Back to Journals » Clinical Ophthalmology » Volume 12
Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes
Authors Katz LJ, Erb C, Carceller Guillamet A, Fea AM , Voskanyan L, Giamporcaro JE, Hornbeak DM
Received 21 September 2017
Accepted for publication 5 December 2017
Published 31 January 2018 Volume 2018:12 Pages 255—262
DOI https://doi.org/10.2147/OPTH.S152268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
L Jay Katz,1 Carl Erb,2 Amadeu Carceller Guillamet,3 Antonio M Fea,4 Lilit Voskanyan,5 Jane Ellen Giamporcaro,6 Dana M Hornbeak6
1Glaucoma Service, Wills Eye Hospital, Philadelphia, PA, USA; 2Glaucoma Service, Eye Clinic Wittenbergplatz, Berlin, Germany; 3Department of Ophthalmology, Hospital Quirón, Barcelona, Spain; 4Department of Surgical Sciences, University of Torino, Torino, Italy; 5Glaucoma Service, S. V. Malayan Ophthalmology Centre, Yerevan, Armenia; 6Department of Clinical Research and Medical Affairs, Glaukos Corporation, San Clemente, CA, USA
Purpose: Evaluate long-term outcomes after one, two, or three trabecular micro-bypass stents implanted in a standalone procedure in eyes with open-angle glaucoma taking ocular hypotensive medication.
Patients and methods: Prospective randomized ongoing study of 119 subjects (109 with 42-month follow-up) with open-angle glaucoma, preoperative intraocular pressure (IOP) 18–30 mmHg on one to three glaucoma medications, and unmedicated (post-washout) IOP 22–38 mmHg. Subjects were randomized to receive one (n=38), two (n=41), or three (n=40) iStent trabecular micro-bypass stents in a standalone procedure. Postoperatively, IOP was measured with medication and annually following washout. Data included IOP, medications, gonioscopy, pachymetry, visual field, visual acuity, adverse events, and slit-lamp and fundus examinations.
Results: Preoperative mean medicated IOP was 19.8±1.3 mmHg on 1.71 medications in one-stent eyes, 20.1±1.6 mmHg on 1.76 medications in two-stent eyes, and 20.4±1.8 mmHg on 1.53 medications in three-stent eyes. Post-washout IOP prior to stent implantation was 25.0±1.2, 25.0±1.7, and 25.1±1.9 mmHg in the three groups, respectively. Postoperatively, Month 42 medicated IOP was 15.0±2.8, 15.7±1.0 and 14.8±1.3 mmHg in the three groups, and post-washout IOP (Months 36–37) was 17.4±0.9, 15.8±1.1 and 14.2±1.5 mmHg, respectively. IOP reduction ≥20% without medication was achieved in 89%, 90%, and 92% of one-, two-, and three-stent eyes, respectively, at Month 12; and in 61%, 91%, and 91% of eyes, respectively, at Month 42. The need for additional medication remained consistent at Months 12 and 42 in multi-stent eyes (four two-stent eyes and three three-stent eyes at both time points), whereas it increased in single-stent eyes (four eyes at Month 12 versus 18 eyes at Month 42). Safety parameters were favorable in all groups.
Conclusion: The standalone implantation of either single or multiple iStent® device(s) produced safe, clinically meaningful IOP and medication reductions through 42 months postoperatively, with incrementally greater and more sustained reductions in multi-stent eyes.
Keywords: microinvasive glaucoma surgery/MIGS, iStent, surgery, standalone, multiple
Introduction
The treatment of glaucoma, a leading cause of irreversible blindness globally, may include a spectrum of medical and/or surgical interventions. Most treatments aim to lower intraocular pressure (IOP), which is widely established as the primary modifiable risk factor for onset and progression of glaucoma. In most clinical practices, patients in the early stages of open-angle glaucoma (OAG) are first prescribed topical medications. As the next treatment step, patients often undergo laser trabeculoplasty, but due to disease progression and/or the well-documented waning efficacy of laser trabeculoplasty over time, this intervention frequently is not a long-term solution.
For patients with mild-to-moderate glaucoma that progresses despite medications and/or laser procedures, there has been, until recently, a paucity of viable intermediate surgical options. Thus, these patients have often been advised to undergo watchful waiting until the glaucomatous damage reached an extent that made the risk-to-benefit profile of traditional filtering surgeries more acceptable. Such risks of traditional filtering surgeries could include, for example, endophthalmitis, fibrosis, bleb leaks or infections, bleb dysesthesia, and hypotony maculopathy; and many of the risks remain throughout life, contributing to a lifelong cumulative postoperative burden for the patient.1–10
The above treatment gap between early and advanced glaucoma has decreased substantially in the past decade with the introduction and increasing adoption of micro-invasive glaucoma surgery (MIGS) procedures. The first US Food and Drug Administration-approved MIGS device was the iStent,® a trabecular micro-bypass stent (Glaukos Corporation, San Clemente, CA, USA). To date, the first-generation iStent and the second-generation iStent inject® (Glaukos Corporation) have been the subject of over 65 peer-reviewed research publications. Most initial studies of these technologies were in OAG patients undergoing concomitant cataract surgery,11–20 while several more recent publications have evaluated standalone implantation of single or multiple iStent or iStent inject devices in OAG.21–31 In both settings, study results have consistently shown durable long-term ability to lower IOP and medications in patients with OAG and in pseudoexfoliative glaucoma. Such findings suggest that reduced IOP and medication burden indeed may be achieved by a minimally invasive approach. Moreover, these reductions have been accompanied by a favorable safety profile, thereby differentiating these procedures from the risks of traditional filtering surgeries such as those discussed previously.1–10
In the present study, the authors examined the long-term safety and IOP- and medication-reducing effects of implanting one, two, or three first-generation iStent trabecular micro-bypass stents in a standalone procedure in eyes with OAG not controlled on ocular hypotensive medication(s). Outcomes through 18 months were presented in a prior publication.31 Briefly, the prior report showed substantial IOP reduction after implantation of one iStent; each additional stent incrementally added more IOP reduction. Likewise, the implantation of additional stents resulted in higher proportions of eyes achieving ≥20% IOP reduction without medication at 12 months, as well as IOP ≤18 and ≤15 mmHg without medication at 12 months.31 Through 18 months, fewer multiple-stent versus single-stent eyes required the addition of ocular hypotensive medication. Based on this earlier publication, the present report summarizes data through 42 months postoperative; subjects will continue to be followed for a total of 60 months.
Materials and methods
Study design
This study is a prospective, randomized, open-label evaluation of the long-term safety and performance of one, two, or three iStent trabecular micro-bypass stents implanted in a standalone procedure (ie, without cataract surgery) in eyes with OAG. As presented in the prior publication, participating surgeons in this study included five visiting MIGS Study Group surgeons from four countries (USA, Italy, Germany, and Spain), in addition to one staff surgeon (Armenia).31 Surgeons received training on the iStent implantation technique and the study protocol prior to their study participation. All surgeries and follow-up visits were completed at the S.V. Malayan Ophthalmological Center in Yerevan, Armenia; the Armenian Ministry of Health provided Ethics Committee approval. The study followed the tenets of the Declaration of Helsinki (2008); all participating subjects provided written informed consent. The study registration number is NCT 01517477 (ClinicalTrials.gov).
The study design specified enrollment of up to 120 phakic or pseudophakic subjects with OAG, cup-to-disc (C:D) ratio ≤0.9, current treatment with one to three medications, preoperative medicated IOP of 18–30 mmHg, and preoperative unmedicated (post-washout) IOP of 22–38 mmHg. The complete inclusion and exclusion criteria were presented in the prior publication.31 Postoperatively, the subjects were examined at Day 1, Week 1, and at Months 1, 3, 6, 12, 13, 18, 24, 25, 30, 36, 37, and 42; subjects are continuing to be followed for a total of 60 months. Postoperative performance was evaluated based on IOP (by Goldmann applanation tonometry) and ocular hypotensive medication use. At each annual postoperative visit, any subject taking an ocular hypotensive medication began a 1-month medication washout, which was followed by unmedicated examinations at Months 13, 25, and 37. For continuous variables such as IOP and medications at each visit, descriptive analyses included mean and SD. Additional calculations included the proportion of eyes with IOP reduced ≥20% versus baseline without medication at Month 12 and Month 42; proportions of eyes with Month 42 best-corrected visual acuity (BCVA) equal to or better than 20/40, 20/100, and 20/200; and a Kaplan–Meier plot showing postoperative time to addition of medication. In addition to BCVA, safety measures in all eyes included visual field (VF); pachymetry; findings from slit-lamp, gonioscopic, and fundus examinations (including optic nerve assessment and C:D ratio); and intraoperative and postoperative adverse events. Examinations were completed by the staff surgeon and glaucoma-trained staff ophthalmologists at the S.V. Malayan Ophthalmological Center. The total duration of study follow-up was fixed at 5 years.
Surgical devices and technique
Study subjects underwent implantation of one, two, or three iStent trabecular micro-bypass stents in a sole procedure. Each single-piece, heparin-coated, titanium stent has a length of 1.0 mm, height of 0.33 mm, and a “snorkel” bore diameter of 120 μm. Figure 1 shows the stent, which is pre-loaded on a single-use inserter that is advanced ab internally through a small temporal clear corneal incision, thereby preserving ocular tissue in case other glaucoma surgery is needed in the future. The inserter is designed to facilitate stent implantation into Schlemm’s canal, thereby bypassing the nasal trabecular meshwork, in order to improve natural physiologic aqueous outflow and reduce IOP. In subjects randomized to receive two or three stents in this study, a second iStent (and third iStent, if applicable) was implanted in the same manner ~2–3 clock hours away from the already-implanted stent(s).
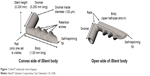  | Figure 1 iStent® trabecular micro-bypass. |
After surgery, the subjects were placed on topical antibiotic medication for 1 week and a topical corticosteroid taper for 4 weeks. Postoperative glaucoma medication was started if IOP exceeded 18 mmHg or in the case of concerning optic nerve or VF changes; any such medication was stopped during annual 1-month medication washouts. The choice of 18 mmHg as the IOP cut-off was consistent with the finding of the landmark Advanced Glaucoma Intervention Study (AGIS) that VF progression is delayed when IOP is consistently maintained below 18 mmHg.32
Results
IOP and medications
A total of 119 qualified subjects were randomized to undergo implantation of one, two, or three stents (n=38, 41, and 40 in the three groups, respectively); follow-up data through 42 months are available for 109 of these subjects (n=33, 38, and 38 in the three groups, respectively). The prior publication from this study provided the complete preoperative demographics and ocular parameters of subjects in this study.31 In the one-, two-, and three-stent groups, preoperative mean medicated IOP was 19.8±1.3 mmHg (range, 18–24 mmHg) on a mean of 1.71 medications, 20.1±1.6 mmHg (range, 18–25 mmHg) on a mean of 1.76 medications, and 20.4±1.8 mmHg (range, 18–28 mmHg) on a mean of 1.53 medications, respectively. Post-washout mean IOP before stent implantation was 25.0±1.2 mmHg (range, 23–28 mmHg), 25.0±1.7 mmHg (range, 22–30 mmHg), and 25.1±1.9 mmHg (range, 22–32 mmHg) for the three respective groups. All but two subjects were phakic.
At Month 42 postoperatively, mean medicated IOP had decreased to 15.0±2.8 mmHg (range, 10.7–19.7 mmHg), 15.7±1.0 mmHg (range, 12.0–17.3 mmHg), and 14.8±1.3 mmHg (range, 12.0–16.7 mmHg) in the three groups, respectively, reflecting 24%, 22%, and 27% reductions versus preoperative mean medicated IOP, respectively (Figure 2). In the absence of medication (ie, post-washout at Month 36–37), mean unmedicated IOP was 17.4±0.9 mmHg (range, 14.7–19.3 mmHg), 15.8±1.1 mmHg (range, 13.7–18.0 mmHg), and 14.2±1.5 mmHg (range, 11.7–17.7 mmHg) for one-, two-, and three-stent eyes, respectively, constituting reductions of 30%, 37%, and 43%, respectively, versus preoperative unmedicated IOP. As shown in Figure 3, postoperative IOP reduction ≥20% without medication was achieved in 89%, 90%, and 92% of one-, two-, and three-stent eyes, respectively, at Month 12; and in 61%, 91%, and 91% of eyes in the three groups, respectively, at Month 42.
Per the study design, all subjects were on one to three medications preoperatively (specific mean values were 1.71, 1.76, and 1.53 medications for the three groups, respectively), and all subjects were placed on zero medications immediately after surgery. Over 42 months of follow-up, medication was added if IOP exceeded 18 mmHg or in cases of glaucomatous optic nerve or VF changes. By Month 42, among eyes without additional surgery, postoperative medication was added in a total of 18 eyes in the one-stent group, four eyes in the two-stent group, and three eyes in the three-stent group. In particular, the need for additional medication remained stable in multi-stent eyes (four two-stent eyes and three three-stent eyes at both Months 12 and 42) whereas it increased in single-stent eyes (four eyes at Month 12 versus 18 eyes at Month 42), as shown in Figure 4.
  | Figure 4 Time to addition of postoperative medication.a |
Safety assessment
No complications occurred intraoperatively or perioperatively, including no hypotony, choroidal effusion, hyphema, nor iridodialysis. During 42 months of postoperative follow-up, no device-related or sight-threatening adverse events occurred; furthermore, no eyes required additional glaucoma surgery. In this cohort of almost entirely phakic subjects (117 of 119) with mean baseline age between 62 and 69 years, the most common (and expected) adverse event over 3.5 years of follow-up was progression of preexisting cataract. By Month 42 postoperatively, a total of eight one-stent eyes, five two-stent eyes, and seven three-stent eyes had BCVA loss ≥1 line due to cataract progression. Of these cases, five one-stent eyes, two two-stent eyes, and three three-stent eyes underwent cataract surgery by Month 42, and their IOP and medication data thereafter were excluded from efficacy analyses; two additional eyes (three-stent group) had cataract surgery shortly after the Month 42 visit. In the remaining eight non-operated eyes, BCVA still remained relatively high: 20/25 or 20/29 in seven eyes and 20/40 in one eye at Month 42. Across all groups, postoperative BCVA appeared generally stable through Month 42; proportional analysis of Month 42 BCVA is shown in Figure 5. In addition, mean C:D ratio and central corneal thickness remained stable over time in all groups through Month 42 (Table 1). Meanwhile, VF mean deviation decreased in all three groups from screening through Month 42; these reductions were compared between the three groups using one-way analysis of variance (ANOVA), and were found to be statistically similar (p=0.40, Table 1).
  | Figure 5 Proportional analysis of Month 42 best-corrected visual acuity. |
Discussion
The 42-month results from this ongoing randomized study demonstrate substantial IOP- and medication-reducing effects of one, two, or three trabecular micro-bypass stents when implanted as a standalone procedure in eyes with mild-to-moderate OAG. Similar to the prior publication from this cohort,31 as well as a study by Belovay et al of two or three iStents during cataract surgery,23 our present findings indicate that the largest portion of IOP reduction results from the first stent: specifically, a 7.6 mmHg (30%) reduction in unmedicated IOP after one stent. After this first stent, each additional stent provided further IOP reduction in an incremental manner: 9.2 mmHg (37%) total IOP decrease with two stents, and 10.9 mmHg (43%) total IOP decrease with three stents.
In addition to confirming these prior clinical reports, the current 42-month IOP findings corroborate data from laboratory investigations of incremental efficacy with multiple iStent devices.33–36 For example, Hunter et al reported 6 mmHg of IOP reduction and 30% decrease in outflow resistance after one stent, and 8.9 mmHg and 44% decrease, respectively, after two stents.33 Similarly, Bahler et al estimated IOP reductions of 6.1 and 9.7 mmHg with one and two stents, respectively.34–35 A possible explanation for this stepwise IOP reduction may be drawn from the biomechanical models of Johnstone, whose laboratory data showed aqueous tissue deformation induced by IOP gradients.36 If such tissue alteration were to decrease the action of a single trabecular stent, the use of multiple stents hypothetically may circumvent this decrease. Thus, in the context of these laboratory data as well as the aforementioned clinical studies, the 42-month IOP reductions seen in the present cohort (30%, 37%, and 43%) appear directionally and quantitatively reasonable.
A distinguishing feature of the 42-month findings versus those of the prior publication31 is the increased use of medications in single-stent versus multi-stent eyes over time. As before, medication reductions were observed in all three groups at all time points, but our current findings suggest an increasing divergence in the degree of medication reduction in single-stent versus multi-stent eyes as time elapsed. As shown in Figure 4, the need for additional medication appeared to stabilize in multi-stent eyes, with equal numbers of subjects on medication at both 12 and 42 months in the two groups (four two-stent eyes and three three-stent eyes); meanwhile, over the same period, the need for additional medication increased in one-stent eyes (four eyes at Month 12 versus 18 eyes at Month 42). This report covers ~3.5 years of postoperative follow-up, so it will be meaningful to observe outcomes through study completion at 5 years.
Safety parameters continued to be favorable in all the three groups, with no intraoperative or perioperative complications (including no hypotony, choroidal effusion, hyphema, nor iridodialysis); no postoperative device-related or sight-threatening adverse events; and no additional glaucoma surgeries throughout 42 months of follow-up. In addition, postoperative BCVA, C:D ratio, and central corneal thickness remained generally stable in all groups through Month 42 (Figure 5; Table 1). VF mean deviation decreased to a similar extent in all the three groups from screening through Month 42 (ANOVA p-value =0.40, Table 1). However, it must be kept in mind that glaucoma is an inherently progressive condition, even in the setting of surgical and/or medication-induced reductions in IOP. For example, the landmark Early Manifest Glaucoma Trial showed that IOP-lowering treatment decreased, but did not stop, VF progression. In this cohort, disease progression occurred in both untreated (62% of eyes) and treated subjects (45% of eyes), despite a mean IOP reduction of 5.1 mmHg.37 Similarly, the foundational Collaborative Normal Tension Glaucoma Study reported VF progression in 35% of untreated eyes (which had no significant IOP reduction), but also in 12% of treated eyes (despite their IOP reduction).38 Furthermore, the landmark AGIS showed VF decline in both treated and untreated eyes, and showed that this decline was progressive over time through seven years postoperatively.32 In this cohort of almost entirely phakic subjects, the most common (and expected) postoperative adverse event was cataract progression. However the cataract rates observed in this study were not dissimilar to US population-based estimates, which document cataract progression in ~18% to 33% of phakic, similarly aged subjects over 3.5 years of follow-up.39 The majority of the cataract cases in our study were rectified by cataract surgery, and BCVA in the remaining non-operated eyes still remained relatively good. Importantly, although multi-stent eyes had undergone a separate insertion for each stent, these additional intraocular entries were not associated with a higher incidence of complications.
This study is not without limitations. The investigation was an open-label, single-site, unmasked study in a solely Caucasian patient population. Baseline and postoperative IOPs were not measured at multiple time points, leaving open the possibility of regression to the mean. No standardized cataract grading system nor threshold for completing cataract surgery was used. One may notice the relatively low values for IOP variance in this study; these appear consistent with variance in prior clinical reports.16,17,29 Finally, this report encompasses data through 42 months; a future report will be able to assess even longer-term outcomes through study completion at 60 months.
Limitations notwithstanding, this long-term randomized study presents clinically informative data on the standalone performance of one, two, and three first-generation trabecular micro-bypass stents through 42 months in subjects with mild-to-moderate OAG not controlled on preoperative medication. The data show substantial IOP reduction in all groups and incrementally greater IOP reduction in multiple-stent eyes, together with an excellent safety profile, thereby confirming results of prior clinical and laboratory work.23,31,33–36 Medication burden also decreased in all groups in an incremental manner, consistent with the 18-month findings from this study.31 Over 42 months of follow-up, fewer multiple-stent versus single-stent eyes ultimately required additional postoperative medication, with an apparent stabilization of medication in multi-stent eyes versus continued increase in single-stent eyes. Thus, these long-term findings corroborate the existing literature showing high efficacy with one stent, and incrementally greater IOP and medication reductions with additional stents.
Acknowledgments
The sponsor, Glaukos Corporation, San Clemente, CA, USA, provided study devices, sponsorship for performing this study, data collection, data management, data analysis, and editorial assistance in the preparation of this manuscript.
Disclosure
LJK is a shareholder in Aerie Pharm., Glaukos, and Mati Ther, he received grant support from Allergan, Aerie Pharm., Mati Therapeutics, Diopsys, Heidelberg Eng., Alcon, and Zeiss; speaker honoraria from Alcon, Allergan, Bausch & Lomb, Aerie Pharm., and Glaukos; and a medical monitor consulting fee from Glaukos; he is a consultant to Allergan, Alcon, Glaukos (Chief Medical Officer), Aerie Pharm., Inotek, Mati Ther., Diopsys, and DSM Biomed. CE is a consultant to Alcon, Zeiss, OmniVision, and Visufarma, and received speaker honoraria from Alcon, Allergan, Bausch & Lomb, Novartis, Thea, Santen, Visufarma, Glaukos, and Zeiss. ACG received financial support from Glaukos for his work as an investigator in this study. AMF received financial support from Glaukos for his work as an investigator in this study. LV received financial support from Glaukos for her work as an investigator in this study. DMH and JEG are employees of Glaukos. The authors report no other conflicts of interest in this work.
References
Jampel HD, Musch DC, Gillespie BW, Lichter PR, Wright MM, Guire KE; Collaborative Initial Glaucoma Treatment Study Group. Perioperative complications of trabeculectomy in the collaborative initial glaucoma treatment study (CIGTS). Am J Ophthalmol. 2005;140(1):16–22. | ||
Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC; Tube Versus Trabeculectomy Study Group. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814. | ||
Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136:464–470. | ||
Rulli E, Biagioli E, Riva I, et al. Efficacy and safety of trabeculectomy versus nonpenetrating surgical procedures: a systematic review and meta-analysis. JAMA Ophthalmol. 2013;131(12):1573–1582. | ||
Kim EA, Law SK, Coleman AL, et al. Long-term bleb-related infections after trabeculectomy: incidence, risk factors, and influence of bleb revision. Am J Ophthalmol. 2015;159(6):1082–1091. | ||
Sharan S, Trope GE, Chipman M, Buys YM. Late-onset bleb infections: prevalence and risk factors. Can J Ophthalmol. 2009;44(3):279–283. | ||
Fontana H, Nouri-Mahdavi K, Caprioli J. Trabeculectomy with mitomycin C in pseudophakic patients with open-angle glaucoma: outcomes and risk factors for failure. Am J Ophthalmol. 2006;141(4):652–659. | ||
Ederer F, Gaasterland DA, Dally LG, et al; AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 13. Comparison of treatment outcomes within race: 10-year results. Ophthalmology. 2004;111(4):651–664. | ||
Zacharia PT, Deppermann SR, Schuman JS. Ocular hypotony after trabeculectomy with mitomycin C. Am J Ophthalmol. 1993;116(3):314–326. | ||
Greenfield DS, Liebmann JM, Jee J, Ritch R. Late-onset bleb leaks after glaucoma filtering surgery. Arch Ophthalmol. 1998;116(4):443–447. | ||
Samuelson TW, Katz LJ, Wells JM, Duh YJ, Giamporcaro JE; US iStent Study Group. Randomized evaluation of the trabecular micro-bypass stent with phacoemulsification in patients with glaucoma and cataract. Ophthalmology. 2011;118(3):459–467. | ||
Craven ER, Katz LJ, Wells JM, Giamporcaro JE; iStent Study Group. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012;38(8):1339–1345. | ||
Fea AM. Phacoemulsification versus phacoemulsification with micro-bypass stent implantation in primary open-angle glaucoma. J Cataract Refract Surg. 2010;36(3):407–412. | ||
Fea AM, Consolandi G, Zola M, et al. Micro-Bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol. 2015;2015:795357. | ||
Arriola-Villalobos P, Martinez-de-la-Casa J, Diaz-Valle D, Fernández-Pérez C, García-Sánchez J, García-Feijoó J. Combined iStent trabecular micro-bypass stent implantation and phacoemulsification for coexistent open-angle glaucoma and cataract: a long-term study. Br J Ophthalmol. 2012;96(5):645–649. | ||
Arriola-Villalobos P, Martinez-de-la-Casa J, Diaz-Valle, Morales-Fernandez L, Fernandez-Perez C, Garcia-Feijoo J. Glaukos iStent inject trabecular micro-bypass implantation associated with cataract surgery in patients with co-existing cataract and open-angle glaucoma or ocular hypertension: a long-term study. J Ophthalmol. 2016;(2016): Article ID 1056573. | ||
Neuhann TH. Trabecular micro-bypass stent implantation during small-incision cataract surgery for open-angle glaucoma or ocular hypertension: long-term results. J Cataract Refract Surg. 2015;41(12):2664–2671. | ||
Gallardo MJ, Supnet RA, Giamporcaro JE, Hornbeak DM. Outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic patient population. Clin Ophthalmol. 2016;10:1931–1937. | ||
Ferguson TJ, Berdahl JP, Schweitzer JA, Sudhagoni RG. Clinical evaluation of a trabecular micro-bypass stent with phacoemulsification in patients with open-angle glaucoma and cataract. Clin Ophthalmol. 2016;10:1767–1773. | ||
Ferguson TJ, Swan R, Ibach M, Schweitzer J, Sudhagoni R, Berdahl JP. Trabecular microbypass stent implantation with cataract extraction in pseudoexfoliation glaucoma. J Cataract Refract Surg. 2017;43(5):622–626. | ||
Ferguson T, Berdahl J, Schweitzer J, Sudhagoni R. Evaluation of a trabecular micro-bypass stent in Pseudophakic patients with open-angle glaucoma. J Glaucoma. 2016;25(11):896–900. | ||
Donnenfeld ED, Solomon KD, Voskanyan L, et al. A prospective 3-year follow-up trial of implantation of two trabecular microbypass stents in open-angle glaucoma. Clin Ophthalmol. 2015;9:2057–2065. | ||
Belovay GW, Naqi A, Chan BJ, Rateb M, Ahmed II. Using multiple trabecular micro-bypass stents in cataract patients to treat open-angle glaucoma. J Cataract Refract Surg. 2012;38(11):1911–1917. | ||
Vold SD, Voskanyan L, Tetz M, et al. Newly diagnosed primary open-angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: outcomes through 36 months. Ophthalmol Ther. 2016;5(2):161–172. | ||
Chang DF, Donnenfeld ED, Katz LJ, et al. Efficacy of two trabecular micro-bypass stents combined with topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 3-year follow-up. Clin Ophthalmol. 2017;11:523–528. | ||
Berdahl J, Voskanyan L, Myers JS, et al. Implantation of two second-generation trabecular micro-bypass stents and topical travoprost in open-angle glaucoma not controlled on two preoperative medications: 18-month follow-up. Clin Exp Ophthalmol. 2017;45(8):797–802. | ||
Fea AM, Belda JI, Rekas M, et al. Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol. 2014;8:875–882. | ||
Voskanyan L, Garcia-Feijoo J, Belda J, Fea A, Jünemann A, Baudouin C; Synergy Study Group. Prospective, unmasked evaluation of the iStent® inject system for open-angle glaucoma: synergy trial. Adv Ther. 2014;31(2):189–201. | ||
Klamann MK, Gonnermann J, Pahlitzsch M, et al. iStent inject in phakic open angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):941–947. | ||
Lindstrom R, Lewis R, Hornbeak H, et al. Outcomes following implantation of two second-generation trabecular micro-bypass stents in patients with open-angle glaucoma on one medication: 18-month follow-up. Adv Ther. 2016;33(11):2082–2090. | ||
Katz LJ, Erb C, Carceller Guillamet AC, et al. Prospective, randomized study of one, two, or three trabecular bypass stents in open-angle glaucoma subjects on topical hypotensive medication. Clin Ophthalmol. 2015;9:2313–2320. | ||
The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. The AGIS Investigators. Am J Ophthalmol. 2000;130(4):429–440. | ||
Hunter K, Fjield T, Heitzmann H, Shandas R, Kahook M. Characterization of micro-invasive trabecular bypass stents by ex vivo perfusion and computational flow modeling. Clin Ophthalmol. 2014;8:499–506. | ||
Bahler CK, Smedley GT, Zhou J, Johnson DH. Trabecular bypass stents decrease intraocular pressure in cultured human anterior segments. Am J Ophthal. 2004;138(6):988–994. | ||
Bahler C, Hann C, Fjield T, Haffner D, Heitzmann H, Fautsch MP. Second-generation trabecular meshwork bypass stent (iStent inject) increases outflow facility in cultured human anterior segments. Am J Ophthal. 2012;153(6):1206–1213. | ||
Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13(5):421–438. | ||
Heijl A, Leske MC, Bengtsson B, et al; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120(10):1268–1279. | ||
Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126(4):487–497. | ||
nei.nih.gov. 2010 U.S. age-specific prevalence rates for Cataract by Age, and Race/Ethnicity. Maryland: National Eye Institute Statistics and Data; c2010. Available from: https://nei.nih.gov/eyedata/cataract. Accessed September 18, 2017. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



