Back to Journals » Clinical Interventions in Aging » Volume 10
Is carotid artery evaluation necessary for primary prevention in asymptomatic high-risk patients without atherosclerotic cardiovascular disease?
Authors Kim G, Youn H, Choi Y , Jung HO, Chung WS, Kim C
Received 21 March 2015
Accepted for publication 7 May 2015
Published 7 July 2015 Volume 2015:10 Pages 1111—1119
DOI https://doi.org/10.2147/CIA.S85216
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Walker
GeeHee Kim,1 Ho-Joong Youn,2 Yun-Seok Choi,2 Hae Ok Jung,2 Wook Sung Chung,2 Chul-Min Kim1
1Department of Internal Medicine, St Vincent’s Hospital, The Catholic University of Korea, Suwon, 2Division of Cardiology, Department of Internal Medicine, School of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Objective: Routine measurement of the carotid intima–media thickness is not recommended in recent clinical practice guidelines for risk assessment of the first atherosclerotic cardiovascular disease (ASCVD) event (the definition of which includes acute coronary syndromes, a history of myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, stroke, transient ischemic attack, or peripheral arterial disease presumed to be of atherosclerotic origin). The aim of the present study was to elucidate the role of carotid artery evaluation for primary prevention of ASCVD in asymptomatic high-risk patients visiting a teaching hospital.
Methods: Eight hundred seventy-three patients (487 male [55.8%], mean age 59.4±11.5 years) who were statin-naive and without ASCVD, which was proven by coronary angiography or coronary CT angiography, were enrolled in this study. The patients underwent carotid scanning in the Medical Department of St Mary’s Hospital from September 2003 to March 2009. ASCVD outcomes were evaluated for median follow-up of 1,402 days.
Results: A total of 119 participants experienced ASCVD events. In multivariate Cox regression analysis, age (hazard ratio [HR] =1.026, 95% confidence interval [CI] =1.002–1.050, P=0.033), history of smoking (HR =1.751, 95% CI =1.089–2.815, P=0.021), statin therapy (HR =0.388, 95% CI =0.205–0.734, P=0.004), and carotid plaques (HR =1.556, 95% CI =1.009–2.400, P=0.045) were associated with ASCVD events. In middle-aged group (45≤ age <65, n=473), history of smoking (HR =1.995, 95% CI =1.142–3.485, P=0.015), statin therapy (HR =0.320, 95% CI =0.131–0.780, P=0.012), and carotid plaques (HR =1.993, 95% CI =1.116–3.560, P=0.020) were associated with ASCVD events.
Conclusion: The presence of carotid plaques, history of smoking, and statin therapy might be important factors for primary prevention of ASCVD in asymptomatic high-risk patients, especially in middle-aged patients. Therefore, the results suggest that carotid artery parameters may have an additional predictive value for primary prevention of ASCVD in the middle-aged high-risk patients.
Keywords: carotid plaque, atherosclerotic cardiovascular event, primary prevention, cholesterol-lowering drug therapy, asymptomatic high-risk patients
Introduction
Research has indicated that the presence of carotid plaque is related to cardiovascular death or nonfatal acute myocardial infarction (MI) in patients with stable angina; however, carotid intima–media thickness (CIMT) is a weak predictor of events.1 Recently, it has been reported that carotid plaque is a strong predictor of future cardiac death and a major adverse cardiac event in patients with coronary artery disease (CAD) confirmed by coronary angiography (CAG).2 Furthermore, CIMT measurement and detection of carotid plaques by ultrasonography are simple and safe methods, use no radiation, and are inexpensive tests to indirectly examine the presence of coronary atherosclerosis.3
In a meta-analysis, the addition of CIMT measurements to the Framingham Risk Score was associated with small improvements in the 10-year risk prediction of first-time MI or stroke, but this improvement was unlikely to be clinically significant.4,5 Furthermore, in 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines for cardiovascular risk assessment indicated that CIMT was not recommended for use in clinical practice as a routine measure of first atherosclerotic cardiovascular disease (ASCVD) event risk assessment.6 However, it has not yet been considered which patient groups could benefit from carotid evaluation for risk assessment of subclinical atherosclerosis.
In the 2013 ACC/AHA guidelines for blood cholesterol treatment to reduce ASCVD in adults, four major statin benefit groups were identified.7 The statin benefit groups included patients 1) with clinical ASCVD; 2) with primary elevations of low-density lipoprotein (LDL) ≥190 mg/dL; 3) with diabetes mellitus (DM) aged 40–75 years with LDL cholesterol (LDL-C) 70–189 mg/dL and without clinical ASCVD; and 4) without clinical ASCVD or DM with LDL-C 70–189 mg/dL and an estimated 10-year ASCVD risk >7.5%. In previous studies, the authors did not discuss the effect of statin therapy after carotid evaluation for prevention in the study population, including statin benefit groups. Additionally, it has not yet been determined which individual patient groups could benefit from carotid evaluation for risk assessment and primary prevention of ASCVD.
The aim of the present study was to elucidate the role of carotid artery evaluation for primary prevention of ASCVD in statin-naive and asymptomatic high-risk patients visiting a teaching hospital.
Methods
Study population
The study population consisted of 985 consecutive patients who underwent carotid scanning and CAG or coronary CT angiography (CCTA) in St Mary’s Hospital CIMT registry from September 2003 to March 2009. Inclusion criteria were age 18 years and above and no chest pain with two or more cardio-cerebrovascular risk factors. High-risk patients were defined as patients with 1) DM or 2) an estimated 10-year ASCVD risk >7.5%7 among statin benefit groups. A CCTA was performed in a patient to evaluate cardiac risk or noncardiac perioperative risk. CAG was also performed when necessary, that is, when CCTA was not performed or when a significant stenosis in CCTA was shown. Additionally, there were 985 patients recruited for this study without a prior history of statin use or ASCVD proven by CCTA or CAG. ASCVD was defined as acute coronary syndromes (including ST elevation MI, non-ST elevation MI, and unstable angina), or a history of MI, stable or unstable angina, coronary or other arterial revascularization, stroke, TIA, or peripheral arterial disease (PAD; defined as ankle–brachial index <0.9, using VP-1000; Omron Healthcare, Kyoto, Japan) presumed to be of atherosclerotic origin. A CAG (n=618, 62.7%) or CCTA (n=367, 37.3%) was performed to evaluate cardiac risk in asymptomatic high-risk patients (Figure 1). The baseline demographic, clinical data were gathered by trained research technicians. The angiographic data were collected and analyzed by cardiologists blinded to the carotid data. After the patients were enrolled, we treated high blood cholesterol in patients according to Adult Treatment Panel III recommendations.8 According to these recommendations, 515 (60%) patients were prescribed cholesterol-lowering drug therapy, followed by statin treatment. We excluded 63 patients who were lost to follow-up, eleven patients with in-hospital events (including noncardiac death), five patients who experienced heart failure (including decreased systolic or diastolic function), and 33 patients who experienced major systemic illness (including septic shock, pneumonia). Finally, a total of 873 statin-naive subjects without ASCVD (487 male [55.8%], mean age 59.4±11.5 years) were enrolled in this study. Exclusion criteria included 1) history of findings of cardiovascular disease, including heart failure symptoms or systolic dysfunction (left ventricle ejection fraction ≤50%); significant valvular heart disease (ie, greater than mild valvular insufficiency or stenosis), hypertrophic cardiomyopathy, and evidence of CAD (defined as CAG with >50% stenosis in one or more of the major coronary arteries); 2) history of findings of cerebrovascular disease, including transient ischemic attack (TIA) or stroke confirmed by a neurologist; 3) history of findings of PAD; 4) pregnancy or lactating; and 5) major systemic illness such as chronic inflammatory disease, active malignancy, and other illnesses.
  | Figure 1 Flowchart of study population. |
Baseline evaluation included a complete history and physical examination with information regarding body mass index (calculated by weight divided by the squared height in meters), hypertension (defined as systolic pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg, based on more than three measurements or current use of antihypertensive drugs), smoking habits (including ex- and current smokers), and DM (controlled with diet, oral hypoglycemic agents, or insulin; or fasting glucose level ≥126 mg/dL or 2 hours oral glucose tolerance test ≥200 mg/dL).
There was no industry involvement in the design, execution, or analysis of the study. The relevant ethics committee approved the use of clinical data for this study, and all patients provided written informed consent (VC14RISI0114).
Clinical and biochemical assessment
Blood specimens were obtained after a 12- to 14-hour fast (8 pm–9.30 am) to reduce the influence of circadian variation. The concentrations of total cholesterol and triglyceride were measured using standard enzyme methods. High-density lipoprotein (HDL) cholesterol was assessed after very LDL with phosphotungstic acid precipitation, and LDL was calculated using the Friedewald formula. Fasting glucose levels were enzymatically examined by the hexokinase method.9 A blood sample was collected from every patient and centrifuged within 30 minutes; the serum samples were stored at -80°C, and high-sensitivity C-reactive protein was measured using an immunoturbidity assay system (Liatest; Stago, Asnières-sur-Seine, France), with an interassay variability coefficient of variation of 6.25%.10
Coronary angiography
Coronary angiogram was performed via the femoral or radial approach according to the ACC/AHA recommendations for CAG. Coronary lesions were assessed with multiple orthogonal views and visually evaluated for morphologic features similar to those reported by the ACC/AHA. Significant coronary artery stenosis was defined as a >50% reduction in the internal diameter at major epicardial coronary arteries and side branches with a diameter ≥2.5 mm.
Coronary CT angiography
Patients were instructed not to eat for at least 4 hours before the examination and to avoid coffee, tea, and tobacco. Patients with DM were asked to stop metformin for 3 days before examination. Contrast-enhanced CCTA was quantified on retrospectively electrocardiography-gated cardiac CT scans using a 64-slice MDCT (Lightspeed VCT; GE Healthcare, Milwaukee, WI, USA). The heart rates of the patients were measured 1 hour before the examination. Our CCTA protocol was applied as follows: slice collimation, 64×0.625 mm; gantry rotation time, 0.5 seconds; pitch, 0.2; scan time, 0.4 seconds; table feed, 6 mm/s; tube voltage, 120 kV; and tube current, 596 mAs. At the time of scanning, patients received 80 mL of contrast agent (Iopromide, Ultravist 300; Schering, Berlin, Germany) at a rate of 5.0 mL/s for 16 seconds, followed by 50 mL of saline solution administered intravenously at a rate of 5.0 mL/s for 10 seconds. Injection was performed through an antecubital vein via an 18-gauge catheter.
CIMT measurement
The carotid arteries were evaluated using high-resolution B-mode ultrasound with a 15-MHz linear array transducer (HP Sonos 5500; Philips, Bothell, WA, USA). Carotid arterial scanning was performed in a dark room by two certified sonographers who were blinded to all clinical information. The CIMT measurements were obtained throughout 10 mm segments across the far wall of the right common carotid arteries at the point most proximal to the carotid bifurcation with patients in the supine position with slight hyperextension and rotation of the neck to the contralateral side. To optimize the image quality, the depth control was fixed at 4 cm. The transducer frequency was set to 15 MHz during the entire analysis with an axial resolution of 0.2 mm. Measurements were acquired at the end-diastole phase (defined as the R wave of an electrocardiogram) because systolic expansion of the lumen causes thinning of CIMT. CIMT composited the intima and media layers and is defined as the distance between the luminal border of the intima and the outer border of the media. The CIMT was assessed by manually measuring each free plaque lesion thickness. Calipers were placed for individual measurements six times and then an average value was taken. The average of the CIMT values of all segments was calculated to determine the mean CIMT per patient. Carotid plaque composited atherosclerotic change and is defined as the presence of focal wall thickening at least 50% greater than that of the surrounding vessel wall or as a focal region with CIMT greater than 1.5 mm that protrudes into the lumen and is distinct from the adjacent boundary.11
Clinical follow-up
Clinical follow-up was performed for a median 1,402 (61–2,858 days), and follow-up data were obtained from all patients. All ASCVD events were adjudicated by a panel of three physicians; the physicians reviewed data collected from in-patient hospitalization, hospital records (559 patients, 64.0%), and telephone interviews (314 patients, 36.0%) by a trained researcher. When a patient could not be contacted, data were obtained by a telephone interview from his or her family members. Primary end points were Hard ASCVD, defined as the first occurrence of nonfatal MI or coronary heart disease, death or fatal or nonfatal stroke, or PAD presumed to be of atherosclerotic origin.
Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are presented as absolute and relative frequencies (%). Data for groups of ASCVD events were compared using the independent Student’s t-test for continuous variables. Categorical variables were compared using the chi-square test. The intraclass correlation coefficient (ICC) is a statistical method to measure intra- and interobserver reliabilities. Reliability was assessed by replicating measurements for 50 patients. Two certified sonographers performed carotid arterial scanning, 1 hour between the first and second measurements. The ICC assesses the consistency of multiple measurements of the same quantity. In general, the ICC ranges from 0.00 (no agreement) to 1.00 (perfect agreement). Additionally, an ICC value of 0.7–0.8 indicates strong agreement, and an ICC value >0.8 indicates excellent agreement. The ICC measures the proportion of the variance that is attributed to different observers. A 95% confidence interval (CI) was estimated for each ICC to estimate the precision and the range of the correlation.
The relationships between the ASCVD events and several risk factors were tested by univariate Cox regression analysis. Multivariate Cox regression analysis was performed to evaluate the association between ASCVD events and several risk factors based on results from univariate analysis. All statistical analyses were conducted using SAS 9.1 statistical software (SAS Institute Inc., Cary, NC, USA).
Results
Characteristics of patients
The mean age of the study subjects was 59.4±11.5 years, and 487 subjects (55.8%) were men. The baseline characteristics of the 873 patients, according to ASCVD events, are shown in Table 1. Among 873 patients, 618 (70.8%) patients underwent CAG and 255 (29.2%) patients underwent CCTA; all results indicated that there was no significant CAD. According to Adult Treatment Panel III recommendations, 515 (60%) patients required cholesterol-lowering drug therapy and were given statin therapy after recruiting for this study.8 Some patients evidenced hypertension (n=454, 52.0%), DM (n=207, 23.7%), smoking (n=209, 23.9%), atrial fibrillation (n=16, 1.8%), or carotid plaque (n=316, 36.2%). Clinical follow-up was performed for a median 1,402 (61–2,858 days). During the follow-up, there were 119 (13.1%) new-onset ASCVD events, and five (0.5%) patients had heart failure (Figure 1). Among 119 patients, 99 (83.2%) patients suffered cardiovascular death and CAD, 17 (14.3%) patients had cerebrovascular disease, and three (2.5%) patients had PAD.
Measurements of intra- or interobserver reliabilities
To determine the reliability of the CIMT measurements, we used ICC, which is a statistical method to measure intra- and interobserver reliabilities. The intra- and interobserver reliabilities for the CIMT measurements were 0.965 and 0.900, respectively.
Comparison of CIMT and plaque measurement according to ASCVD events
The carotid artery measurements of 873 patients according to ASCVD events are shown in Table 2. Patients with ASCVD events had thicker maximum and mean CIMT compared with those without ASCVD events. Additionally, patients with ASCVD events had an increased incidence of carotid artery plaques compared with those without ASCVD events (Table 2).
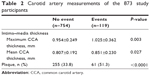  | Table 2 Carotid artery measurements of the 873 study participants |
Prognostic evaluation for primary prevention
Univariate and multivariate Cox regression analyses are shown in Table 3. The predictors of new-onset ASCVD events were age (hazard ratio [HR] =1.026, 95% CI =1.002–1.050, P=0.033), history of smoking (HR =1.751, 95% CI =1.089–2.815, P=0.021), statin therapy (HR =0.388, 95% CI =0.205–0.734, P=0.004), and carotid plaques (HR =1.556, 95% CI =1.009–2.400, P=0.045). Because there was an age-specific effect for primary prevention (Figure 2), we performed subgroup analysis according to age. We categorized the subjects into three groups: group 1 (age <45, n=91), group 2 (45≤ age <65, n=473), and group 3 (65≤ age ≤90, n=309). There were four (4.4%) ASCVD events in group 1, 61 (12.9%) ASCVD events in group 2, and 54 (17.5%) ASCVD events in group 3. In group 2, history of smoking (HR =1.995, 95% CI =1.142–3.485, P=0.015), statin therapy (HR =0.320, 95% CI =0.131–0.780, P=0.012), and carotid plaques (HR =1.993, 95% CI =1.116–3.560, P=0.020) were associated with hard ASCVD events (Table 4). In group 3, HDL (HR =0.969, 95% CI =0.841–0.998, P=0.035) was associated with hard ASCVD events.
Discussion
This study sought to examine the role of carotid artery evaluation for primary prevention of ASCVD in statin-naive and asymptomatic high-risk patients visiting a teaching hospital. The role of carotid artery evaluation for ASCVD risk assessment is controversial. Furthermore, current guidelines for cardiovascular risk assessment do not recommend CIMT for routine assessment. However, CIMT measurement and detection of carotid plaques are very useful methods and have a fluent research results. Therefore, we suggest that it is necessary to evaluate carotid artery measurement in asymptomatic high-risk patients without ASCVD.
The present study demonstrates that carotid plaque was a more important predictive factor for primary prevention of ASCVD after adjusting for the effect of treatment in groups that required statin therapy and, also, after adjusting for cardiac risk factors, including age and sex. Therefore, carotid artery measurement may prove to be useful for assessing the risk of ASCVD in groups that could benefit from statin therapy, especially in middle-aged patients.
These findings are similar to those of a previous study that reported carotid plaque is associated with cardiovascular events in the general population.12 In addition, a multiethnic study of atherosclerosis reported that carotid plaque independently predicts cardiovascular events and improves risk prediction for coronary heart disease when used in combination with Framingham risk factors.13 In this study, carotid plaque was associated with ASCVD events; this was assessed in subjects without ASCVD confirmed by several standard diagnostic tools, including CAG or CCTA. We used the new concept of ASCVD in this study because risk estimates for a more broadly based ASCVD outcome are more relevant to contemporary populations.
The results of the present study are different than those of an earlier report that indicated that CIMT, a surrogate marker of subclinical atherosclerosis, is consistently linked to cardiovascular and cerebrovascular disease.14–17 In this study, CIMT was not associated with ASCVD events after adjusting for cardiac risk factors and treatments. In this study, CIMT comprised the thickness of the intima (IT) and thickness of the media (MT) layers, and in a previous study, it was found that the increase in the IT was associated with smoking.18,19 In patients with hypertension, the CIMT increment is caused by increased MT.20 Furthermore, with advancing age and through the development of atherosclerosis, distinct changes occur in the IT and MT layers.19 Because carotid plaque causes atherosclerotic changes in the intima layer, even after adjusting for risk factors and the effect of treatments, we suggest that carotid plaque is a powerful predictive marker for primary prevention of ASCVD events in asymptomatic high-risk patients.
Justification for the Atherosclerosis Regression Treatment Extension Study reported that 2-year treatment with rosuvastatin inhibits the progression of CIMT and also improves carotid plaque composition.21 However, several previous studies did not specify the effect of medication for primary prevention of ASCVD, despite including study populations that required statin therapy. Furthermore, in current guidelines,6 DM or an estimated 10-year ASCVD risk ≥7.5% using the Pooled Cohort Equation should be used to guide statin therapy for primary prevention. Therefore, we suggest that a longitudinal study of carotid assessment needs be conducted to evaluate the long-term effects of statin therapy.
To our knowledge, CIMT increases nearly threefold between the ages of 20 and 90 (25, 26). Postmortem studies indicate that age-associated increases in thickening of carotid wall are primarily caused by intima thickening.22 In this study, in subgroup analysis, we divided the subjects into three age-groups and considered an age-associated increase in CIMT. There were rare ASCVD events in group 1. In group 3, there were higher amounts of ASCVD events than in the other groups; however, carotid plaque and statin therapy were not associated with these events, although HDL was associated with ASCVD events. We do not currently have an explanation for this observation. Therefore, we propose that additional studies assess the primary prevention of ASCVD events in elderly patients.
There were some limitations to this present study. First, this was a prospective, observational, longitudinal study and was not randomized. Additionally, we did not evaluate participants to determine a therapeutic target after statin therapy. However, without a reference to determine the therapeutic target, the presence of carotid plaques was a prognostic factor for primary prevention of ASCVD. Second, this study did not analyze automatic carotid artery measurement. Instead, we manually performed CIMT measurements, and ultrasound imaging was an operator-dependent modality. To evaluate intra- and interobserver reliabilities, we used ICC, which is a statistical method for intra- and interobserver reliability measurements. An ICC value of 0.7–0.8 indicates strong agreement, and an ICC value >0.8 indicates excellent agreement. Third, in 2013, the ACC/AHA guidelines for treatment of blood cholesterol identified four major statin benefit groups to reduce ASCVD in adults.7 However, in this study, we treated high blood cholesterol in patients according to Adult Treatment Panel III recommendations,8 because data were collected from September 2003 to March 2009. Moreover, we did not describe the effect of statin and duration, because we gathered only data for ASCVD events using hospital records or telephone interviews. Fourth, in this study, the CIMT of enrolled patients was thicker than that observed in the general Korean population.23 We hypothesized that an elevated number of patients with risk factors may be visiting the tertiary hospital for screening.
Our CIMT registry was designed to evaluate real-world outcomes in consecutive participants without ASCVD. This large, observational registry included clinical, CAG or CCTA, and long-term outcome patient data. Although enrolled patients of our registry were selected based on the need to undergo CAG or CCTA for the evaluation of CVD in asymptomatic high-risk patients, we suggest that carotid artery assessment is a noninvasive, useful tool for patients with high-risk factors.
Conclusion
In conclusion, the presence of carotid plaques, history of smoking, and statin therapy are important prognostic factors for primary prevention of ASCVD in individuals not receiving cholesterol-lowering drug therapy, especially in middle-aged individuals. Therefore, we suggest that carotid artery parameters may have additional predictive value for primary prevention of ASCVD in asymptomatic high-risk patients.
Acknowledgment
This research received no grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
Held C, Hjemdahl P, Eriksson SV, Björkander I, Forslund L, Rehnqvist N. Prognostic implications of intima-media thickness and plaques in the carotid and femoral arteries in patients with stable angina pectoris. Eur Heart J. 2001;22:62–72. | ||
Park HW, Kim WH, Kim KH, et al. Carotid plaque is associated with increased cardiac mortality in patients with coronary artery disease. Int J Cardiol. 2013;166:658–663. | ||
Nguyen-Thanh HT, Benzaquen BS. Screening for subclinical coronary artery disease measuring carotid intima media thickness. Am J Cardiol. 2009;104:1383–1388. | ||
Den Ruijter HM, Peters SA, Anderson TJ, et al. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. | ||
Peters SA, den Ruijter HM, Bots ML, Moons KG. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98:177–184. | ||
Goff DC Jr, Lloyd-Jones DM, Bennett G, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. | ||
Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. | ||
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. | ||
Tzou WS, Douglas PS, Srinivasan SR, et al. Increased subclinical atherosclerosis in young adults with metabolic syndrome: the Bogalusa Heart Study. J Am Coll Cardiol. 2005;46:457–463. | ||
Mattsson N, Ronnemaa T, Juonala M, et al. Arterial structure and function in young adults with the metabolic syndrome: the cardiovascular risk in Young Finns Study. Eur Heart J. 2008;29:784–791. | ||
Stein JH, Korcarz CE, Hurst RT, et al; American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography carotid intima-media thickness task force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. quiz 189–190. | ||
Polak JF, Pencina MJ, Pencina KM, O’Donnell CJ, Wolf PA, D’Agostino RB Sr. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. | ||
Polak JF, Szklo M, Kronmal RA, et al. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2013;2: e000087. | ||
Finn AV, Kolodgie FD, Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler Thromb Vasc Biol. 2010;30:177–181. | ||
Johnson HM, Douglas PS, Srinivasan SR, et al. Predictors of carotid intima-media thickness progression in young adults: the Bogalusa Heart Study. Stroke. 2007;38:900–905. | ||
Li R, Duncan BB, Metcalf PA, et al. B-mode-detected carotid artery plaque in a general population. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Stroke. 1994;25:2377–2383. | ||
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. | ||
Semba I, Funakoshi K, Kitano M. Histomorphometric analysis of age changes in the human inferior alveolar artery. Arch Oral Biol. 2001;46: 13–21. | ||
Bae JH, Kim WS, Rihal CS, Lerman A. Individual measurement and significance of carotid intima, media, and intima-media thickness by B-mode ultrasonographic image processing. Arterioscler Thromb Vasc Biol. 2006;26:2380–2385. | ||
Won HK, Kim WS, Kim KY, Hyun DW, Kwon TG, Bae JH. Increased carotid intima-media thickness in hypertensive patients is caused by increased medial thickness. Korean J Med. 2008;75:179–185. | ||
Nohara R, Daida H, Hata M, et al; Justification For Atherosclerosis Regression Treatment (JART) Investigators. Effect of long-term intensive lipid-lowering therapy with rosuvastatin on progression of carotid intima-media thickness – Justification for Atherosclerosis Regression Treatment (JART) extension study. Circ J. 2013;77:1526–1533. | ||
Virmani R, Avolio AP, Mergner WJ, et al. Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis. Comparison between occidental and Chinese communities. Am J Pathol. 1991;139:1119–1129. | ||
Bae JH, Seung KB, Jung HO, et al. Analysis of Korean carotid intima-media thickness in Korean healthy subjects and patients with risk factors: Korea multi-center epidemiological study. Korean Circ J. 2005;35: 513–524. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




