal artery. She underwent SFA angioplasty with a 6×40 mm angioplasty balloon and Supera 5×60 mm stent insertion, with above-knee popliteal artery 5×20 mm balloon angioplasty. She presented 9 months later with recurrent symptoms: her proximal and mid-SFA had developed de novo disease of 50% with instent restenosis up to 80%. She had angioplasty with a 4×120 mm balloon and drug-eluting 5×120 mm balloon.
She returned 3 years later with debilitating short-distance right-calf claudication again, and duplex and intraoperative angiography found mid-SFA to above-knee popliteal artery 50%–70% restenosis (Tosaka class II2), total of about 8 cm, and 50% restenosis of the below-knee popliteal artery (Figure 2A and B). We used an ipsilateral antegrade femoral approach with a 6 Fr sheath, with the target lesion crossed with 0.018 wire with a 5 Fr Quick-Cross catheter. A filter was placed in the distal below-knee popliteal artery. The above-knee popliteal stenosis had angioplasty with a 4×40 mm balloon (Chocolate PTA balloon catheter; Medtronic), and 5×60 mm drug-eluting balloon (In.Pact Admiral), and the mid-SFA was treated with OCT-guided atherectomy with good radiological results (Figure 2C). Neointimal hyperplasia and atheroma material was removed (Figure 2D). The lesions received angioplasty with a 5×80 mm drug-eluting balloon (In.Pact Admiral, Figure 2E). Her claudication resolved and has recovered well since. She remained well with patent artery up to last follow-up at 6 months.
Discussion
These two cases demonstrated our experience in using OCT-guided atherectomy in conjunction with drug-eluting balloons in treatment of multiple recurrent femoropopliteal occlusive disease with in-stent restenosis. OCT-guided atherectomy offers accurate real-time visualization of the arterial lesion before and during the procedure, and atherectomy aims to precisely remove the stenotic lesion whilst avoiding stent struts. The combination therapy of OCT-guided atherectomy plus drug-eluting balloons may lead to a synergistic effect and help pave the way for more effective local delivery of the antiproliferative agent coating the balloon during angioplasty.
The main advantage with the use of OCT is its high resolution in delineating the microscopic structure of vessels. Understanding the morphology and characteristics of disease processes in primary atherosclerosis, in-stent restenosis, and reocclusion after angioplasty is important, as two-dimensional angiographic images may not fully capture the characteristics of plaques. Eberhardt et al1 reported that owing to its noninvasive high-resolution visualization of the microstructure of the vessels, OCT could accurately discriminate the three layers of the vessel wall, as well as characterize the composition of atherosclerotic plaques, including calcifications, lipids, and fibrosis, which is conventionally demonstrated by histology obtained through invasive means. OCT is useful in showing neointimal hyperplasia following angioplasty, stent insertion, and drug-eluting stent placement.3,4 Our patients had plaques in native vessels and neointimal hyperplasia in stent restenosis consisting of fibrous tissue and some calcium, and OCT was helpful in vessel-wall assessment in terms of plaque characterization and quantification during the intent-to-treat atherectomy procedure. OCT can be used in patients with in-stent restenosis, as the images are able to distinguish between homogeneous and nonhomogeneous substances, such as lipids, fibrosis, and calcium. For homogeneous lesions, recurrent target-lesion revascularization can be achieved with thrombectomy and angioplasty, whil nonhomogeneous lesions often require balloon angioplasty and stent implantation. Other applications include OCT to gain insight into the possible mechanism of re-occlusion after Fogarty thrombectomy.5
OCT is an adjunctive modality for quality control during the atherectomy procedure in detecting complications of endovascular procedures. Results of angioplasty or atherectomy, including dissections, intimal tears, thrombi, and media ruptures can be seen. In cases of dissection, the flap can be removed by atherectomy under real-time OCT imaging (Figure 3). In cases when stenting is required, results and complications, including intrastent tissue prolapse, stent thrombosis, and stent-strut malapposition, can also be assessed with OCT.
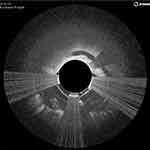 |
Figure 3 Local dissection during atherectomy was identified using OCT, and the flap removed. This is one of the intraoperative screens captured during case 1. |
The VISION trial6 has been the largest multicenter cohort study to date to assess OCT-guided imaging or atherectomy for femoropopliteal occlusive disease. This was a single-arm, multicenter, global investigational device exemption study enrolling 158 subjects across 20 participating sites. The average lesion length of 53±40 mm was treated using the Pantheris catheter alone or Pantheris + adjunctive therapy. Of the 198 target lesions, 104 (52.5%) were treated with the Pantheris alone, 84 (42.4%) with the Pantheris + adjunctive angioplasty, and ten (5.1%) with the Pantheris + angioplasty + stenting. The composite major adverse-effect outcome throughout 6 months occurred in 25 (16.6%) of 151 subjects. There were no clinically significant perforations, but there was one catheter-related dissection, four embolic events, and a 6.4% clinically driven target lesion–revascularization rate at 6 months. Characterization of the OCT-guided lesions revealed evidence of an underestimation of disease burden when using fluoroscopy. The incidence of distal emboli during lower-limb endovascular intervention is generally low (1%–2%), but the use of different atherectomy devices may increase the incidence of distal emboli to 4%–5% compared to angioplasty and stenting.7 As a precaution, we always prefer to use distal filters in patients requiring lower-limb atherectomy.
Our initial experience and contemporary evidence supports the view that OCT-guided atherectomy is safe and efficacious, with promising short-term outcomes, as an adjunct to be used in the field of peripheral arterial disease and also in the treatment of recurrent occlusion disease with or without in-stent restenosis. It offers clear visualization of vascular pathophysiology, but Secca et al8 reported significant additional contrast required for OCT evaluation of large muscular arteries, and this might increase the risk of contrast nephropathy. As the high backscatter of red blood cells may require contrast flush infusions, which are generally responsible for higher final-contrast dose, Secca et al9 showed it was safe to use selective saline infusions instead of contrast to achieve sufficient substitution of blood and gain better-quality OCT images during peripheral interventions. The cost-effectiveness has not been thoroughly assessed, as these devices are expensive, especially in countries without reimbursement policies. Although there is a lack of high-level evidence concerning its long term outcome compared to other devices, OCT-guided atherectomy nonetheless adds to the armamentarium of endovascular technology in treating recurrent femoropopliteal occlusive disease.
Ethics
IRB reference UW 19-488. This project has HAMSINP reference F19/46 (http://cto.home/Technology-Assessment/HAMSINP/HAMSINP-Register.aspx) and local IRB approval (reference UW 19-477).
Disclosure
This paper received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Purchase of consumables was from our own division’s funding. The authors declare no conflicts of interest in this work.
References
1. Eberhardt KM, Treitl M, Boesenecker K, Maxien D, Reiser M, Rieger J. Prospective evaluation of optical coherence tomography in lower limb arteries compared with intravascular ultrasound. J Vasc Interv Radiol. 2013;24(10):1499–1508. doi:10.1016/j.jvir.2013.06.015
2. Tosaka A, Soga Y, Iida O, et al. Classification and clinical impact of restenosis after femoropopliteal stenting. J Am Coll Cardiol. 2012;59:16–23. doi:10.1016/j.jacc.2011.09.036
3. Mandelias K, Tsantis S, Spiliopoulos S, et al. Automatic quantitative analysis of in-stent restenosis using FD-OCT in vivo intra-arterial imaging. Med Phys. 2013;40(6):063101. doi:10.1118/1.4803461
4. Lichtenberg MK, Carr JG, Golzar JA. Optical coherence tomography: guided therapy of in-stent restenosis for peripheral arterial disease. J Cardiovasc Surg. 2017;58(4):518–527. doi:10.23736/S0021-9509.17.09946-3
5. Yamamoto Y, Kawarada O, Sakamoto S, et al. Progression of intimal hyperplasia and multiple-channel formation after fogarty thrombectomy: insight into vasculopathy from optical coherence tomography and intravascular ultrasound findings. JACC Cardiovasc Interv. 2015;8(15):e251–e253. doi:10.1016/j.jcin.2015.07.036
6. Schwindt AG, Bennett JG, Crowder WH, et al. Lower extremity revascularization using optical coherence tomography-guided directional atherectomy: final results of the evaluation of the pantheris optical coherence tomography ImagiNg Atherectomy system for use in the peripheral vasculature (VISION) study. J Endovasc Ther. 2017;24(3):355–366. doi:10.1177/1526602817701720
7. Ochoa Chaar CI, Shebl F, Sumpio B, Dardik A, Indes J, Sarac T. Distal embolization during lower extremity endovascular interventions. J Vasc Surg. 2017;66(1):143–150. doi:10.1016/j.jvs.2017.01.032
8. Secco GG, Grattoni C, Parisi R, et al. Saline vs contrast infusion during optical coherence tomography imaging of peripheral percutaneous intervention. Int J Cardiol. 2014;172(1):246–248. doi:10.1016/j.ijcard.2013.12.288
9. Secco GG, Grattoni C, Parisi R, et al. Optical coherence tomography guidance during peripheral vascular intervention. Cardiovasc Intervent Radiol. 2015;38:768–772. doi:10.1007/s00270-014-0868-3
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.