Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Lentigo Maligna: Clinical Presentation and Appropriate Management
Authors Iznardo H, Garcia-Melendo C , Yélamos O
Received 25 June 2020
Accepted for publication 15 October 2020
Published 13 November 2020 Volume 2020:13 Pages 837—855
DOI https://doi.org/10.2147/CCID.S224738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Helena Iznardo,1,* Cristina Garcia-Melendo,1,* Oriol Yélamos1,2
1Dermatology Service, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain; 2Dermatology Service, Centro Médico Teknon – Quirónsalud, Barcelona, Spain
*These authors contributed equally to this work
Correspondence: Oriol Yélamos
Dermatology Service, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, C/Mas Casanovas 90, Block A, 5th Floor, Module 3, Barcelona 08041, Spain
Tel +34 935537007
Fax +34 935537008
Email [email protected]
Abstract: Lentigo maligna (LM) is a type of melanoma in situ that has distinctive characteristics regarding epidemiology, risk factors and clinical features. In addition, LM has a potential to progress to an invasive tumor with potentially aggressive behavior: lentigo maligna melanoma (LMM). Overall, LM has a very good prognosis, whereas LMM has the same prognosis as other invasive melanomas with similar Breslow thickness. LM/LMM represents a challenging entity not only regarding the diagnosis but also regarding the management. Diagnostic criteria are not well established, and there is an overlap of clinical, dermoscopic and pathological features with other benign pigmented skin lesions such as lentigines, pigmented actinic keratoses or macular seborrheic keratoses. LM/LMM’s common appearance within photodamaged skin makes lesion border identification difficult. Wide excisions are often required, but since LM/LMM typically appears on cosmetically sensitive areas such as the face, sometimes large excisions are not possible nor desirable. In this sense, specialized approaches have been developed such as margin-controlled surgery or image-guided treatment using reflectance confocal microscopy. Other treatments for LM such as cryosurgery, imiquimod, radiotherapy or photodynamic therapy have been proposed, although recurrence/persistence is common. The current manuscript reviews extensively the published data regarding the diagnosis, treatment and management of both complex entities LM and LMM.
Keywords: lentigo maligna, melanoma, lentigo maligna melanoma, dermoscopy, dermatoscopy, reflectance confocal microscopy, staged excision, Mohs surgery, imiquimod
Introduction and Epidemiology
Lentigo maligna (LM) is a type of melanoma in situ (MIS), recognized both by the American Committee on Cancer and the World Health Organization. Despite being classified as a type of MIS, LM has distinctive characteristics regarding epidemiology, risk factors and clinical features. In addition, LM has a potential to progress to an invasive tumor with potentially aggressive behavior: lentigo maligna melanoma (LMM).1,2 The lifetime risk of progression from LM to LMM ranges from 5% to 50% and increases with time.3 Overall, LM has a very good prognosis, whereas LMM has the same prognosis as other invasive melanomas with similar Breslow thickness.4
Regarding incidence, LM and LMM account for approximately 4–15% of all melanomas, representing up to 10–26% of head and neck melanomas, as they most commonly present on these areas.4 They typically appear on chronically sun-damaged skin in elderly people, as opposed to the most common subtype of melanoma, the superficial spreading type, which typically occurs on areas acutely exposed to UV radiation.5 Mean presentation age of LM/LMM ranges from 66 to 72 years, approximately a decade older than for other melanoma subtypes.1 Men are more likely to be diagnosed with LM/LMM, although in some series a predominance in females has been observed.6,7
Incidence has increased over the past few decades: Swetter et al8 found that LM was the most prevalent (79–83%) form of MIS in North Carolina. In a recent study in Catalonia (northeast Spain) with 4999 MM cases (282 cases of LM and 136 of LMM), a significant increase in incidence was observed during an 8-year period, from 6.9% in 2000 to 13.1% in 2007. Several factors may explain this increase such as increased chronic sun-exposure. Because LM appears on chronically sun-exposed skin, there is a higher incidence in southern latitudes compared to northern latitudes (1.3 cases/100.000 person-years in Australia vs. 0.8 cases/100.000 person-years in the United States). Other factors such as Fitzpatrick skin types and skin cancer awareness must be taken into account as they can influence the reported incidence of LM. This increase has been attributed to aging of the population and changes in sun exposure patterns,6–12 as well as lack of recognition of LM as a distinct histologic subtype in the past.9 It must be noted that the increase in the number of LM cases was significantly higher than the increase of the invasive ones, and mean Breslow thickness of LMM has remained stable.7
Risk factors associated to LM/LMM include increased age, chronically sun-exposed areas of sun-damaged skin, increased number of lentigines, increased number of actinic keratosis and history of previous keratinocyte carcinomas.13 Genetic conditions such as xeroderma pigmentosum, oculocutaneous albinism, Werner syndrome and porphyria cutanea tarda are also associated with LM.13 Larger size of LM has been proposed as a risk factor for transformation to LMM.
The pathogenesis of LM/LMM is complex. Whiteman et al14 proposed in 2003 that cutaneous melanomas arise through 2 distinct pathways, with chronic solar exposure being responsible for LMM. Elder et al15 have recently expanded on this concept suggesting a new classification of melanoma with different genomic characteristics. Contrary to the observed in superficial spreading melanoma, BRAFV600E mutations are infrequent in skin with marked solar elastosis.16,17 Driver mutations in LMM include NF1, BRAFV600K, NRAS and KIT. In addition, gain- or loss- of function mutations such as CCND1, MITF and TP53 have also been implicated in melanomas on sun-damaged skin.18
LM represents a challenging entity not only regarding the diagnosis but also regarding the management. Diagnostic criteria are not well established, and there is an overlap of clinical, dermoscopic and pathological features with other benign pigmented skin lesions such as lentigines, pigmented actinic keratoses and macular seborrheic keratoses.12,19 LM common appearance within photodamaged skin makes lesion border identification difficult and they often extend beyond what is visible with the naked eye. Wide excisions are often required, with appropriate surgical margins appearing to be around 9 mm on the trunk and extremities, and greater than 1 cm on the head and neck.20,21 However, sometimes these large excisions are not possible nor desirable in cosmetically-sensitive areas such as the face. In this sense, margin-controlled surgery such as Mohs micrographic surgery or staged-excision offers the highest cure rates with the minimal scarring necessary to achieve curation. However, the location of LM/LMM on the head and neck region and their often large size frequently represent obstacles to these therapeutic options. In addition, margin-controlled surgical techniques are not always available since they require trained surgeons and dedicated facilities, thus limiting its availability. Other treatments such as cryosurgery, imiquimod, radiotherapy and photodynamic therapy have been proposed in order to decrease morbidity and recurrence rates. Furthermore, a wait-and-see strategy has also been suggested in select patients such as patients with numerous comorbidities and patients with limited life expectancy.
The current manuscript reviews the published data on the diagnosis, treatment and management of LM/LMM.
Clinical Presentation
LM/LMM arises on chronically sun damaged skin predominantly of the head and neck area, which hosts 78.3% of the cases22 and with the cheeks accounting for 53.7% of the cases.23
However, extrafacial LM/LMM can also be encountered. According to a study analyzing 71 cases of LM,24 extrafacial lesions account for 17.5% of all LM/LMM cases, with patients being significantly younger when compared to head and neck LM/LMM patients. Extrafacial LM/LMM is usually seen on the trunk in men or extremities in women.25,26
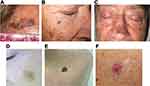
LM/LMM usually presents as a slowly growing isolated large pigmented macule or patch with irregular borders. At times it can have a discontinuous appearance on clinical examination with ill-defined borders. Additionally, LM/LMM can also present as an amelanotic/hypomelanotic macule or patch, especially in fair-skinned individuals (Figure 1). Repigmentation of previous white or gray hair may rise suspicion towards the possibility of LM in the scalp, and it has been described as an early sign of LM.27
Because of this slow growth and sometimes hypomelanotic presentation, it is not uncommon that LM/LMM diagnosis is delayed, with 48.8% of lesions being >10mm in diameter at the time of biopsy.23 With time, LM may evolve into papules, nodules or thick plaques which correspond to invasive foci thus becoming LMM and conferring risk of metastatic disease (Figure 1).
The clinical differential diagnosis includes solar lentigo, seborrheic keratosis, lichen planus-like keratosis (LPLK) and pigmented actinic keratosis which are summarized in Table 1.
 |
Table 1 Lentigo Maligna (LM)/LM Melanoma (LMM) Differential Diagnosis: Clinical Presentation and Dermoscopy |
Non-Invasive Diagnostic Tools
Non-invasive imaging methods including Wood’s lamp, dermoscopy and reflectance confocal microscopy can improve the diagnostic accuracy for LM/LMM, improve margin delineation, aid in biopsy site selection and also become an important tool for monitoring treatment.
Wood’s Lamp
The Wood’s light emits ultraviolet (UV) light in a wavelength of 320–400nm with a peak irradiance at 365nm. Epidermal melanin absorbs most of the light emitted from the Wood’s light with little reflectance, making superficially pigmented lesions appear darker than the surrounding normal epidermis.
Rather than for the diagnosis, Wood’s lamp is especially useful for estimating LM/LMM size – particularly areas of subclinical disease invisible to the naked-eye- and therefore becomes an essential part of physical examination.28 Wood’s light examination has been demonstrated to be useful for margin assessment of melanoma in situ (MIS) before biopsy,29 and is particularly useful in determining margins of LM/LMM before surgical excision.30,31
However, Walsh et al30 prospectively studied the accuracy of preoperative Wood’s light examination for margin assessment of MIS after excisional biopsy. They concluded that it is not reliable to assess subclinical disease with Wood's light in these patients since many false positive and false negatives can occur. This is not surprising since surrounding photodamaged skin can harbor numerous activated melanocytes. Hence, this may limit Wood’s lamp utility to delineate the LM/LMM margins as these melanocytes either isolated or within benign lesions (seborrheic keratoses, pigmented actinic keratoses) may be highlighted as well. Also, it must be born in mind that dermal melanin is not accentuated by the Wood’s light and may lead to false negatives regarding a deeper atypical melanocytic component.32
Dermoscopy
Dermoscopy is a non-invasive technique that allows for the visualization of skin structures not visible to the naked eye and therefore improves diagnostic accuracy for both pigmented and non-pigmented skin lesions. Dermoscopy consists of a handheld magnifier lens (normally around 10x) which is coupled to a light source that can be polarized or non-polarized. Polarized and non-polarized dermoscopy provide complementary information for the diagnosis of LM/LMM and it has been found to be superior to Wood’s lamp examination to delineate the borders of LM/LMM.28
When assessing facial LM/LMM it is important to take into account that facial skin has numerous terminal hair follicles, sweat gland ostia, and attenuated rete ridges. These special features in facial skin create a pseudo-network appearance: a structureless pigment area interrupted by nonpigmented adnexal openings.33–35
According to the third consensus conference of the International Society of Dermoscopy36 both metaphoric and descriptive terminology can be used to describe lentigo maligna dermoscopy. LM/LMM dermoscopy will be briefly discussed herein by using metaphoric and descriptive terminology, as well as their histopathological correlates.
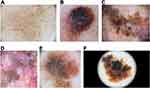
Annular-Granular Pattern
Dots and structureless brown to blue-gray areas scattered throughout the lesion or arranged adnexal openings (Figure 2A). Brown dots correspond on histopathology to aggregates of melanocytes and small nests at the dermoepidermal junction between the follicles. Blue-gray granularity corresponds to melanophages in the upper dermis.
Polygons/Rhomboids/Zig-Zag Pattern (angulated lines)
They correspond to gray-brown lines that are connected at an angle or coalescing to form polygons (Figure 2E). The term “rhomboids” was introduced to describe gray-brown angulated lines around adnexal ostial openings in LM. This term is reserved for facial skin, and they are more frequently seen in LM located on the upper part of the face.23,36,37 However, the term “zig-zag pattern” can be regarded as a variant of rhomboids on facial skin.38
Whereas the terms “rhomboids” and “zig-zag pattern” are reserved for facial skin, angulated lines39 and polygons40 are the appropriate terms to characterize pigmented lines that form angles in melanomas on nonfacial skin,39,41 although many use “angulated lines” as a single term that encompasses polygons and rhomboids, regardless of their location.
These structures correspond histologically to confluent junctional atypical melanocytes together with underlying melanophages in the papillary dermis.33,42,43
Asymmetric Pigmented Follicular Openings
The presence of asymmetric distribution of pigment surrounding the follicular openings is characteristic, especially in LM located on the lower part of the face23 (Figure 2). A fine circle, semicircle, signet ring-like circle, irregular circle and double circle can be seen.
On histopathology, it corresponds to atypical melanocytes as single units or small nests in the epidermis that are surrounding and/or extending down hair follicles.35
Circle Within a Circle
When concentric pigmented circles surrounding follicular openings are present it is called the circle-within-a-circle structure, which is identified in 5% and 25.4% of cases.23,41,44
Regarding extrafacial LM/LMM, the most common dermoscopic structures are granularity (67.7%), angulated lines (44.1%), and atypical dots (36.6%).45 However, because of preserved rete ridges and significantly fewer adnexal openings in extrafacial skin, focal islands of network without an annular granular pattern can be observed46 (Figure 2F).
Schiffner et al33 described a model of the dermoscopic progression for LM/LMM (Figure 3). Initially, asymmetric pigmentation and dots around the follicle are seen, which later evolve to rhomboidal structures and finally form homogeneous pigmented areas and cause obliteration of the follicular openings. Pralong et al47 found that at least one of the structures previously described are present in 87% of LMM. The combination of asymmetrically pigmented follicular openings, gray dots, gray globules, or rhomboidal structures located anywhere within a lesion has a sensitivity of 89% and a specificity of 96% for LM/LMM.33 Another diagnostic clue for LM/LMM diagnosis is the darkening at dermoscopic examination.47 In fact, in 25% of LM/LMM the pigment appears darker and with different shades of brown and gray when viewed with dermoscopy compared to naked-eye examination.47 Moreover, it should be taken into account that increased density of the vascular network within the lesion when compared to adjacent skin is found in 58% of cases,47 and should rise suspicion for invasive melanoma.48
Differential Diagnosis
It must be born in mind that gray color, gray circles, and annular granular structures can be seen in both pigmented actinic keratoses (PAK) and LM/LMM. Similarly, the hyperpigmented rim of follicular openings of LM/LMM can be mistaken for pseudofollicular openings of seborrheic keratoses.49 The differential diagnosis among these entities is further assessed in Table 1.
Some algorithms have been proposed to differentiate pigmented lesions of the face.
Micantonio et al50 proposed an algorithm to distinguish between facial LM and PAK with an excellent accuracy and a high positive predictive value for early facial LM. Eight dermoscopic criteria achieved the highest discriminative power to distinguish LM from PAK: light brown color, brown-to-gray incomplete circles, brown-to-black structureless zone obscuring the hair follicles, structureless brown eccentric zone, structureless blue zone, brown-to-black structureless zone, in-focus brown discontinued lines and scales. Tschandl et al51 focused on the evaluation of non-melanoma dermatoscopic features in order to differentiate LM/LMM from other pigmented lesions in the face. These features include scales, white follicles, erythema/reticular vessels, reticular and/or curved lines/fingerprints, structureless brown color, sharp demarcation and classic criteria of seborrheic keratosis. If no prevalent non-melanoma patterns are seen then the suspicion for melanoma increases.
Reflectance Confocal Microscopy
Reflectance confocal microscopy (RCM) is a non-invasive imaging tool that uses near-infrared laser light obtaining horizontal quasi-histological images.52 RCM improves diagnostic accuracy of multiple skin tumors.52,53 It is very useful for diagnosing and monitoring LM/LMM since it has cellular resolution and allows the visualization of very small amounts of melanin which are invisible to the naked eye or dermoscopy.52 Therefore, RCM is an ideal tool to differentiate LM/LMM from benign macules and solar damage.54,55
RCM increases the physician’s diagnostic confidence and increases diagnostic sensitivity, therefore improving the management of difficult lesions.53 In fact, RCM is more sensitive (overall sensitivity of RCM 0.93) and specific (overall specificity 0.89) than dermoscopy (overall sensitivity 0.73, and overall specificity was 0.84) for the diagnosis of LM.56 Moreover, the integration of dermoscopy and RCM increases the diagnostic accuracy of both these techniques used alone for facial tumors.57
RCM is remarkably useful when assessing lesions located on the head and neck area,58 and is particularly suitable for the identification of hypomelanotic/amelanotic and recurrent LM/LMM.59,60
Menge et al found the RCM and histopathology results were consistent in 89% of the cases when evaluating suspected LM, but skin damage may limit the specificity of the diagnosis.61
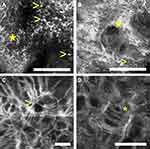
The proliferation of atypical melanocytes at the DEJ may be visualized on RCM as atypical round or dendritic cells, typically large (twice the size of the adjacent keratinocytes)59 (Figure 4). As LM becomes more extensive, pagetoid spread of large pleomorphic cells are seen through all layers of the epidermis, causing epidermal disarray. Poorly defined dermal papillae and atypical cells may be seen at the dermal-epidermal junction and can form bridges resembling mitochondrial structures.62 Other characteristic findings include junctional swelling with infiltration of the hair follicle, resembling caput medusae (Figure 4), which was found to be indicative of LM/LMM compared to nonmelanocytic skin neoplasms, with an overall sensitivity of 96% and specificity of 83%.59,63,64
Guitera et al59 developed a LM score to distinguish LM from benign lesions of the face. The score consists of 2 major and 4 minor criteria, represented in Table 2. A LM score of greater than 2 resulted in a sensitivity of 85% and specificity of 76% for the diagnosis of LM, and the algorithm was equally effective in the diagnosis of amelanotic lesions. Some of the features included in the algorithm are the presence of round and large pagetoid cells and the presence of more than three atypical cells at the dermoepidermal junction in five images. However, Champin et al65 consider that the presence of a single large bright dendritic cell-predominantly found around follicle openings – should be considered part of the tumor when assessing LM margins. Moreover, Yélamos et al66 believe atypical melanocytes continuing from the center of a LM/LMM, regardless of their size, should be considered as positive for LM as they may reflect the trailing edge of a LM/LMM (Table 2).
 |
Table 2 In vivo Confocal Features for LM Diagnosis |
RCM is also useful for margin definition in ill-defined lesions45 and to map the extent of LM/LMM before treatment.67,68 Champin et al65 described a new procedure that combined the “spaghetti” technique (described in the treatment section) with in vivo RCM to evaluate LM margins, with better results than with the “spaghetti” technique alone.
Handheld RCM (HRCM) together with the use of videomosaics (static mosaics obtained from dynamic videos) has allowed an accurate evaluation of large lesions in curved areas of the body, including the face. HRCM has been reported to be useful for detecting subclinical margins, and presence of invasion,69 thus becoming a valuable tool for deciding the optimal treatment. Yélamos et al66 combined adhesive rings and radial videomosaics obtained with HRCM to calculate clinical extension and presurgical margins of LM/LMM, and to identify LM/LMM extending beyond the dermoscopic and Wood’s lamp margins showing excellent accuracy when compared to margin-controlled surgery using staged excision.
A prospective study evaluating the correlation of LM/LMM subclinical extension defined by RCM compared to the gold standard histopathology showed an overall agreement of 85.9% between RCM imaging and histopathology of staged excision margins.70 Diagnostic accuracy for detection of residual melanoma in the tumor debulking (after biopsy) had a sensitivity of 96.7% and a specificity of 66.7% when compared to the histopathology.
Future Directions
Machine learning (ML) is a form of artificial intelligence which uses computer algorithms to help with clinical decisions. An interesting subfield of ML is deep learning, in which large datasets are scaled, allowing to improve themselves with more data.71 Deep learning convolutional neural networks (CNNs) have further improved the accuracy of ML in melanoma screening, even exceeding some dermatologists.71,72 These algorithms could improve LM/LMM diagnosis in the future,73 although some limitations have to be addressed. Winkler et al74 investigated the diagnostic performance of a CNN across different melanoma subtypes, including LMM. The authors used a dermatoscopic image set containing 30 LMM and 100 benign lesions (flat, macular solar lentigines, seborrheic keratoses and nevi), matched for localization and morphology. A “malignancy score” ranging from 0 to 1 (higher scores indicating a higher probability of melanoma) was obtained by the CNN. Average score was 0.98 for LMM and 0.37 for solar lentigines, seborrheic keratoses and nevi. Sensitivity and specificity (95% confidence interval) for diagnosis of LMM (a priori cut-off of >0.5) was 100% (88.7–100%) and 65.0% (55.3–73.6%), respectively. These good results are promising, although the authors admit that their dermatoscopic images were of greater quality than those obtained in a clinical routine setting. In addition, most images were derived from fair-skinned patients. Images of LMM in individuals of other ethnic background are scarce, therefore these can suppose additional limitations for pattern recognition by CNNs. In addition, some characteristics of pigmented skin lesions make them unavailable for ML analysis.75–77 The most relevant is the difficulty in assessing the border of the lesion (lack of pigmentation, lack of surrounding normal skin, presence of hair and lesions appearing in volar skin). Another important limitation is large lesions that do not fit into the field of view of the dermatoscopic camera. In addition, a recent study78 evaluated the limitations in image selection for ML analysis. Authors found that 66.7% of the LM included in the study showed exclusion criteria, with only 33.3% of them being eligible for ML analysis.78 Therefore, although ML and CNN will probably play an important role in the future management of LM/LMM, there are still limitations that need to be addressed by the use of larger image datasets that better represent different skin types, include benign lesions as well as images obtained with consumer cameras in a rather uncontrolled manner.
Histopathology
Biopsy Technique
When a lesion has been identified as suggestive of LM/LMM, a skin biopsy typically allows for a definitive diagnosis. Different biopsies can be performed regarding the purpose (incisional/excisional) or the technique (shave/punch/ellipse). The choice between one or another depends on the size of the lesion, location, cosmesis and patient’s and physician’s preferences.
Agarwal-Antal et al80 reported that 16% of LM contain invasive melanoma. Diagnostic excisional biopsy with narrow margins has been considered the gold standard for diagnosing melanoma because incisional biopsy can underestimate the depth of the lesion due to sampling error.81 Also, a broad shave biopsy extending into the deep papillary dermis or superficial reticular dermis can be acceptable for LM/LMM since it allows the evaluation of a large piece of tissue.82,83
However, RCM enables the physician to assess the invasive components of LMM and target biopsies to the thickest areas, thus potentially reducing sampling error.68 In a retrospective study by Mataca et al,84 RCM selected sites showed more histopathologic criteria when compared to dermoscopy selected sites.
HRCM can guide mapping biopsies by detecting features of LM with high sensitivity, thus reducing sample bias inherent to blind mapping biopsies.67 Also, mapping of LM/LMM with HRCM-videomosaicing can reduce the number of biopsies needed in doubtful areas.69
Histopathology Analysis
The diagnosis of LM/LMM is usually made by histopathologic examination. The dermis may show solar elastosis1 and may display a patchy or band-lymphocytic infiltrate. Some authors suggest that the presence of melanophages is a helpful to differentiate LM from melanocytic hyperplasia of chronically sun-damaged skin.86 The epidermis of LM/LMM is often atrophic and it can have hyperpigmentation of basal keratinocytes.4,87 It is important to highlight that no histological differences have been found between facial and extrafacial LM/LMM.88
Initially, LM is characterized by an increased density of melanocytes along the dermal-epidermal junction, although they may also be found in the spinous layer1 (Figure 5). These melanocytes can have variable nuclear atypia1 and can show dendritic appearance.89 Some melanocytes can be multinucleated and have prominent dendritic processes, known as “starburst giant cell”,90 which is not specific for LM and can also be seen in benign melanocytic nevi.91 The adnexa can be involved by neoplastic melanocytes,92 and is first seen in the infundibular part of the follicle. At the next stage, pagetoid spread, melanocytes with hyperpigmented and angulated nucleus in the basal layer, and involvement of the deeper portion of the follicles can be seen.93
Regarding LMM, some histologic variables have been identified. These include melanocytes forming rows, subepidermal clefts, a lesser degree of solar elastosis and nests.94 Nests of pleomorphic melanocytes – resembling dysplastic junctional nevus – predominate in 43% of LM/LMM.95
The vertical growth phase often shows spindle cell morphology. Up to two-thirds of desmoplastic melanomas are associated with LM,96 and neurotropism is not uncommon in deeply invasive LMM86 Immunohistochemical markers such as MART-1 may help identify a dermal invasive component.
Treatment
Even though LM has good prognosis, treatment is necessary in order to reduce the potential for LMM transformation and its associated mortality. The classic treatment which remains the mainstay is surgical excision with safety margins. However, due to the cosmetically-sensitive location on sun-exposed areas such as the face, surgery is not always possible or desirable. Herein we will review the different treatment modalities with their grades of recommendation and levels of evidence (Table 3).81,97 A big limitation is that there have been no randomized controlled trials to date comparing the efficacy of all LM treatments. Treatment choice is complex and depends on factors such as anatomic location and previous treatments, as well as patient comorbidities, age and treatment adherence.98 In fact, Fosko et al have proposed a personalized treatment plan in which patients with no comorbidities are suited for surgical excision, whereas patients who do not tolerate surgery are offered off-label imiquimod or radiotherapy.99 Patient care would benefit from a multidisciplinary team composed by dermatologists, surgeons and both medical and radiation oncologists.100
 |
Table 3 Evidence-Based Recommendations for Lentigo Maligna (LM) |
Surgical Excision
Current guidelines suggest that surgical excision is the treatment of choice for both LM and LMM.81 The primary goal of surgical excision of LM/LMM is to achieve histologically-negative margins and prevent local recurrence because of persistent disease, as stated by the latest guidelines for the treatment of melanoma published by the American Academy of Dermatology and the National Comprehensive Cancer Network.81,101 Therefore, a surgical excision technique that carefully examines the entire tumor to exclude areas of invasion and complete surgical margins for accurate tumor clearance must be chosen.102 A standard elliptical wide excision examines a very small percentage of the total margin, with the risk of false-negative margins increasing as the number of bread loaf sections decreases. Margin-controlled surgical techniques such as staged excision or Mohs micrographic surgery offer low recurrence rates of 0.5% to 5%, and they also provide tissue-sparing in these cosmetically-sensitive sites.102
Conventional Surgery
The conventional melanoma margins of 5 mm for in situ disease and 10 mm for invasive disease have been demonstrated to be inadequate in LM and LMM,21,81,102–104 especially in the head and neck region. Kunishige et al21 observed clearance rates of 79.4%, 94.4%, 97.4% and 98.7% for 6, 9, 12 and 15 mm margins, respectively, in overall LM. Microscopic assessment of LM is difficult because adjacent actinically damaged skin frequently shows actinic melanocytic hyperplasia, which can simulate atypical junctional melanocytic proliferations.105
Mohs Micrographic Surgery (MMS)
It consists of excision of the lesion with immediate microscopic frozen section examination of 100% of the peripheral and deep excision margin. The main limitations are its high costs, that it’s a time-consuming technique, and that histologic interpretation of melanocytic atypia is difficult since freezing leads to artefactual changes in the melanocytes.106 Sensitivity and specificity rates for diagnosing LM/LMM on frozen sections vary from 100% and 90% to 73% and 68%, respectively.107,108 However, immunohistochemical stains such as MART-1 or MITF can be used in frozen sections, thus improving the diagnostic accuracy. Local recurrence rates for melanoma treated with MMS with MART-1 are between 0.2% and 0.49%.109,110 If tumor is detected at the margin, targeted excision or a second stage is performed around the residual tumor, repeating the process until clear margins are achieved. A recent systematic review including 27 articles showed a 1.35% recurrence rate with MMS.111 Another potential benefit of MMS is that LMM can be detected prior to reconstruction, allowing accurate sentinel lymph node biopsy (SNLB) prior to complex reconstructions that would otherwise compromise SNLB. In addition, some authors advise that the central tumor debulking specimen should be sent for formalin-fixed paraffin embedded sections, to detect possible invasion and eventual LMM upstaging.112
Staged Excision
This technique is a variation of MMS and allows confirmation of negative margins with delayed repair of the surgical site. The usual procedure involves vertical excision with initial narrow margins examined by formalin-fixed permanent sections. Further excision is guided by histology over subsequent days. A variation of this technique which only assesses a rim of peripheral tissue has also been described and named “spaghetti technique”. A correlation with increased lesion size and with recurrent lesions and increasing surgical margin required for tumor clearance has been found with staged excision.102–104,112
Margin-controlled surgical techniques require an expert multidisciplinary team formed by expert dermatologists, Mohs micrographic surgeons and pathologists with close communication and clinical-pathological correlation that is only achievable in referral centers.100
Non-Surgical Treatment
As stated before, LM common location on the head and neck region, big size and advanced age of the patients frequently represent obstacles to surgical treatment. Alternative minimally-invasive treatments have been proposed in LM in order to decrease morbidity and recurrence rates. The greatest limitation of all these techniques is that LMM cannot be completely excluded, as the whole specimen is not examined histologically.
Cryotherapy with liquid nitrogen (cryosurgery) is an alternative approach in select patients, with recurrence rates ranging between 0% and 40%.113,114 Melanocytes are highly sensitive to low temperatures, being destroyed between −4 and −7ºC.115 A depth of at least 3 mm must be achieved in order to destroy atypical melanocytes that may have extended into the hair follicles.116 Study comparison is limited by variability in treatment regimens and duration of follow-up. A study with 18 patients with LM used two freezing cycles of 1 minute each, separated by a thaw cycle of at least 2 minutes, with frozen areas extending 1 cm beyond the borders of the lesions.117 No recurrences or metastasis were observed during a mean follow up of 75.5 months.117
Immunocryosurgery
Combination of cryosurgery sessions with imiquimod application has been successfully used for LM. This method increases local inflammation and therefore the effectiveness of the treatment in those patients who do not respond with vivid inflammation to imiquimod alone.118 Different regimes have been applied with variable results. Matas-Nadal et al119 used topical imiquimod 5% cream once daily on the lesion plus 1 cm along the lesion edge for 3 weeks, followed by one session of cryosurgery (2 cycles of 20 seconds) on the lesion plus 1 cm along the lesion edge. This was followed by 6 months of topical imiquimod cream 3 times per week. Clinical clearance was observed in 3/3 patients.
Imiquimod
Imiquimod is a topical immune-response modifier that acts through binding Toll-like receptors 7 and 8 on dendritic cells, macrophages and neutrophils, recruiting CD68+ macrophages and cells involved in cytotoxic T cell responses.120 In addition, it may interfere with adenosine receptor pathways, increasing adenylyl cyclase activity; and may have proapoptotic activity against tumor cells.13,121 Although it is off label, topical 5% imiquimod cream for LM has been extensively used, with variable results.122–125 Complete histopathologic responses (based on targeted biopsies) range from 50% to 93%, recurrence rates range from 7.1% up to 50% and mean follow-up durations range from 6 months to 4.5 years.122–126 Once again, variability in treatment regimes, assessment of outcome and duration of follow-up make comparison between studies difficult. The inability to eliminate atypical melanocytes within hair follicles due to limited penetration has been proposed as an important limiting factor for imiquimod’s higher recurrence rates.81 A recent study has investigated the role of the host immune response in order to identify responders to imiquimod.127 Samples were taken prior to starting imiquimod 5 days per week for 12 weeks. At 16 weeks, lesions were excised and assessed histologically. No differences between immune cell subset densities were observed between responders and non-responders pre-treatment. Post-treatment, responders showed up-regulated antigen presentation, type I interferon, T cell activation and tolerance induction signaling pathways.127 The optimal regimen treatment remains to be determined, but a typical regimen is an application for 5–7 days per week for 12 weeks, within a 1–2cm margin of clinically normal-appearing skin.124 A systematic review with 471 patients treated with imiquimod in different treatment schedules observed that a treatment schedule using 6–7 applications per week, with at least 60 applications total, had the greatest odds of complete clinical and histological clearance of LM.128
Imiquimod as a neoadjuvant therapy has also been used in LM, demonstrating to be useful for decreasing the necessary margin for complete clearance.121,129 In a study with 334 patients treated with imiquimod 5% cream 5 nights per week for 2 to 3 months followed by staged excisions, there was a median final margin of 2 mm with a recurrence rate of 3.9%, a mean time to recurrence of 4.3 years and a mean length of follow-up of 5.5 years.121 Addition of tazarotene 0.1% gel to imiquimod cream 5% prior to staged excision has been assessed in a randomized trial of 47 patients in order to improve imiquimod’s response.129 Complete response after 12 weeks was observed histologically in 78% of lesions treated with combined therapy versus 64% treated with monotherapy (p = 0.17). Residual LM was observed in 22% of the lesions treated with combined therapy versus 36% with monotherapy on subsequent staged excision, without recurrences at a mean follow-up of 42 months.129
Assessment of treatment response is complicated although RCM may be useful (as discussed later in the section “management”). Clinical inflammation is generally associated with adequate histologic response, although there are cases in which this was not observed and actually showed the opposite results (inflammatory reaction without histopathologic clearance).129,130 In addition, the development of LMM after imiquimod treatment for LM has been described.129,131 Whether imiquimod had a role in tumor progression or focal microinvasion was already present in these lesions is unknown.
Advantages of imiquimod include superior cosmetic result, home application, avoidance of surgical morbidity, and treatment of potential atypical melanocytes in surrounding skin. However, the main limitation of this treatment is the potential severe inflammatory reactions observed. However, these adverse effects are usually limited to dose-dependent local skin reactions that subside after stopping of treatment, and post-inflammatory pigmentation. Systemic side effects, although rare, have been described and include flu-like symptoms. The latest American guidelines for the treatment of melanoma consider imiquimod as a second-line treatment for LM when surgery is not possible (primary setting) or when optimal surgery has been performed (adjuvant setting), after carefully discussing of its limitations and associated risks with the patient and family.81
Radiotherapy
Radiotherapy (RT) can be used in LM as primary treatment or as adjuvant treatment when positive margins are found after surgery. The latest American guidelines for the treatment of melanoma support the use of radiotherapy for LM as a primary treatment when complete surgical excision is not possible81 and the European Society of Medical Oncology states that “RT for local tumor control should be considered in cases of inadequate resection margins of LMM”. Superficial RT (Grenz ray or Miescher technique) was initially used, with recurrence rates ranging from 7% to 12% in 2 large retrospective studies.132,133 However, this modality is not preferred currently due to insufficient dermal penetration of Grenz rays (1 mm into the dermis) and therefore lack of adnexal treatment, where residual atypical melanocytes can remain.134 A different approach with a slightly deeper penetration has been increasingly used, with reported cure rates of 86–91%.135 A concern with deeper penetration is scarring and pigmentary changes, which may be difficult to differentiate from recurrence. A literature review including 349 irradiated patients found a crude 5% LM recurrence rate, with a median study follow-up of 3 years.136 Resolution of pigmentation occurred after 2–24 months in the same review. Differing treatments, parameters, and dosages limit specific recommendations. The field should be expanded 1 cm beyond the area marked as involved by RCM.136 Another systematic review examined 10 studies with a total cohort of 454 patients treated with RT. Complete response rates varied between 80% and 100%, and mean recurrence rate was of 11.5%.137 Regarding electronic surface brachytherapy, it is not recommended by the latest American guidelines for the treatment of melanoma.81 A multicenter-randomized trial of imiquimod versus definitive RT is currently active and will provide results in the following years (NCT02394132).134
Photodynamic Therapy
Photodynamic therapy (PDT) is an effective, non-invasive treatment for precancerous and cancerous skin lesions. Studies have shown that PDT with 5-aminolaevulinic acid induces in vitro lysis of melanocytes.138 Karam et al139 treated 15 LM with PDT with methyl-aminolaevulinate, achieving a cure rate of 80% (12/15). They used a higher light dose (average 60 J/cm2) than the dose for non-pigmented carcinomas (37 J/cm2), as melanin restricts the diffusion of red light into deep layers of the epidermis. The response was assessed by clinical follow-up (18–50 months) and multiple biopsies.139 A recent study investigated the efficacy of ablative fractional laser-assisted PDT (average dose 90 J/cm2) with 5-aminolaevulinic acid nanoemulsion for treating LM.140 The rationale for using ablative fractional laser is that it allows for the photosensitizer precursor to penetrate deep enough to reach all the atypical melanocytes. Seven out of ten lesions (70%) were histologically completely cleared after three sessions. These promising results suggest that PDT could be an alternative therapy for inoperable LM.140
Laser Therapy
Besides the use of fractional lasers to increase the penetration of PDT, described in the previous section, various forms of laser therapy have been used for the treatment of LM, with the majority of evidence derived from anecdotal reports and case studies. Carbon dioxide, Q-switched ruby, argon, neodymium-doped yttrium aluminium garnet, alexandrite laser or combinations of the above have shown poor results, with high recurrence rates.137 Lee et al reported a recurrence rate of 6.7% for carbon dioxide laser (1/15), with an initial response of 100%.141 Further studies with accurate treatment protocols are needed to confirm these results.
Ingenol Mebutate
Although ingenol mebutate has shown to induce apoptosis in melanoma cells in vitro,142 clinical data are nonconsistent. One case reported its efficacy in a 91-year-old patient with a recurrent LMM.143 Recently, a prospective multicenter study with 12 patients treated with ingenol mebutate at 150 μg/g daily for 3 days has been published, with disappointing results. Only 2 achieved complete response and no correlation between ingenolol mebutate-induced inflammation and clinical/histological clearance was observed.144
Other Non-Surgical Treatments
Other treatments such as azelaic acid, intralesional interferon alpha, fluorouracil and retinoids have proven to be ineffective for the treatment of LM and therefore are not currently recommended.27
Monitoring
Monitoring is crucial in order to detect persistence, recurrence or progression in treated LM/LMM patients. The mean time to local recurrence of primary LM and thin LMM was identified to be at least 57.5 months.145 The surgical cure rate is considered after 5 years; therefore, a long term follow-up is required. Surgery and other nonsurgical treatments can cause inflammation, pigmentation and scarring which should be taken into account when assessing treated LM/LMM. Also, identifying local recurrence can be challenging in patients with chronically sun damaged skin.
Clinically, recurrent/persistent LM/LMM can be identified by the onset of a papule, nodule or change over a treated area, which typically tends to be amelanotic or lightly pigmented with nonspecific clinical and dermoscopic features.146 Dermoscopy can sometimes improve the detection of recurrence/persistence and assist in post-treatment monitoring. The presence of “dust-like dots” is a clue for treatment failure in cases of LMM treated with imiquimod or radiotherapy.146
Follow-up after treatment can also be assisted by RCM. It is recommended to wait at least 3 months after surgery to assess the treated area with RCM to avoid inflammation and early scarring changes.147 HRCM has been reported to be useful for detecting persistence or recurrence of LM/LMM, and also for monitoring after surgical and nonsurgical treatment,148 especially in large, amelanotic or poorly pigmented lesions.68
Alarcon et al149 found no statistically significant differences between RCM and histopathology when evaluating the response of LM to imiquimod. RCM identified 70% of patients as responders to imiquimod without false-negative results, whereas response was overestimated with clinical evaluation and underestimated when using dermoscopy.
Clinical follow-up and examinations frequency depend on the AJCC primary tumor stage and the presence of additional risk factors, such as family history of melanoma, history of sunburns, dysplastic nevi, among others. LM tends to have a slower growth compared to other subtypes of melanoma; however, when LM transforms into LMM and therefore becomes invasive, its prognosis and management are the same as the other melanoma subtypes.
According to the latest 2019 European melanoma guidelines,150 follow-up for recurrence detection should be performed for at least 5 years, and follow-up for the detection of new melanomas and other skin cancers for at least 10 years. In stage 0 and IA melanoma a dermatological examination should be performed every 6 months during the first 3 years. In stage IB-IIB melanoma clinical examination is recommended every 3–6 months, together with lymph node sonography every 6 months, during the first 3 years. In stage IIC-IV with no evidence of disease, dermatological examination is advisable every 3 months during the first 3 years, together with lymph node sonography and laboratory examination (including LDH and if possible S-100) every 3–6 months. Moreover, a CT scan including neck, thorax, abdomen and pelvis or a PET-CT, and head MRI should be performed every 6 months in stage IIC-IIIC melanoma, every 3–6 months in stage IIID melanoma and every 3 months in stage IV with no evidence of disease melanoma during the first three years.150
After 4 years an annual clinical examination for stage IA melanoma seems to be adequate. For stage IB-IV with no evidence of disease from year 4 to 10 clinical examination is advisable every 6 months, and annually thereafter. Stage IV melanoma with distant metastasis follow-up should be individualized depending on symptoms, therapy and examinations.150
Conclusion
LM/LMM incidence has been increasing in the last decades. Since LM has the potential to progress into invasive LMM, careful diagnosis, treatment and management is required. Diagnosis can be difficult but non-invasive tools may help increase diagnostic accuracy. The gold-standard treatment is still surgical excision with histologically-negative margins, although alternative minimally-invasive treatments in selected patients with limited disease (LM) may help reduce morbidity and recurrence rates. However, in all cases careful monitoring after treatment with ancillary tests such as dermoscopy of RCM is fundamental, to exclude and identify persistent or recurrent disease. Randomized clinical trials comparing the efficacy of different treatments, including surgical and minimally-invasive, are needed to provide evidence-based data to improve the management of this challenging skin cancer.
 |
Figure 3 Schiffner progression model fo LM/LMM. Initially asymmetric pigmentation and dots around the follicle are seen (A), which later evolve to rhomboidal structures (B) and finally form homogeneous pigmented areas and cause obliteration of the follicular openings (C). Adapted with permission from Dermoscopedia (https://creativecommons.org/licenses/by-nc-sa/4.0/).158 |
Disclosure
Dr Oriol Yélamos reports personal fees from Leo Pharma and Almirall, outside the submitted work. The authors report no other potential conflicts of interest for this work.
References
1. Clark WH, Mihm MC. Lentigo maligna and lentigo-maligna melanoma. Am J Pathol. 1969;55(1):39–67.
2. Scolyer RA, Long GV, Thompson JF. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol Oncol. 2011;5(2):124–136. doi:10.1016/j.molonc.2011.03.002
3. McKenna JK, Florell SR, Goldman GD, Bowen GM. Lentigo maligna/lentigo maligna melanoma: current state of diagnosis and treatment. Dermatol Surg. 2006;32(4):493–504.
4. Cohen LM. Lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 1995;33(6):
5. Shain AH, Bastian BC. From melanocytes to melanomas. Nat Rev Cancer. 2016;16(6):345–358. doi:10.1038/nrc.2016.37
6. Greveling K, Wakkee M, Nijsten T, van den Bos RR, Hollestein LM. Epidemiology of lentigo maligna and lentigo maligna Melanoma in the Netherlands, 1989–2013. J Invest Dermatol. 2016;136(10):1955–1960. doi:10.1016/j.jid.2016.06.014
7. Matas‐Nadal C, Malvehy J, Ferreres JR, et al. Increasing incidence of lentigo maligna and lentigo maligna melanoma in Catalonia. Int J Dermatol. 2019;58(5):577–581. doi:10.1111/ijd.14334
8. Swetter SM, Boldrick JC, Jung SY, Egbert BM, Harvell JD. Increasing incidence of lentigo maligna melanoma subtypes: northern California and national trends 1990–2000. J Invest Dermatol. 2005;125(4):685–691. doi:10.1111/j.0022-202X.2005.23852.x
9. Forman SB, Ferringer TC, Peckham SJ, et al. Is superficial spreading melanoma still the most common form of malignant melanoma? J Am Acad Dermatol. 2008;58(6):1013–1020. doi:10.1016/j.jaad.2007.10.650
10. Little JH, Holt J, Davis N. Changing epidemiology of malignant melanoma in Queensland. Med J Aust. 1980;1(2):66–69.
11. Mirzoyev SA, Knudson RM, Reed KB, et al. Incidence of lentigo maligna in Olmsted County, Minnesota, 1970 to 2007. J Am Acad Dermatol. 2014;70(3):443–448. doi:10.1016/j.jaad.2013.11.008
12. Guitera P, Collgros H, Madronio CM, et al. The steadily growing problem of lentigo maligna and lentigo maligna melanoma in Australia: population-based data on diagnosis and management. Australas J Dermatol. 2019;60(2):118–125. doi:10.1111/ajd.12928
13. Nehal KS, Busam KJ. Lentigo maligno melanoma.
14. Whiteman DC, Watt P, Purdie DM, Hughes MC, Hayward NK, Green AC. Melanocytic nevi, solar keratoses, and divergent pathways to cutaneous melanoma. J Natl Cancer Inst. 2003;95(11):806–812. doi:10.1093/jnci/95.11.806
15. Elder DE, Bastian BC, Cree IA, Massi D, Scolyer RA. The 2018 World Health Organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med. 2020;144(4):500–522. doi:10.5858/arpa.2019-0561-RA
16. Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst. 2003;95(24):1878–1890. doi:10.1093/jnci/djg123
17. Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353(20):2135–2147. doi:10.1056/NEJMoa050092
18. DeWane ME, Kelsey A, Oliviero M, Rabinovitz H, Grant-Kels JM. Melanoma on chronically sun-damaged skin: lentigo maligna and desmoplastic melanoma. J Am Acad Dermatol. 2019;81(3):823–833. doi:10.1016/j.jaad.2019.03.066
19. Weyers W, Bonczkowitz M, Weyers I, Bittinger A, Schill WB. Melanoma in situ versus melanocytic hyperplasia in sun-damaged skin. Assessment of the significance of histopathologic criteria for differential diagnosis. Am J Dermatopathol. 1996;18(6):560–566. doi:10.1097/00000372-199612000-00002
20. Kunishige JH, Brodland DG, Zitelli JA. Surgical margins for melanoma in situ. J Am Acad Dermatol. 2012;66(3):438–444. doi:10.1016/j.jaad.2011.06.019
21. Kunishige JH, Doan L, Brodland DG, Zitelli JA. Comparison of surgical margins for lentigo maligna versus melanoma in situ. J Am Acad Dermatol. 2019;81(1):204–212. doi:10.1016/j.jaad.2019.01.051
22. Fröhlich SM, Cazzaniga S, Kaufmann LS, Hunger RE, Seyed Jafari SM, Retrospective Cohort A. Study on patients with lentigo maligna melanoma. Dermatology. 2019;235(4):340–345. doi:10.1159/000499689
23. Tiodorovic-Zivkovic D, Argenziano G, Lallas A, et al. Age, gender, and topography influence the clinical and dermoscopic appearance of lentigo maligna. J Am Acad Dermatol. 2015;72(5):801–808. doi:10.1016/j.jaad.2015.01.030
24. Cox, Aitchison Mackie. Extrafacial lentigo maligna melanoma: analysis of 71 cases and comparison with lentigo maligna melanoma of the head and neck: extrafacial lentigo maligna melanoma. Br J Dermatol. 1998;139(3):439–443. doi:10.1046/j.1365-2133.1998.02407.x
25. Wee E, Wolfe R, Mclean C, Kelly JW, Pan Y. The anatomic distribution of cutaneous melanoma: a detailed study of 5141 lesions. Australas J Dermatol. 2020;61(2):125–133.
26. Higgins HW, Lee KC, Galan A, Leffell DJ. Melanoma in situ. J Am Acad Dermatol. 2015;73(2):181–190. doi:10.1016/j.jaad.2015.04.014
27. Chan C, Magro CM, Pham AK, et al. Spontaneous hair repigmentation in an 80-year-old man: a case of melanoma-associated hair repigmentation and review of the literature. Am J Dermatopathol. 2019;41(9):671–674. doi:10.1097/DAD.0000000000001353
28. Robinson JK. Use of digital epiluminescence microscopy to help define the edge of lentigo maligna. Arch Dermatol. 2004;140(9). Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.140.9.1095. Accessed October 20, 2020.
29. Robinson JK. Margin control for lentigo maligna. J Am Acad Dermatol. 1994;31(1):79–85. doi:10.1016/S0190-9622(94)70140-7
30. Walsh SB, Varma R, Raimer D, et al. Utility of Wood’s light in margin determination of melanoma in situ after excisional biopsy. Dermatol Surg. 2015;41(5):572–578. doi:10.1097/DSS.0000000000000345
31. Atwan AA, Ziaj S, Mills CM. Defining surgical margins with Wood lamp. Dermatol Pract Concept. 2020;10(1):e2020018.
32. Paraskevas L-R, Halpern AC, Marghoob AA. Utility of the Wood’s light: five cases from a pigmented lesion clinic. Br J Dermatol. 2005;152(5):1039–1044. doi:10.1111/j.1365-2133.2005.06346.x
33. Schiffner R, Schiffner-Rohe J, Vogt T, et al. Improvement of early recognition of lentigo maligna using dermatoscopy. J Am Acad Dermatol. 2000;42(1):25–32. doi:10.1016/S0190-9622(00)90005-7
34. Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008. doi:10.1111/j.1365-2133.2008.08713.x
35. Cognetta AB, Stolz W, Katz B, Tullos J, Gossain S. Dermatoscopy of lentigo maligna. Dermatol Clin. 2001;19(2):307–318. doi:10.1016/S0733-8635(05)70268-0
36. Kittler H, Marghoob AA, Argenziano G, et al. Standardization of terminology in dermoscopy/dermatoscopy: results of the third consensus conference of the international society of dermoscopy. J Am Acad Dermatol. 2016;74(6):1093–1106. doi:10.1016/j.jaad.2015.12.038
37. Stolz W, Schiffner R, Burgdorf WHC. Dermatoscopy for facial pigmented skin lesions. Clin Dermatol. 2002;20(3):276–278. doi:10.1016/S0738-081X(02)00221-3
38. Slutsky JB, Marghoob AA. The zig-zag pattern of lentigo maligna. Arch Dermatol. 2010;146(12):1444. doi:10.1001/archdermatol.2010.307
39. Jaimes N, Marghoob AA, Rabinovitz H, et al. Clinical and dermoscopic characteristics of melanomas on nonfacial chronically sun-damaged skin. J Am Acad Dermatol. 2015;72(6):1027–1035. doi:10.1016/j.jaad.2015.02.1117
40. Keir J. Dermatoscopic features of cutaneous non-facial non-acral lentiginous growth pattern melanomas. Dermatol Pract Concept. 2014;4(1):77–82.
41. Lallas A, Argenziano G, Moscarella E, Longo C, Simonetti V, Zalaudek I. Diagnosis and management of facial pigmented macules. Clin Dermatol. 2014;32(1):94–100. doi:10.1016/j.clindermatol.2013.05.030
42. Pehamberger H, Binder M, Steiner A, Wolff K. In vivo epiluminescence microscopy: improvement of early diagnosis of melanoma. J Invest Dermatol. 1993;100(3):356S–362S. doi:10.1038/jid.1993.63
43. Vanden Daelen A, Ferreira I, Marot L, Tromme I, Digital Dermoscopy A. Follow-up illustration and a histopathologic correlation for angulated lines in extrafacial lentigo maligna. JAMA Dermatol. 2016;152(2):200–203. doi:10.1001/jamadermatol.2015.4132
44. Tschandl P, Rosendahl C, Kittler H. Dermatoscopy of flat pigmented facial lesions. J Eur Acad Dermatol Venereol. 2015;29(1):120–127. doi:10.1111/jdv.12483
45. Curiel-Lewandrowski C, Williams CM, Swindells KJ, et al. Use of in vivo confocal microscopy in malignant melanoma: an aid in diagnosis and assessment of surgical and nonsurgical therapeutic approaches. Arch Dermatol. 2004;140(9). Available from: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.140.9.1127. Accessed October 20, 2020.
46. Lau YN, Affleck AG, Fleming CJ. Dermatoscopic features of extrafacial lentigo maligna. Clin Exp Dermatol. 2013;38(6):612–616. doi:10.1111/ced.12063
47. Pralong P, Bathelier E, Dalle S, Poulalhon N, Debarbieux S, Thomas L. Dermoscopy of lentigo maligna melanoma: report of 125 cases: dermoscopy of lentigo maligna melanoma. Br J Dermatol. 2012;167(2):280–287. doi:10.1111/j.1365-2133.2012.10932.x
48. Yélamos O, Braun RP, Liopyris K, et al. Usefulness of dermoscopy to improve the clinical and histopathologic diagnosis of skin cancers. J Am Acad Dermatol. 2019;80(2):365–377. doi:10.1016/j.jaad.2018.07.072
49. Lallas A, Tschandl P, Kyrgidis A, et al. Dermoscopic clues to differentiate facial lentigo maligna from pigmented actinic keratosis. Br J Dermatol. 2016;174(5):1079–1085. doi:10.1111/bjd.14355
50. Micantonio T, Neri L, Longo C, et al. A new dermoscopic algorithm for the differential diagnosis of facial lentigo maligna and pigmented actinic keratosis. Eur J Dermatol. 2018;28(2):162–168.
51. Tschandl P, Gambardella A, Boespflug A, et al. Seven non-melanoma features to rule out facial melanoma. Acta Derm Venereol. 2017;97(10):1219–1224. doi:10.2340/00015555-2759
52. Rajadhyaksha M, Grossman M, Esterowitz D, Webb RH, Rox Anderson R. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104(6):946–952. doi:10.1111/1523-1747.ep12606215
53. Guilera JM, Barreiro Capurro A, Carrera Alvárez C, Puig Sardá S. The role of reflectance confocal microscopy in clinical trials for tumor monitoring. Dermatol Clin. 2016;34(4):519–526. doi:10.1016/j.det.2016.06.001
54. Busam KJ, Charles C, Lee G, Halpern AC. Morphologic features of melanocytes, pigmented keratinocytes, and melanophages by in vivo confocal scanning laser microscopy. Mod Pathol. 2001;14(9):862–868. doi:10.1038/modpathol.3880402
55. Guitera P, Li L-XL, Scolyer RA, Menzies SW. Morphologic features of melanophages under in vivo reflectance confocal microscopy. Arch Dermatol. 2010;146(5):492–498. doi:10.1001/archdermatol.2009.388
56. Hao T, Meng X, Li C. A meta-analysis comparing confocal microscopy and dermoscopy in diagnostic accuracy of lentigo maligna. Skin Res Technol. 2019;srt.12821.
57. Cinotti E, Fiorani D, Labeille B, et al. The integration of dermoscopy and reflectance confocal microscopy improves the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol. 2019;33(10). doi:10.1111/jdv.15669
58. Yélamos O, Manubens E, Jain M, et al. Improvement of diagnostic confidence and management of equivocal skin lesions by integration of reflectance confocal microscopy in daily practice: prospective study in 2 referral skin cancer centers. J Am Acad Dermatol. 2019;S0190962219309697.
59. Guitera P, Pellacani G, Crotty KA, et al. The impact of in vivo reflectance confocal microscopy on the diagnostic accuracy of lentigo maligna and equivocal pigmented and nonpigmented macules of the face. J Invest Dermatol. 2010;130(8):2080–2091. doi:10.1038/jid.2010.84
60. Cinotti E, Labeille B, Debarbieux S, et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol. 2018;32(8):1284–1291. doi:10.1111/jdv.14791
61. Borsari S, Pampena R, Lallas A, et al. Clinical Indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152(10):1093. doi:10.1001/jamadermatol.2016.1188
62. Braga JCT, Macedo MP, Pinto C, et al. Learning reflectance confocal microscopy of melanocytic skin lesions through histopathologic transversal sections. PLoS One. 2013;8(12):e81205. doi:10.1371/journal.pone.0081205
63. Persechino F, De Carvalho N, Ciardo S, et al. Folliculotropism in pigmented facial macules: differential diagnosis with reflectance confocal microscopy. Exp Dermatol. 2018;27(3):227–232. doi:10.1111/exd.13487
64. Ahlgrimm-Siess V, Massone C, Scope A, et al. Reflectance confocal microscopy of facial lentigo maligna and lentigo maligna melanoma: a preliminary study. Br J Dermatol. 2009;161(6):1307–1316. doi:10.1111/j.1365-2133.2009.09289.x
65. Champin J, Perrot J-L, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40(3):247–256. doi:10.1111/dsu.12432
66. Yélamos O, Cordova M, Blank N, et al. Correlation of handheld reflectance confocal microscopy with radial video mosaicing for margin mapping of lentigo maligna and lentigo maligna melanoma. JAMA Dermatol. 2017;153(12):1278. doi:10.1001/jamadermatol.2017.3114
67. Chen C-SJ, Elias M, Busam K, Rajadhyaksha M, Marghoob AA. Multimodal in vivo optical imaging, including confocal microscopy, facilitates presurgical margin mapping for clinically complex lentigo maligna melanoma. Br J Dermatol. 2005;153(5):1031–1036.
68. Guitera P, Moloney FJ, Menzies SW, et al. Improving management and patient care in lentigo maligna by mapping with in vivo confocal microscopy. JAMA Dermatol. 2013;149(6):692. doi:10.1001/jamadermatol.2013.2301
69. Hibler BP, Yélamos O, Cordova M, et al. Handheld reflectance confocal microscopy to aid in the management of complex facial lentigo maligna. Cutis. 2017;99(5):346–352.
70. Navarrete-Dechent C, Cordova M, Aleissa S, et al. Lentigo maligna melanoma mapping using reflectance confocal microscopy correlates with staged excision: a prospective study. J Am Acad Dermatol. 2019;S0190962219331500.
71. Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115–118. doi:10.1038/nature21056
72. Marchetti MA, Codella NCF, Dusza SW, et al. Results of the 2016 international skin imaging collaboration international symposium on biomedical imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78(2):270–277.e1. doi:10.1016/j.jaad.2017.08.016
73. Safran T, Viezel-Mathieu A, Corban J, Kanevsky A, Thibaudeau S, Kanevsky J. Machine learning and melanoma: the future of screening. J Am Acad Dermatol. 2018;78(3):620–621. doi:10.1016/j.jaad.2017.09.055
74. Winkler JK, Sies K, Fink C, et al. Melanoma recognition by a deep learning convolutional neural network-performance in different melanoma subtypes and localisations. Eur J Cancer. 2020;127:21–29. doi:10.1016/j.ejca.2019.11.020
75. Celebi ME, Schaefer G, Iyatomi H, Stoecker WV. Lesion border detection in dermoscopy images. Comput Med Imaging Graph. 2009;33(2):148–153. doi:10.1016/j.compmedimag.2008.11.002
76. Tschandl P, Rosendahl C, Kittler H. The HAM10000 dataset, a large collection of multi-source dermatoscopic images of common pigmented skin lesions. Sci Data. 2018;5:180161. doi:10.1038/sdata.2018.161
77. Gareau DS, JC da R, Yagerman S, et al. Digital imaging biomarkers feed machine learning for melanoma screening. Exp Dermatol. 2017;26(7):615–618. doi:10.1111/exd.13250
78. González-Cruz C, Jofre MA, Podlipnik S, et al. Machine learning in melanoma diagnosis. Limitations About to Be Overcome. Actas Dermosifiliogr. 2020;111(4):313–316.
79. Machine Learning in Melanoma Diagnosis. Limitations About to be overcome - pubmed [internet]. [cited September 30, 2020]. Available from: https://pubmed.ncbi.nlm.nih.gov/32248945/.
80. Agarwal-Antal N, Bowen GM, Gerwels JW. Histologic evaluation of lentigo maligna with permanent sections: implications regarding current guidelines. J Am Acad Dermatol. 2002;47(5):743–748. doi:10.1067/mjd.2002.124085
81. Swetter SM, Tsao H, Bichakjian CK, et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208–250. doi:10.1016/j.jaad.2018.08.055
82. Dalton SR, Gardner TL, Libow LF, Elston DM. Contiguous lesions in lentigo maligna. J Am Acad Dermatol. 2005;52(5):859–862. doi:10.1016/j.jaad.2004.11.063
83. Megahed M, Schön M, Selimovic D, Schön MP. Reliability of diagnosis of melanoma in situ. Lancet. 2002;359(9321):1921–1922. doi:10.1016/S0140-6736(02)08741-X
84. Mataca E, Migaldi M, Cesinaro AM. Impact of dermoscopy and reflectance confocal microscopy on the histopathologic diagnosis of lentigo maligna/lentigo maligna melanoma. Am J Dermatopathol. 2018;40(12):884–889. doi:10.1097/DAD.0000000000001212
85. Menge TD, Hibler BP, Cordova MA, Nehal KS, Rossi AM. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74(6):1114–1120. doi:10.1016/j.jaad.2015.12.045
86. Massi G, LeBoit PE. Histological Diagnosis of Nevi and Melanoma [Internet].
87. Green A, Little JH, Weedon D. The diagnosis of hutchinson’s melanotic freckle (lentigo maligna) in Queensland. Pathology. 1983;15(1):33–35. doi:10.3109/00313028309061399
88. Martínez-Leboráns L, Garcías-Ladaria J, Oliver-Martínez V, Alegre de Miquel V. Lentigo maligno extrafacial. Serie de 14 casos y revisión de la literatura. Actas Dermosifiliogr. 2016;107(8):e57–e63.
89. Mishima Y. Melanosis circumscripta praecancerosa (Dubreuilh), a non-nevoid premelanoma distinct from junction nevus. J Invest Dermatol. 1960;34:361–375. doi:10.1038/jid.1960.63
90. Cohen LM. The starburst giant cell is useful for distinguishing lentigo maligna from photodamaged skin. J Am Acad Dermatol. 1996;35(6):962–968. doi:10.1016/S0190-9622(96)90121-8
91. Katz SK, Guitart J. Starburst giant cells in benign nevomelanocytic lesions. J Am Acad Dermatol. 1998;38(2):283. doi:10.1016/S0190-9622(98)70253-1
92. McGuire LK, Disa JJ, Lee EH, Busam KJ, Nehal KS. Melanoma of the lentigo maligna subtype: diagnostic challenges and current treatment paradigms. Plast Reconstr Surg. 2012;129(2):288e–99e. doi:10.1097/PRS.0b013e31823aeb72
93. Fensterseifer GS, Lodi AP, Dantas ML, Boff AL, Lovatto L. Lentigo maligna of the face: the importance of clinical, dermoscopic, and histological correlation. Dermatol Pract Concept. 2019;9(4):292–294. doi:10.5826/dpc.0904a08
94. Moreno A, Manrique-Silva E, Virós A, et al. Histologic features associated with an invasive component in lentigo maligna lesions. JAMA Dermatol. 2019;155(7):782. doi:10.1001/jamadermatol.2019.0467
95. Farrahi F, Egbert BM, Swetter SM. Histologic similarities between lentigo maligna and dysplastic nevus: importance of clinicopathologic distinction. J Cutan Pathol. 2005;32(6):405–412. doi:10.1111/j.0303-6987.2005.00355.x
96. Chen LL, Jaimes N, Barker CA, Busam KJ, Marghoob AA. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68(5):825–833. doi:10.1016/j.jaad.2012.10.041
97. Robinson M, Primiero C, Guitera P, et al. Evidence-based clinical practice guidelines for the management of patients with lentigo maligna. Dermatology (Basel). 2019;1–6.
98. De Luca EV, Perino F, Di Stefani A, Coco V, Fossati B, Peris K. Lentigo maligna: diagnosis and treatment. G Ital Dermatol Venereol. 2020;155(2):179–189. doi:10.23736/S0392-0488.18.06003-0
99. Fosko SW, Navarrete-Dechent CP, Nehal KS. Lentigo maligna—challenges, observations, imiquimod, confocal microscopy, and personalized treatment. JAMA Dermatol. 2018;154(8):879. doi:10.1001/jamadermatol.2018.0531
100. Mori S, Navarrete-Dechent C, Petukhova TA, et al. Tumor board conferences for multidisciplinary skin cancer management: a survey of US cancer centers. J Natl Compr Canc Netw. 2018;16(10):1209–1215. doi:10.6004/jnccn.2018.7044
101. National Comprehensive Cancer Network. Cutaneous melanoma (version 1.2020 December 19, 2019). Available from: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf.
102. Hazan C, Dusza SW, Delgado R, Busam KJ, Halpern AC, Nehal KS. Staged excision for lentigo maligna and lentigo maligna melanoma: a retrospective analysis of 117 cases. J Am Acad Dermatol. 2008;58(1):142–148. doi:10.1016/j.jaad.2007.09.023
103. Huilgol SC, Selva D, Chen C, et al. Surgical margins for lentigo maligna and lentigo maligna melanoma: the technique of mapped serial excision. Arch Dermatol. 2004;140(9):1087–1092. doi:10.1001/archderm.140.9.1087
104. Bub JL, Berg D, Slee A, Odland PB. Management of lentigo maligna and lentigo maligna melanoma with staged excision: a 5-year follow-up. Arch Dermatol. 2004;140(5):552–558. doi:10.1001/archderm.140.5.552
105. Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. J Am Acad Dermatol. 2011;65(5):1032–1047. doi:10.1016/j.jaad.2011.04.031
106. Barlow RJ, White CR, Swanson NA. Mohs’ micrographic surgery using frozen sections alone may be unsuitable for detecting single atypical melanocytes at the margins of melanoma in situ. Br J Dermatol. 2002;146(2):290–294. doi:10.1046/j.1365-2133.2002.04661.x
107. Cohen LM, McCall MW, Hodge SJ, Freedman JD, Callen JP, Zax RH. Successful treatment of lentigo maligna and lentigo maligna melanoma with Mohs’ micrographic surgery aided by rush permanent sections. Cancer. 1994;73(12):2964–2970.
108. Zitelli JA, Moy RL, Abell E. The reliability of frozen sections in the evaluation of surgical margins for melanoma. J Am Acad Dermatol. 1991;24(1):102–106. doi:10.1016/0190-9622(91)70020-3
109. Zitelli JA, Brown C, Hanusa BH. Mohs micrographic surgery for the treatment of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37(2 Pt 1):236–245. doi:10.1016/S0190-9622(97)80131-4
110. Bricca GM, Brodland DG, Ren D, Zitelli JA. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52(1):92–100. doi:10.1016/j.jaad.2004.08.038
111. Sharma AN, Foulad DP, Doan L, Lee PK, Atanaskova Mesinkovska N. Mohs surgery for the treatment of lentigo maligna and lentigo maligna melanoma - a systematic review. J Dermatolog Treat. 2019;1–7. doi:10.1080/09546634.2019.1690624
112. Ellison PM, Zitelli JA, Brodland DG. Mohs micrographic surgery for melanoma: a prospective multicenter study. J Am Acad Dermatol. 2019;81(3):767–774. doi:10.1016/j.jaad.2019.05.057
113. Böhler-Sommeregger K, Schuller-Petrovic S, Neumann R, Müller E, Rhodes R. Cryosurgery of lentigo maligna. Plast Reconstr Surg. 1992;90(3):
114. Kuflik EG, Gage AA. Cryosurgery for lentigo maligna. J Am Acad Dermatol. 1994;31(1):75–78. doi:10.1016/S0190-9622(94)70139-3
115. Gage AA, Meenaghan MA, Natiella JR, Greene GW. Sensitivity of pigmented mucosa and skin to freezing injury. Cryobiology. 1979;16(4):348–361. doi:10.1016/0011-2240(79)90048-8
116. Stevenson O, Ahmed I. Lentigo maligna: prognosis and treatment options. Am J Clin Dermatol. 2005;6(3):151–164. doi:10.2165/00128071-200506030-00002
117. de Moraes AM, Pavarin LB, Herreros F, de Aguiar Michelman F, Velho PENF, de Souza EM. Cryosurgical treatment of lentigo maligna. J Dtsch Dermatol Ges. 2007;5(6):477–480. doi:10.1111/j.1610-0387.2007.06331.x
118. Gaitanis G, Bassukas IM. Long-term outcomes of imiquimod-treated lentigo maligna: add on cryosurgery to induce inflammation and increase efficacy? Clin Exp Dermatol. 2019.
119. Matas-Nadal C, Sòria X, García-de-la-Fuente MR, et al. Immunocryosurgery as monotherapy for lentigo maligna or combined with surgical excision for lentigo maligna melanoma. J Dermatol. 2018;45(5):564–570. doi:10.1111/1346-8138.14248
120. Schön MP, Schön M. Imiquimod: mode of action. Br J Dermatol. 2007;157(Suppl 2):8–13. doi:10.1111/j.1365-2133.2007.08265.x
121. Donigan JM, Hyde MA, Goldgar DE, Hadley ML, Bowling M, Bowen GM. Rate of recurrence of lentigo maligna treated with off-label neoadjuvant topical imiquimod, 5%, cream prior to conservatively staged excision. JAMA Dermatol. 2018;154(8):885–889. doi:10.1001/jamadermatol.2018.0530
122. Cotter MA, McKenna JK, Bowen GM. Treatment of lentigo maligna with imiquimod before staged excision. Dermatol Surg. 2008;34(2):147–151. doi:10.1097/00042728-200802000-00002
123. Fleming CJ, Bryden AM, Evans A, Dawe RS, Ibbotson SH. A pilot study of treatment of lentigo maligna with 5% imiquimod cream. Br J Dermatol. 2004;151(2):485–488. doi:10.1111/j.1365-2133.2004.05983.x
124. Kirtschig G, van Meurs T, van Doorn R. Twelve-week treatment of lentigo maligna with imiquimod results in a high and sustained clearance rate. Acta Derm Venereol. 2015;95(1):83–85. doi:10.2340/00015555-1861
125. Ly L, Kelly JW, O’Keefe R, et al. Efficacy of imiquimod cream, 5%, for lentigo maligna after complete excision: a study of 43 patients. Arch Dermatol. 2011;147(10):1191–1195. doi:10.1001/archdermatol.2011.260
126. Tio DCKS, van Montfrans C, Ruijter CGH, Hoekzema R, Bekkenk MW. Effectiveness of 5% topical imiquimod for lentigo maligna treatment. Acta Derm Venereol. 2019;99(10):884–888. doi:10.2340/00015555-3241
127. Halse H, Caramia F, McLean CA, et al. A Distinct pretreatment immune gene signature in lentigo maligna is associated with imiquimod response. J Invest Dermatol. 2019.
128. Tio D, van der Woude J, Prinsen CAC, Jansma EP, Hoekzema R, van Montfrans C. A systematic review on the role of imiquimod in lentigo maligna and lentigo maligna melanoma: need for standardization of treatment schedule and outcome measures. J Eur Acad Dermatol Venereol. 2017;31(4):616–624. doi:10.1111/jdv.14085
129. Hyde MA, Hadley ML, Tristani-Firouzi P, Goldgar D, Bowen GM. A randomized trial of the off-label use of imiquimod, 5%, cream with vs without tazarotene, 0.1%, gel for the treatment of lentigo maligna, followed by conservative staged excisions. Arch Dermatol. 2012;148(5):592–596. doi:10.1001/archdermatol.2012.270
130. Swetter SM, Chen FW, Kim DD, Egbert BM. Imiquimod 5% cream as primary or adjuvant therapy for melanoma in situ, lentigo maligna type. J Am Acad Dermatol. 2015;72(6):1047–1053. doi:10.1016/j.jaad.2015.02.008
131. Fisher GH, Lang PG. Treatment of melanoma in situ on sun-damaged skin with topical 5% imiquimod cream complicated by the development of invasive disease. Arch Dermatol. 2003;139(7):945–947. doi:10.1001/archderm.139.7.945
132. Farshad A, Burg G, Panizzon R, Dummer R. A retrospective study of 150 patients with lentigo maligna and lentigo maligna melanoma and the efficacy of radiotherapy using Grenz or soft X-rays. Br J Dermatol. 2002;146(6):1042–1046. doi:10.1046/j.1365-2133.2002.04750.x
133. Hedblad M-A, Mallbris L. Grenz ray treatment of lentigo maligna and early lentigo maligna melanoma. J Am Acad Dermatol. 2012;67(1):60–68. doi:10.1016/j.jaad.2011.06.029
134. Fogarty GB, Hong A, Economides A, Guitera P. Experience with treating lentigo maligna with definitive radiotherapy. Dermatol Res Pract. 2018;2018:7439807. doi:10.1155/2018/7439807
135. Harwood AR. Conventional fractionated radiotherapy for 51 patients with lentigo maligna and lentigo maligna melanoma. Int J Radiat Oncol Biol Phys. 1983;9(7):1019–1021. doi:10.1016/0360-3016(83)90391-7
136. Fogarty GB, Hong A, Scolyer RA, et al. Radiotherapy for lentigo maligna: a literature review and recommendations for treatment. Br J Dermatol. 2014;170(1):52–58. doi:10.1111/bjd.12611
137. Read T, Noonan C, David M, et al. A systematic review of non-surgical treatments for lentigo maligna. J Eur Acad Dermatol Venereol. 2016;30(5):748–753.
138. Córdoba F, Braathen LR, Weissenberger J, et al. 5-aminolaevulinic acid photodynamic therapy in a transgenic mouse model of skin melanoma. Exp Dermatol. 2005;14(6):429–437. doi:10.1111/j.0906-6705.2005.00303.x
139. Karam A, Simon M, Lemasson G, Misery L. The use of photodynamic therapy in the treatment of lentigo maligna. Pigment Cell Melanoma Res. 2013;26(2):275–277. doi:10.1111/pcmr.12044
140. Räsänen JE, Neittaanmäki N, Jeskanen L, Pölönen I, Snellman E, Grönroos M. Ablative fractional laser-assisted photodynamic therapy for lentigo maligna: a prospective pilot study. J Eur Acad Dermatol Venereol. 2019.
141. Lee H, Sowerby LJ, Temple CL, Yu E, Moore CC. Carbon dioxide laser treatment for lentigo maligna: a retrospective review comparing 3 different treatment modalities. Arch Facial Plast Surg. 2011;13(6):398–403. doi:10.1001/archfacial.2011.66
142. Gillespie SK, Zhang XD, Hersey P. Ingenol 3-angelate induces dual modes of cell death and differentially regulates tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in melanoma cells. Mol Cancer Ther. 2004;3(12):1651–1658.
143. Mansuy M, Nikkels-Tassoudji N, Arrese JE, Rorive A, Nikkels AF. Recurrent in situ melanoma successfully treated with ingenol mebutate. Dermatol Ther (Heidelb). 2014;4(1):131–135. doi:10.1007/s13555-014-0051-4
144. Montaudié H, Le Duff F, Butori C, et al. Ingenol mebutate to treat lentigo maligna of the head (face and scalp): a prospective, multicenter, single-arm Phase 2 trial indicates no benefit. J Am Acad Dermatol. 2020;82(3):731–733. doi:10.1016/j.jaad.2019.07.035
145. Connolly KL, Hibler BP, Lee EH, Rossi AM, Busam KJ, Nehal KS. Locally recurrent lentigo maligna and lentigo maligna melanoma: characteristics and time to recurrence after surgery. Dermatol Surg. 2017;43(6):792–797. doi:10.1097/DSS.0000000000001118
146. Guitera P, Haydu LE, Menzies SW, et al. Surveillance for treatment failure of lentigo maligna with dermoscopy and in vivo confocal microscopy: new descriptors. Br J Dermatol. 2014;170(6):1305–1312. doi:10.1111/bjd.12839
147. Star P, Guitera P. Lentigo maligna, macules of the face, and lesions on sun-damaged skin. Dermatol Clin. 2016;34(4):421–429. doi:10.1016/j.det.2016.05.005
148. Nadiminti H, Scope A, Marghoob AA, Busam K, Nehal KS. Use of reflectance confocal microscopy to monitor response of lentigo maligna to nonsurgical treatment. Dermatol Surg. 2010;36(2):177–184. doi:10.1111/j.1524-4725.2009.01421.x
149. Alarcon I, Carrera C, Alos L, Palou J, Malvehy J, Puig S. In vivo reflectance confocal microscopy to monitor the response of lentigo maligna to imiquimod. J Am Acad Dermatol. 2014;71(1):49–55. doi:10.1016/j.jaad.2014.02.043
150. Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: diagnostics – update 2019. Eur J Cancer. 2020;126:141–158. doi:10.1016/j.ejca.2019.11.014
151. Akay BN, Kocyigit P, Heper AO, Erdem C. Dermatoscopy of flat pigmented facial lesions: diagnostic challenge between pigmented actinic keratosis and lentigo maligna: dermatoscopy of pigmented actinic keratosis. Br J Dermatol. 2010;163(6):1212–1217. doi:10.1111/j.1365-2133.2010.10025.x
152. Tanaka M, Sawada M, Kobayashi K. Key points in dermoscopic differentiation between lentigo maligna and solar lentigo: dermoscopy of lentigo maligna. J Dermatol. 2011;38(1):53–58. doi:10.1111/j.1346-8138.2010.01132.x
153. Braun RP. Dermoscopy of pigmented seborrheic keratosis: a Morphological Study. Arch Dermatol. 2002;138(12):1556.
154. Braun RP, Rabinovitz H, Oliviero M, Kopf AW, Saurat JH. Dermoscopic diagnosis of seborrheic keratosis. Clin Dermatol. 2002;20(3):270–272. doi:10.1016/S0738-081X(02)00228-6
155. Kopf AW, Rabinovitz H, Marghoob A, et al. “Fat fingers:” a clue in the dermoscopic diagnosis of seborrheic keratoses. J Am Acad Dermatol. 2006;55(6):1089–1091. doi:10.1016/j.jaad.2006.08.028
156. Bugatti L, Filosa G. Dermoscopy of lichen planus-like keratosis: a model of inflammatory regression. J Eur Acad Dermatol Venerol. 2007;21(10):1392–1397. doi:10.1111/j.1468-3083.2007.02296.x
157. Zaballos P, Ara M, Puig S, Malvehy J. Clinical and dermoscopic image of an intermediate stage of regressing seborrheic keratosis in a lichenoid keratosis. Dermatol Surg. 2006;31(1):102–103. doi:10.1111/j.1524-4725.2005.31017
158. Ralph P Braun. Two step algorithm. dermoscopedia; 2017. Available from: https://dermoscopedia.org/File:2step_4.jpg. Accessed November 3, 2020.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.