Back to Journals » International Journal of Women's Health » Volume 12
Laparoscopic Hysterectomy and Bilateral Salpingectomy in a Patient with Microduplication Syndrome (20p13p12.1) and a Bicornuate Uterus: An Unreported Association
Authors Pachajoa H , Perafan L , Ramos I , Escobar ÁJ
Received 14 March 2020
Accepted for publication 23 May 2020
Published 25 August 2020 Volume 2020:12 Pages 675—679
DOI https://doi.org/10.2147/IJWH.S253885
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Everett Magann
Harry Pachajoa,1,2 Lina Perafan,1,3 Isabella Ramos,1,4 Álvaro J Escobar4
1Centro de Investigaciones en Anomalías Congénitas y Enfermedades Raras (CIACER), Faculty of Health Sciences, Universidad Icesi, Cali, Colombia; 2Department of Medical Genetics, Fundación Valle del Lili, Cali, Colombia; 3Hospitalization Service, Fundación Valle del Lili, Cali, Colombia; 4Department of Obstetrics and Gynecology, Fundación Valle del Lili, Cali, Colombia
Correspondence: Harry Pachajoa Tel +57 2 331 90 90, Extension: 7486
Fax +57 2 3319090
Email [email protected]
Abstract: Trisomy 20p is a chromosomal anomaly resulting from whole or partial duplication of the short arm of chromosome 20. It is a rarely reported syndrome and it is estimated that there are only a few cases of this condition worldwide, which hampers the phenotypic characterization of this entity. Conversely, müllerian anomalies include a group of congenital malformations of the uterus, vagina, cervix, and fallopian tubes resulting from alterations in the embryological development of the müllerian ducts. We report a case of pure trisomy 20p diagnosed using array comparative genomic hybridization (CGH) accompanied by a müllerian anomaly in a female patient with abnormal growth pattern, round face, coarse hair, broad nose, long palpebral fissure, epicanthus, and megaureter.
Keywords: trisomy, trisomy 20p, chromosome duplication, duplication 20p, comparative genomic hybridization, uterine anomalies
Introduction
Trisomy 20p or trisomy of the short arm of chromosome 20 is an infrequent chromosomal anomaly caused by the duplication of a fraction of the short arm of this chromosome, resulting in an extra copy of genetic material.1 This is an extremely rare genetic condition, with roughly 40 cases reported worldwide, which is not sufficient to characterize fully the specific phenotypes of patients. This is because this anomaly often presents simultaneously with additional chromosomal alterations, with monosomy of another region or chromosome being the comorbidity described more broadly in the literature.1,2 There are a few case reports of pure trisomy 20p,3,4 which, together with the limited number of molecularly characterized cases, explains the complexity of the definition of a syndrome such as trisomy 20p.2
The müllerian anomalies include a wide range of congenital malformations that arise from alterations during the embryogenesis of the müllerian ducts and affect the morphology of the uterus, cervix, fallopian tubes, and vagina. These anomalies have an estimated prevalence of 4%–7% and are usually associated with an increased risk of obstetric complications, morbidity, and mortality.5,6 A bicornuate uterus is a müllerian anomaly that is characterized by the presence of an external cleft with a size >1 cm located in the midline of the uterine fundus and results in a partial or complete division of the uterine corpus.5
In this review, we present a case of a patient with trisomy 20p diagnosed using comparative genomic hybridization (array-CGH) in coexistence with a müllerian anomaly. This association was not reported previously. Moreover, abnormal uterine bleeding (AUB) secondary to von Willebrand disease was observed, thus requiring a gynecological laparoscopy procedure, which led to the finding of a uterus with a bicornuate morphology.
Case Report
We report a case of a female patient who was the first child of non-consanguineous parents (mother, 45 years old; and father, 43 years old) with a healthy pregnancy and no relevant data regarding antenatal care visits. At birth, the child exhibited fetal macrosomia, weighed 6000 g (P > 99), and measured 60 cm (P > 99); no data pertaining to head circumference were available. During the first 2 months of life, she presented multiple urinary tract infections, leading to the diagnosis of right megaureter, which was surgically intervened at 20 months of age. At 3 years old, she was referred to pediatric neurology who diagnoses mild mental retardation and sluggish cognitive tempo.
At the age of 10, she reached menarche, debuting with hypermenorrhea, which was treated with oral combined contraceptives (levonorgestrel/ethinylestradiol) and tranexamic acid cycles. Ferropenic anemia was documented later, which required clinical management with parenteral iron therapy because of intolerance to oral therapy and referral to a pediatric hematologist, who proposed that her clinical course was compatible with von Willebrand Disease (VWD). VWD type I was confirmed at the age of 15. Because of persistent overweight, she was referred to a pediatric endocrinologist; however, no underlying endocrine disorders where found.
One year later, during a visit to a pediatric nephrologist, a ureteral re-obstruction was documented on abdominal magnetic resonance imaging (MRI), together with the finding of a bicornuate unicollis uterus. She underwent pyeloplasty with the previous administration of Factor VIII and von Willebrand factor (FvW).
Because of her past medical history of disorders affecting multiple organ systems, she was referred to the Medical Genetics department at the age of 17. Her medical findings included stage 1 obesity (weight, 87 kg (P > 95); height, 1.65 m (P34); and body mass index (BMI), 31.96 kg/m2) and a head circumference of 59 cm (P > 99) (Figure 1). Physical examination evidenced a round face, broad nose, xanthelasmas in both lower lids, epicanthal fold, long palpebral fissures, an inner canthal distance of 3.5 cm (P75–97), and an outer canthal distance of 11.5 cm (P > 97) (Figures 2 and 3); as well as an abundant adipose panicle, speech delay, poor coordination, coarse hands with a length of 19 cm, and a third finger measuring 8.5 cm. Brain MRI was normal and the initial karyotype evidenced the presence of 46,XX,add(5)(q35); hence, array-CGH was performed (Method: CMA (Oligo V8.1.1) Slide 253568913808–1). The previous test demonstrated a gain of 20p13p12.1 (102422–15240554)x3, which led to the establishment of a diagnosis of trisomy 20p.
 |
Figure 1 Patient’s phenotype at age 17. |
 |
Figure 2 Patient’s round face. |
 |
Figure 3 Increased outer canthal distance, xanthelasmas, and epicanthal folds. |
Because of the lack of control of the patient’s AUB, which persisted with each menstrual cycle, reducing her quality of life; she was evaluated by a gynecologist, who proposed programming a multidisciplinary medical board with the participation of genetics and hematology too. The goal was to offer and analyze various therapeutic strategies for persistent AUB. However, after receiving the explanation of the different advantages and disadvantages, the patient opted for the definitive laparoscopic approach and additionally, she asked for a salpingectomy because of her own decision of not having children in the future. It was a consented and informed decision, discussed with the presence of the patient’s legal representative.
At the age of 20, and with the previous administration of Factor VIII and FvW, a laparoscopic total hysterectomy with bilateral salpingectomy was performed using advanced bipolar energy and closing the cupula with barbed sutures (Figure 4). The operative time was 42 min. A heart-shaped uterus measuring 12 x 7 x 3 cm and weighing 164.1 g was removed. The procedure and the follow-up were uneventful. The pathological examination of the specimen confirmed the diagnosis of bicornuate uterus, corresponding to the previous radiological findings (Figure 5).
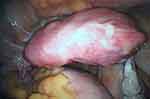 |
Figure 4 Laparoscopic total hysterectomy. |
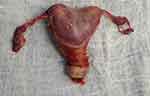 |
Figure 5 Bicornuate uterus. |
Currently, the patient is being treated by a multidisciplinary team with regular follow-up visits.
Discussion
The phenotypic characterization of the duplication syndrome or trisomy 20p has not been successful because of the low incidence of this condition and the strong association with other chromosomal anomalies. The early detection of trisomy 20p is as important for its diagnosis as it is for proper genetic counseling.7 The severity of the symptoms of trisomy 20p depends on the extent of the duplication, ie, larger duplications are associated with more severe symptoms.7 To date, the most frequent findings correspond to mental retardation, developmental and speech delays, coordination impairment, short neck, coarse hands, and facial dysmorphism (including round face with prominent cheeks, broad nose, arched eyebrows, and thick hair).3,7–11 All of the above correspond to the clinical findings of the patient.
Additional traits that are frequently associated with this condition include osteoporosis; dental, cardiac, and vertebral anomalies (none of which were present in this patient); and unspecific renal anomalies,3,4 which could explain the patient’s right megaureter. As to the patient’s growth, most patients with trisomy 20p present a normal birth weight and tend to maintain it during adult life, which delays diagnosis.4,7 Unlike previous reports, this patient had a tendency toward higher percentiles according to her age (P > 99 at birth and P > 95 at the time of diagnosis).
Furthermore, VWD has been associated, in all of its subtypes, with mutations in the VWF gene, which is located on 12p13.31 (OMIM: 613160) and is not related with the trisomy 20p phenotype. Nonetheless, it is worth noting that there are no reports in the literature describing VWD coexisting with trisomy 20p, other than the fact that the latter does not change the clinical treatment of this hematologic condition.
Moreover, no müllerian anomalies have been linked to trisomy 20p or described as the common phenotypical traits associated with this chromosomal anomaly. Most women with müllerian anomalies have 46 XX karyotypes, and it has been estimated that only 7.7% of them have an abnormal karyotype.12 Genes implicated in the development of müllerian anomalies have been reported, such as HNF1B and HOXA13, which are located on chromosome 17q13 and 7p12, respectively, and not on chromosome 20. Although many of these anomalies have been associated with certain syndromes, most of them are not likely to be syndromic.13
The most common müllerian anomalies are uterine malformations, with a bicornuate uterus being the most common of them, as it accounts for 37% of the cases. This anomaly is the result of an incomplete fusion of the müllerian ducts.13 Moreover, uterine malformations have been correlated (20%–30%) with urological and renal anomalies,12,14 thus contributing, together with the phenotype of trisomy 20p, to the urological findings of the patient.
This patient is one of the few cases of pure trisomy 20p described in the literature with molecular characterization through array-CGH. As stated above, the patient shared many of the phenotypic traits described in the previous literature and exhibits the comorbid conditions that have not been associated with the chromosomal anomaly.
Conclusion
In conclusion, trisomy 20p is an unusual genetic condition with a limited number of cases around the world which makes difficult its clinical suspicion and thus, the diagnoses. This case report broadens this entity’s clinical spectrum; however, additional cases are required for a proper and more precise phenotypical and genotypic characterization.
Consent and Ethics
Written informed consent has been provided by the patient and her parents, to have the case details and any accompanying images published. This case report was approved by the Institutional Review Board (IRB) in the Fundación Valle del Lili.
Acknowledgment
We thank the family for their interest and cooperation with the publication of this report.
Disclosure
The authors report no conflicts of interest for this work.
References
1. de Ravel TJ, Vermeesch JR, Fryns JP. De novo interstitial tandem duplication of chromosome 20p12.1p13. Am J Med Genet A. 2003;117A(1):76–79. doi:10.1002/ajmg.a.10825
2. Bartolini L, Sartori S, Lenzini E, et al. De novo trisomy 20p characterized by array comparative genomic hybridization: report of a novel case and review of the literature. Gene. 2013;524(2):368–372. doi:10.1016/j.gene.2013.04.033
3. Sidwell RU, Pinson MP, Gibbons B, et al. Pure trisomy 20p resulting from isochromosome formation and whole arm translocation. J Med Genet. 2000;37(6):454–458. doi:10.1136/jmg.37.6.454
4. Grammatico P, Cupilari F, Di Rosa C, Falcolini M, Del Porto G. 20 p duplication as a result of parental translocation: familial case report and a contribution to the clinical delineation of the syndrome. Clin Genet. 1992;41(6):285–289. doi:10.1111/j.1399-0004.1992.tb03398.x
5. Grimbizis GF, Gordts S, Di Spiezio Sardo A, et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod. 2013;28(8):2032–2044. doi:10.1093/humrep/det098
6. Reyes-Muñoz E, Vitale SG, Alvarado-Rosales D, et al. Müllerian anomalies prevalence diagnosed by hysteroscopy and laparoscopy in mexican infertile women: results from a cohort study. Diagnostics (Basel). 2019;9:4.
7. Choi J, Yoon SY, Park BG, Eun BL, Kim M, Kwon JA. De novo pure trisomy 20p: report of a novel case of a marker chromosome and literature review. Ann Lab Med. 2020;40(3):277–280. doi:10.3343/alm.2020.40.3.277
8. Oppenheimer S, Dignan P, Soukup S. Partial trisomy 20p: familial occurrence. Am J Med Genet. 2000;95(4):316–319. doi:10.1002/1096-8628(20001211)95:4<316::AID-AJMG4>3.0.CO;2-Z
9. Wieczorek D, Bartsch O, Gillessen-Kaesbach G. Distal monosomy 18p/distal trisomy 20p–a recognizable facial phenotype? Am J Med Genet A. 2003;120A(3):429–433. doi:10.1002/ajmg.a.20060
10. Molina-Gomes D, Nebout V, Daikha-Dahmane F, Vialard F, Ville Y, Selva J. Partial trisomy 20p resulting from recombination of a maternal pericentric inversion: case report of a prenatal diagnosis by chorionic villus sampling. Prenat Diagn. 2006;26(3):239–241. doi:10.1002/pd.1389
11. Leclercq S, Maincent K, Baverel F, et al. Molecular cytogenetic characterization of the first reported case of inv dup del 20p compatible with a U-type exchange model. Am J Med Genet A. 2009;149A(3 Mar):437–445. doi:10.1002/ajmg.a.32640
12. Lin PC, Bhatnagar KP, Nettleton GS, Nakajima ST. Female genital anomalies affecting reproduction. Fertil Steril. 2002;78(5):899–915. doi:10.1016/S0015-0282(02)03368-X
13. Bhagavath B, Ellie G, Griffiths KM, et al. Uterine malformations: an update of diagnosis, management, and outcomes. Obstet Gynecol Surv. 2017;72(6):377–392. doi:10.1097/OGX.0000000000000444
14. Oppelt P, von Have M, Paulsen M, et al. Female genital malformations and their associated abnormalities. Fertil Steril. 2007;87(2):335–342. doi:10.1016/j.fertnstert.2006.07.1501
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
