Back to Journals » Clinical Interventions in Aging » Volume 13
Low skeletal muscle mass as a risk factor for postoperative delirium in elderly patients undergoing colorectal cancer surgery
Authors Mosk CA, van Vugt JLA, de Jonge H , Witjes CDM, Buettner S , Ijzermans JNM , van der Laan L
Received 31 May 2018
Accepted for publication 13 July 2018
Published 24 October 2018 Volume 2018:13 Pages 2097—2106
DOI https://doi.org/10.2147/CIA.S175945
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Christina Alexa Mosk,1 Jeroen LA van Vugt,2 Huub de Jonge,1 Carlijn DM Witjes,2 Stefan Buettner,2 Jan NM Ijzermans,2 Lijckle van der Laan1
1Department of Surgery, Amphia Hospital, Breda, the Netherlands; 2Department of Surgery, Erasmus MC University Medical Center, Rotterdam, the Netherlands
Background: Both low skeletal muscle mass (LSMM) and delirium are frequently seen in elderly patients. This study aimed to investigate the association between preoperative LSMM and postoperative delirium (POD) in elderly patients undergoing colorectal cancer (CRC) surgery and to design a model to predict POD.
Patients and methods: This is a retrospective observational cohort study. Patients aged 70 years or older undergoing CRC surgery from January 2013 to October 2015 were included in this study. The cross-sectional skeletal muscle area at the level of the third lumbar vertebra using computed tomography was adjusted for patients’ height, resulting in the skeletal muscle index. The lowest quartile per sex was defined as LSMM. Short Nutritional Assessment Questionnaire for Residential Care and KATZ-Activities of Daily Living were used to define malnourishment and physical dependency, respectively. POD was diagnosed using the Delirium Observational Screening Scale and geriatricians’ notes.
Results: Median age of the 251 included patients was 76 years (IQR, 73–80 years), of whom 56% of patients were males, 24% malnourished, and 15% physically impaired. LSMM and POD were diagnosed in 65 and 33 (13%) patients, respectively. POD occurred significantly more in patients with LSMM (25%) compared with patients without LSMM (10%), P=0.006. In the multivariate analysis, age, history of delirium, and LSMM were significantly associated with POD. In addition, this effect increased in patients with LSMM and malnourishment (P=0.019) or physical dependency (P=0.017).
Conclusion: Age, history of delirium, LSMM, and malnourishment or physical dependency were independently associated with POD. Our nomogram could be used to identify patients at an increased risk for delirium. These patients may benefit from intensive monitoring to prevent POD.
Keywords: skeletal muscle mass, sarcopenia, colorectal surgery, postoperative delirium, elderly, nomogram
Introduction
Recently, many risk factors for postoperative delirium (POD) have been identified in colorectal cancer (CRC) patients.1 However, after colorectal surgery in elderly patients, POD occurs in 8.2%–54% of patients.2 POD is associated with adverse long-term outcomes, such as increased cognitive decline, more institutionalization, and an increased mortality rate, compared to patients who did not experience delirium.3,4 In our population of patients aged 65 years or older, 15% developed POD after elective colorectal surgery. The mortality after surgery was significantly higher in patients suffering from delirium compared to that in non-delirious patients.5 This underlines the need to seek preventive measures for POD. It is presumed that 30%–40% of delirium cases could be prevented. Known predictors of delirium are frailty (ie, the inability to adequately respond to stressors),6 increased age, history of psychiatric disorders, previous delirium in medical history, cognitive decline, acute hospital admission, and intensive care unit admission.4 In addition, a poor nutritional state and functional dependency have been described as important risk factors for delirium.3,4,7 Both these latter factors might also influence the occurrence of low skeletal muscle mass (LSMM): involuntary loss of skeletal muscle mass (SMM), leading to decreased physical performance.8–10 Increased age and several diseases (eg, cancer) are contributing factors to skeletal muscle wasting.8,10 The relationship between LSMM and adverse postoperative outcomes has been described before in patients with CRC.11–13 Furthermore, it is associated with decreased long-term health-related quality of life.14 Particularly, older cancer patients are at an increased risk for frailty, functionally dependency, or malnourishment due to inadequate food intake and metabolic changes caused by underlying diseases.15
When taking into account the similar risk factors for LSMM and delirium, LSMM might be an interesting and strong predictor of POD. In addition, LSMM can be measured objectively, and it could be solved by training. To our knowledge, only one study with a small sample size described LSMM as an independent risk factor for POD.16 Therefore, additional research should be conducted. The aim of this study is to investigate whether there is an association between preoperative LSMM and POD in older patients undergoing CRC surgery and, if so, to design a model to predict POD.
Patients and methods
Patient selection
All consecutive patients who underwent elective colorectal surgery for histologically proven CRC at the Amphia Hospital, Breda, the Netherlands, from January 2013 to October 2015 were identified in a retrospective database. The patients included were aged 70 years or older on the day of surgery and had a contrast-enhanced computed tomography (CT) examination up to 4 months (16 weeks) before surgery. All CTs were routinely performed as a part of the preoperative staging for surgical workup.
Data collection
Demographics, disease, and treatment characteristics were collected. Medical history, in particular history of delirium, was derived from medical geriatric notes. Psychiatric disease history was derived from the electronic patient file. Comorbidity was classified using the American Society of Anesthesiologists (ASA) physical status classification.17 Activities of daily living (ADL) and dependency were assessed using the KATZ-ADL.18 Patients with a KATZ-ADL less than 6 points were considered impaired, whereas patients with a score of less than 3 points were considered severely impaired. The Short Nutritional Assessment Questionnaire for Residential Care (SNAQ-RC) was used to screen for malnutrition. A patient was considered as malnourished when ≥3 points were scored on the SNAQ-RC.19 Both KATZ-ADL and SNAQ-RC were screened at admission. Perioperative blood transfusion was noted before, during, and until 1 week after surgery.
SMM and density measurements
The cross-sectional skeletal muscle area (in cm2; ie, the psoas, rectus abdominis, transversus abdominis, internal and external abdominal oblique muscles) was measured on CT images at the level of the third lumbar vertebra (L3) using a Hounsfield unit (HU) threshold of −30 to +150, as previously described by van Vugt et al.20 Measurements were performed on the image on which both transverse processes were depicted. The cross-sectional skeletal muscle area was adjusted for patients’ height, as it is conventional for body composition measures, resulting in the skeletal muscle index (SMI; cm2/m2). This is a validated measure of total body SMM.21 Furthermore, the mean radiodensity (HU) of the cross-sectional skeletal muscle area, a measure for intramuscular adiposity (ie, myosteatosis), was noted.20
Patients in the lowest sex-specific quartile for SMM were considered to have LSMM, whereas patients in the lowest sex-specific quartile for skeletal muscle density were considered to have myosteatosis. In sensitivity analysis, we also classified patients has having LSMM or density using the cutoff values defined by Martin et al.22
This study was approved by the institutional review board of Erasmus MC, Rotterdam, the Netherlands. A waiver for informed consent was granted due to the retrospective nature of this study. All data were collected anonymously and stored in a secured environment.
Primary outcome: POD
The primary outcome was the occurrence of POD. The Delirium Observational Screening Scale (DOSS) was used to screen for POD.23 Daily screening for delirium is a standard procedure in our hospital. DOSS was performed daily during each nurse shift, three times a day. The DOSS score ≥3 during three or more nurse shifts in a row was considered as highly specific for delirium, in which case the geriatrician was consulted. Geriatric consultation was routinely performed in case of any doubt about the diagnosis and in all patients with known cognitive impairment. For the current study, the DOSS score and clinical notes of the geriatric department were analyzed to confirm the diagnosis of delirium. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria were used to define delirium.13 Treatment of delirium was performed according to a local protocol, using both medical (starting with 0.5 mg haloperidol) and non-medical (eg, support of day–night rhythm, support of orientation, or the appropriate use of visual and hearing aids) treatments.
If a patient was discharged with the DOSS score ≥3, the duration of the delirium was counted until the day of discharge.
Prophylactic medication was used according to the protocol; when a patient had a high risk (eg, previous delirium, psychiatric disorders, cognitive impairment, high comorbidity) to develop delirium, 0.5 mg haloperidol was started 1 day before surgery and continued until 3 days postoperatively. When no delirium has occurred, medication was stopped. Nevertheless, physicians could deviate from the protocol depending on clinical presentations.
Secondary outcomes
The secondary outcomes were length of stay and all other postoperative adverse events occurring during hospital stay or within 30 days from surgery. All postoperative complications were noted, and the severity was scored according to the Clavien–Dindo classification.24 Furthermore, 6- and 12-month mortality rates were recorded. Date of mortality was retrieved from the municipal records database.
Statistical analyses
Categorical data were presented as numbers and percentages and continuous data as the mean±SD or median and IQR, depending on the normality of data distribution. Differences between groups were assessed using the chi-squared test or Fisher’s exact test. Differences in continuous variables were assessed using the Mann–Whitney U-test or Student’s t-test, where appropriate. Univariate logistic regression analysis was performed. Then, all clinically relevant and statistically significant preoperative variables were entered into a multivariate logistic regression analysis using a forward enter method to identify independent preoperative risk factors for POD. Data were presented as the OR and 95% CI. Since LSMM is highly associated with malnourishment and physical dependency, analyses were performed combining these parameters (ie, LSMM–malnourishment and LSMM–physically impaired) to identify most vulnerable patients.
Two separate prognostic models were made using the combined parameters. Regression coefficients from the multivariate models were used to construct nomograms to predict chances of developing delirium. A nomogram is a graphical depiction of the regression analysis and can serve as a tool for calculating each individual patient’s risk. Performance of these nomograms was assessed using Harrell’s concordance index (c-index) and the Brier score. The c-index provides the probability that, in a randomly selected pair of patients, in which one patient develops POD, the patient who develops delirium had the worse predicted outcome from the nomogram. The Brier score measures the mean squared difference between predicted outcomes and actual outcomes. Two-sided P-value<0.05 was considered as statistically significant. All analyses were performed using SPSS (Version 23.0; IBM Corporation, Armonk, NY, USA) software and the rms package for R version 3.3.3 (http://www.r-project.org).
Results
Baseline characteristics
All baseline characteristics are shown in Table 1. Of the 355 consecutive patients, 251 patients (56% males) were included. Patients were excluded for the following reasons: the CT scan was older than 4 months (and further preoperative workup was by a magnetic resonance imaging [MRI]), CT was not compatible to define sarcopenia (ie, no contrast CT), patients were operated on elsewhere, or baseline information concerning height or weight was missing. The median age was 76 years (IQR, 73–80 years). The majority of patients (65%) had an ASA classification of 1–2. At admission, 60 patients (24%) were considered as malnourished and 38 patients (15%) as physically impaired and severely impaired. Median time between preoperative CT scan and surgery was 0.95 months (IQR, 0.69–1.31), with a range of 0.13–3.84 months. Eleven patients had a longer waiting time than 3 months: seven patients with rectal cancer, five patients who underwent neoadjuvant therapy, and two patients who endured an incomplete total mesorectal excision (TME), and four patients with colon cancer with a diversity of reasons. Only one of these 11 patients became delirious.
SMM
In female patients, the SMI cutoff value for the lowest quartile was 35.71 cm2/m2. The highest SMI quartile ranged from 43.27 to 62.75 cm2/m2. In male patients, the SMI cutoff value for the lowest quartile was 43.19 cm2/m2. The highest SMI quartile ranged from 54.55 to 93.89 cm2/m2. LSMM was diagnosed in 61 patients based on these cutoff values (Table 2).
  | Table 2 Delirium prevalence stratified for SMI quartiles |
The association between LSMM and POD
In total, POD occurred in 33 patients (13%). Significantly, more patients with LSMM experienced POD compared to patients without LSMM (15 patients, 25% vs 18 patients, 10%; OR, 3.11; 95% CI, 1.46–6.65; P=0.002; Table 2). When using the cutoff values defined by Martin et al,22 the prevalence of POD was higher in patients with LSMM (17.9% vs 10.2%, P=0.079) and low skeletal muscle density (16.7% vs 5.6%, P=0.038).
Age (P<0.001), delirium in medical history (P<0.001), physical dependency (KATZ 5–3, P=0.029; KATZ <3, P=0.006), malnourished patients (P=0.001), blood transfusion (P=0.005), and LSMM based on SMI (P=0.002) were significantly associated with delirium in univariate analysis. No association was found between low skeletal muscle density and POD (OR, 0.88; 95% CI, 0.37–2.07, P=0.774). In multivariate analyses, only preoperative risk factors were used. When combining age (P=0.002) and delirium in medical history (P=0.003) in multivariate analyses, LSMM (based on SMI) remained significant (OR, 2.29; 95% CI, 1.01–5.20, P=0.047; Table 3).
Regarding malnourished patients with LSMM or physically dependent patients with LSMM, an independent association with the occurrence of POD was shown (Tables 4 and 5). Factors from these two analyses were used to develop nomograms (Figures 1 and 2). With the regression coefficients from the factors that were independently associated with developing delirium in the two models (a model with LSMM/malnourishment and a model with LSMM/dependency), weighted points scales were created. The nomograms were based on the two LSMM metrics as well as age (in years) and a history of delirium (no or yes). Higher total points, based on the sum of the assigned number of points for each factor in the nomogram, were associated with higher chances of developing POD. For example, a 70-year-old patient with a history of delirium (56 points) in addition to LSMM/malnourishment (41 points) would have a 60% chance of developing delirium. In contrast, a 73-year-old patient without history of delirium and without LSMM/malnourishment would have only a 5% chance of developing delirium.
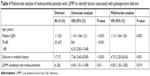  | Table 4 Multivariate analysis of malnourished patients with LSMM to identify factors associated with postoperative delirium |
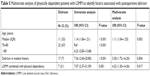  | Table 5 Multivariate analysis of physically dependent patients with LSMM to identify factors associated with postoperative delirium |
  | Figure 1 Nomogram to calculate chances of developing postoperative delirium in patients undergoing colorectal surgery with LSMM and malnourishment. |
  | Figure 2 Nomogram to calculate chances of developing postoperative delirium in patients undergoing colorectal surgery with LSMM and physical dependency. |
The two models showed a good discriminatory ability (a c-index of 0.795 for the model with LSMM/malnourishment and a c-index of 0.783 for model with LSMM/dependency). Brier scores also indicated good performance (0.096 for the LSMM/malnourishment model and 0.099 for the LSMM/dependency model).
The association between LSMM and other postoperative complications and mortality
Postoperative complications and mortality are summarized in Table 6. No significant differences were observed between patients with and without LSMM.
  | Table 6 Postoperative outcomes |
Discussion
Both LSMM and delirium are related to frailty, the inability to adequately respond to stressors, and may be related to each other.4,6 The current study primarily aimed to investigate if any association exists between LSMM and density and the incidence of POD in elderly CRC patients undergoing surgery. The secondary aim of this study was to compare other postoperative adverse outcomes between patients with and without LSMM.
We found an incidence of POD of 13% in our study cohort. Of the patients with LSMM, 23% experienced delirium after surgery. Factors that were associated with POD in univariate analysis were older age, delirium in medical history, blood transfusion, LSMM, malnourishment, and physical dependency. After multivariate analysis, both age and delirium in medical history factors remained significantly correlated with the incidence of POD. LSMM was also associated with POD, and only the significant association was much stronger when patients were also malnourished or physically dependent.
Previous studies showed similar POD incidence in cancer patients, up to 16%. In addition, the risk factors for delirium found in this study are in line with those described in previous studies.5,16 In our study, LSMM was defined based on the lowest quartile of the SMI. Recently, an article was published by Miller et al,16 describing a pilot observational cohort study concerning the relationship between POD and preoperative psoas muscle sign measured on CT. They described similar results by defining LSMM as the lowest tertile but found a slightly higher delirium incidence (15.5%) and a substantial influence on delirium when patients have a low psoas muscle (OR=3.51; 95% CI, 1.37–8.95).16 However, measurements of the psoas muscle only show less than 10% of total trunk muscles and could underestimate the real total muscle loss.25 Furthermore, the surgical population and delirium protocol are not described.
By using the lowest quartile to define LSMM, at least 25% of the patients were considered to have LSMM. A range in the prevalence of LSMM of 17%–60% in CRC patients undergoing surgery was described.26,27 The review by Levolger et al describes a great diversity of methods to measure SMM on CT images and refers to several definitions of sarcopenia, in the current study defined as LSMM. Low skeletal muscle mass is most often used in oncological studies to define sarcopenia. Measuring skeletal muscle mass on CT is considered the golden standard.15,28 However, other studies also describe sarcopenia more extensively: combining radiological measurements and physical performance.25,29 Therefore, in this study, we consider the CT measurements as LSMM, instead of sarcopenia.
In our multivariate model, malnourished patients with LSMM were associated with POD, as well as physically dependent patients with LSMM. The combinations are made because all three factors are connected. Malnourishment in cancer patient is often disease related. Gastrointestinal cancer and its therapy could cause malnourishment.30 Cachexia, loss of body weight and muscle strength, is caused due to aging and acute or chronic diseases and frequently seen in cancer patients. This leads to functional dependency, further spiraling down a vicious circle of muscle loss and weakness.15,29,31
When comparing patients with and without LSMM, no differences were found in other adverse postoperative outcomes, which is in line with a previous study that included only gastric cancer patients.32 Other studies describe the relationship of LSMM and worse postoperative outcome, like postoperative infections or delayed recovery in colorectal patients.13,27,33,34 These studies included cancer patients of all ages. Lieffer et al34 also included acutely admitted patients due to tumor obstruction or perforation. The chances of developing postoperative infection or complications are, admissibly, much higher in these patient groups compared to that in elective planned patients. The study by Huang et al33 described a small patient group, but it is the only study that described a broader definition of sarcopenia. The systematic review by Levolger et al26 found a decreased morbidity rate in patients with LSMM. A higher psoas density would even protect against overall complications and infectious complications in specifically CRC patients.26
Several studies showed significant differences within the mortality rates.35,37 However, these studies used other definitions of LSMM, using a cutoff value of the L3 muscle index of 52.4 cm2/m2 for men and 38.5 cm2/m2 for women based on the study by Prado et al.36 In our study, the cutoff based on the lowest quartile was slightly lower for both male and female and therefore might show less mortality. In addition, Reisinger et al included more patients, but they also included patients in need of acute colorectal surgery, which have a higher chance for mortality.
Tailored surgery, especially for elderly surgical cancer patients, is more often described as a necessity to prevent postoperative adverse outcomes.38,39 This is important for not only infectious complications, readmissions, or mortality but also to prevent delirium. Defining LSMM on CT images could be an easy tool to add in the risk assessment for POD and other adverse outcomes, especially combined with muscle power and function. Rehabilitation projects (either before or after surgery), integrating muscle mass, function, and power could optimize postoperative outcomes. Prediction of delirium for individual patients, based on the prognostic models, could be of additional relevance in tailored surgery.
Previous studies showed a correlation between inflammatory status, body composition, and postoperative outcome in various surgical oncology populations.40,41 Indeed, inflammation is thought to be a key mechanism underpinning LSMM. Furthermore, inflammation is associated with cognitive impairment in both community dwelling elderly and cancer patients.42,43 Consequently, it may be an important cofounding factor. Unfortunately, these data were not available for the current cohort.
Several other limitations may be noted. First, the retrospective nature of this study and the relatively small number of patients may have led to selection bias and a lack of statistical power. Furthermore, preoperative screening was not a standard procedure. Therefore, preoperative psychiatric testing was not available. Second, the definition of LSMM was based on muscle mass investigated on CT, and we did not investigate muscle power or function. Defining LSMM as the lowest quartile might show a low incidence of LSMM and therefore underestimate several outcomes. Nevertheless, as the debate remains which cutoff values to use, and previously established cutoff values were created with (overall) survival as a study outcome, in our opinion, the lowest quartile is an appropriate measure. Third, a few CT examinations were older then 3 months before surgery. In case of chemotherapy or radiotherapy, the patient could have LSMM or could have started exercises and a refeeding program and have a higher SMM, and this was not analyzed in this study. Fourth, malnourishment and physical dependency are analyzed at admission. Finally, the relatively low number of events in this study limited multivariate analyses. Therefore, future studies should validate our results in larger cohorts.
Conclusion
LSMM, particularly in malnourished or physically dependent patients, is an important risk factor for POD in elderly patients undergoing CRC surgery. Preoperative interventions may reduce skeletal muscle wasting and consequently decrease POD rates.
Disclosure
The authors report no conflicts of interest in this work.
References
van der Sluis FJ, Buisman PL, Meerdink M, et al. Risk factors for postoperative delirium after colorectal operation. Surgery. 2017;161(3):704–711. | ||
Scholz AF, Oldroyd C, Mccarthy K, Quinn TJ, Hewitt J. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103(2):e21–e28. | ||
Bellelli G, Morandi A, di Santo SG, et al. “Delirium Day”: a nationwide point prevalence study of delirium in older hospitalized patients using an easy standardized diagnostic tool. BMC Med. 2016;14:106. | ||
Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. | ||
Raats JW, van Eijsden WA, Crolla RM, Steyerberg EW, van der Laan L. Risk Factors and Outcomes for Postoperative Delirium after Major Surgery in Elderly Patients. PLoS One. 2015;10(8):e0136071. | ||
Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. | ||
Raats JW, Steunenberg SL, de Lange DC, van der Laan L. Risk factors of post-operative delirium after elective vascular surgery in the elderly: A systematic review. Int J Surg. 2016;35:1–6. | ||
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. | ||
Hu X, Zhang L, Wang H, Hao Q, Dong B, Yang M. Malnutrition-sarcopenia syndrome predicts mortality in hospitalized older patients. Sci Rep. 2017;7(1):3171. | ||
Fearon K, Evans WJ, Anker SD. Myopenia-a new universal term for muscle wasting. J Cachexia Sarcopenia Muscle. 2011;2(1):1–3. | ||
Joglekar S, Nau PN, Mezhir JJ. The impact of sarcopenia on survival and complications in surgical oncology: A review of the current literature. J Surg Oncol. 2015;112(5):503–509. | ||
Mei KL, Batsis JA, Mills JB, Holubar SD. Sarcopenia and sarcopenic obesity: do they predict inferior oncologic outcomes after gastrointestinal cancer surgery? Perioper Med. 2016;5:30. | ||
Hanaoka M, Yasuno M, Ishiguro M, et al. Morphologic change of the psoas muscle as a surrogate marker of sarcopenia and predictor of complications after colorectal cancer surgery. Int J Colorectal Dis. 2017;32(6):847–856. | ||
van Roekel EH, Bours MJL, Te Molder MEM, et al. Associations of adipose and muscle tissue parameters at colorectal cancer diagnosis with long-term health-related quality of life. Qual Life Res. 2017;26(7):1745–1759. | ||
Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. | ||
Miller AL, Englesbe MJ, Diehl KM, et al. Preoperative psoas muscle size predicts postoperative delirium in older adults undergoing surgery: a pilot cohort study. J Am Geriatr Soc. 2017;65(1):e23–e24. | ||
Cohen MM, Duncan PG, Tate RB. Does anesthesia contribute to operative mortality? JAMA. 1988;260(19):2859–2863. | ||
Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of adl: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. | ||
Kruizenga HM, Seidell JC, de Vet HC, Wierdsma NJ, van Bokhorst-de van der Schueren MA. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ). Clin Nutr. 2005;24(1):75–82. | ||
van Vugt JL, Levolger S, Gharbharan A, et al. A comparative study of software programmes for cross-sectional skeletal muscle and adipose tissue measurements on abdominal computed tomography scans of rectal cancer patients. J Cachexia Sarcopenia Muscle. 2017;8(2):285–297. | ||
Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–2338. | ||
Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539–1547. | ||
Schuurmans MJ, Shortridge-Baggett LM, Duursma SA. The Delirium Observation Screening Scale: a screening instrument for delirium. Res Theory Nurs Pract. 2003;17(1):31–50. | ||
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. | ||
Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle. 2017;8(4):527–528. | ||
Levolger S, van Vugt JL, de Bruin RW, Ijzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102(12):1448–1458. | ||
Nakanishi R, Oki E, Sasaki S, et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today. 2018;48(2):151–157. | ||
Mourtzakis M, Prado CM, Lieffers JR, Reiman T, Mccargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. | ||
Rier HN, Jager A, Sleijfer S, Maier AB, Levin MD. The Prevalence and Prognostic Value of Low Muscle Mass in Cancer Patients: A Review of the Literature. Oncologist. 2016;21(11):1396–1409. | ||
Arends J, Baracos V, Bertz H, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017;36(5):1187–1196. | ||
Reijnierse EM, Trappenburg MC, Leter MJ, et al. The Association between Parameters of Malnutrition and Diagnostic Measures of Sarcopenia in Geriatric Outpatients. PLoS One. 2015;10(8):e0135933. | ||
Tegels JJ, van Vugt JL, Reisinger KW, et al. Sarcopenia is highly prevalent in patients undergoing surgery for gastric cancer but not associated with worse outcomes. J Surg Oncol. 2015;112(4):403–407. | ||
Huang DD, Wang SL, Zhuang CL, et al. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17(11):O256–O264. | ||
Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107(6):931–936. | ||
Buettner S, Wagner D, Kim Y, et al. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1,326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J Am Coll Surg. 2016;222(4):e392:397–407. | ||
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008 Jul 1;9(7):629–35. | ||
Reisinger KW, van Vugt JL, Tegels JJ, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261(2):345–352. | ||
Audisio RA. Tailoring surgery to elderly patients with cancer. Br J Surg. 2016;103(2):e10–e11. | ||
Huisman MG, Kok M, de Bock GH, van Leeuwen BL. Delivering tailored surgery to older cancer patients: Preoperative geriatric assessment domains and screening tools – A systematic review of systematic reviews. Eur J Surg Oncol. 2017;43(1):1–14. | ||
Reisinger KW, Derikx JP, van Vugt JL, et al. Sarcopenia is associated with an increased inflammatory response to surgery in colorectal cancer. Clin Nutr. 2016;35(4):924–927. | ||
Richards CH, Roxburgh CS, Macmillan MT, et al. The relationships between body composition and the systemic inflammatory response in patients with primary operable colorectal cancer. PLoS One. 2012;7(8):e41883. | ||
Trollor JN, Smith E, Agars E, et al. The association between systemic inflammation and cognitive performance in the elderly: the Sydney Memory and Ageing Study. Age. 2012;34(5):1295–1308. | ||
Williams AM, Shah R, Shayne M, et al. Associations between inflammatory markers and cognitive function in breast cancer patients receiving chemotherapy. J Neuroimmunol. 2018;314:17–23. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


