Back to Journals » International Journal of Women's Health » Volume 12
Is Cabergoline Safe and Effective for Postpartum Lactation Inhibition? A Systematic Review
Authors Yang Y , Boucoiran I , Tulloch KJ, Poliquin V
Received 29 September 2019
Accepted for publication 7 February 2020
Published 9 March 2020 Volume 2020:12 Pages 159—170
DOI https://doi.org/10.2147/IJWH.S232693
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Yang Yang,1 Isabelle Boucoiran,2 Karen J Tulloch,3 Vanessa Poliquin4
1University of Manitoba, Winnipeg, MB, Canada; 2Department of Obstetrics & Gynecology, Université de Montréal, Montreal, QC, Canada; 3BC Women’s Hospital, Vancouver, BC, Canada; 4Department of Obstetrics & Gynecology, University of Manitoba, Winnipeg, MB, Canada
Correspondence: Vanessa Poliquin
Health Sciences Centre, RS430 – 810 Sherbrook St, Winnipeg, MB R3A 1R8, Canada
Tel +1 204 612-3867
Fax +1 204 787-2314
Email [email protected]
Background: Despite its benefits, there are some situations where breastfeeding is impossible or not recommended. Breast milk secretion and engorgement can be distressing to these non-breastfeeding women. There is currently no universal guideline on the most appropriate management for these women. Our objective is to evaluate the effectiveness and safety of cabergoline, a dopamine agonist, in lactation inhibition in postpartum women.
Methods: Studies were identified through electronic database searching (Cochrane library, EMBASE, Medline, IPA and Scopus) to identify all relevant studies that evaluated the use of cabergoline as a lactation inhibitor in postpartum women. Citations were screened and a narrative synthesis was undertaken given the heterogeneity of study designs.
Results: A total of six randomized trials met the inclusion criteria. Majority of the studies recruited healthy postpartum women electing for lactation inhibition for personal reasons. A range of 0.4 mg to 1 mg of cabergoline was given within 0 to 50 hrs of delivery. Dose–response relationship is established, and the highest rate of complete success was achieved with 1 mg of cabergoline, with time to cessation between 0 and 1 day. Cabergoline is non-inferior to bromocriptine for lactation inhibition while also associated with fewer rebound symptoms and adverse effects. Commonly reported adverse effects of cabergoline (eg, dizziness, headache and nausea) are self-limited.
Conclusion: Cabergoline is simple, effective and generally safe when given to postpartum women either wishing or needing to suppress lactation. Further research is needed to improve postpartum care of these women.
Keywords: cabergoline, dostinex, lactation suppression, lactation inhibition, postpartum
Background
There are many well-known benefits of breast milk for mother and infants; however, there are also instances that necessitate avoidance of breastfeeding. These may include the birth of a still born baby, neonatal death, maternal infection, such as HIV, which may be transmitted to the baby via breastmilk, and maternal illness that requires toxic therapy that may be excreted in the breastmilk.1 According to the Canadian Community Health Survey in 2012, 11% of women do not breastfeed their newborn infants, and in 23% of cases it was because of a medical condition of the mother or child.2 Women may also seek lactation inhibition for social or personal reasons. In the absence of breast stimulation from infant suckling, lactation will eventually cease in the span of days to weeks.3 However, up to two-thirds of non-breastfeeding women may experience moderate to severe breast engorgement.1 The physical pain can further compound the emotional pain in women who experienced fetal loss or sometimes grief over the inability to breastfeed. Breast binding, icing, fluid restriction, avoidance of tactile breast stimulation are techniques trialed in the past to help these women relieve physical symptoms; however, their efficacy is few and inconclusive.1 Pharmacologic options such as estrogen preparations and bromocriptine are available. Their use is limited due to potential serious side effects such as cerebral accidents, myocardial infarction and postpartum psychosis.4,5 Cabergoline is a newer synthetic ergoline that acts on the dopamine D2 receptors and is commonly used for the treatment of hyperprolactinemia. It has a Health Canada indication for “the prevention of the onset of physiological lactation in the puerperium for clearly defined medical reasons,” but its use has not been adopted into routine practice in North America. For women living with HIV, there is a consensus in developed countries that exclusive formula feeding is recommended over breastfeeding in infants born to mothers with HIV. This is endorsed by the Society of Obstetricians and Gynaecologists of Canada (SOGC) 2014 guideline,6 the Centers for Disease Control and Prevention (CDC) in the United States7 and the British HIV Association (BHIVA).8 Interestingly, the BHIVA 2018 guideline has a level 1C recommendation that cabergoline should be offered to suppress lactation in women not breastfeeding their infant by choice or who have high viral load >50 copies/mL.8 The Royal College of Obstetricians and Gynaecologist (RCOG) in UK has discussed options for lactation suppression particularly for women experienced late intrauterine fetal death and stillbirth, and suggests that women should be advised that dopamine agonists successfully suppress lactation in a very high proportion of women and are well tolerated by a very large majority; cabergoline is superior to bromocriptine.9 The purpose of this literature review is to evaluate the safety and effectiveness of cabergoline in lactation inhibition so that it may become a routine part of postpartum care for women in need of lactation inhibition.
Methods
We systematically reviewed studies that evaluated the use of cabergoline as a lactation inhibitor in postpartum women.
Search Strategy
Studies were identified through electronic database searching (Cochrane library, EMBASE, Medline, IPA and Scopus) in collaboration with a librarian at Neil John McLean Library, University of Manitoba. The search was updated until March 10, 2019. See Appendix for search detail (Prospero number CRD42019128987).
Inclusion and Exclusion Criteria
Studies eligible for inclusion were published in English or French, peer-reviewed, related to cabergoline and lactation inhibition, without publication date restrictions. Studies that focused on the use of cabergoline for other indications than lactation inhibition were excluded from the review, as were non-randomized controlled trials (RCT), editorials and clinical guidelines.
Citation Screening
Citations identified through our searches were exported to Zotero (https://www.zotero.org/). After de-duplication, titles and abstracts were screened independently by two authors. Articles were excluded based on the inclusion and exclusion criteria. All potentially includable papers underwent full-text review to ensure inclusion criteria were met. Any that did not were discarded prior to data extraction and analysis. See Figure 1 for PRISMA Flow Diagram for search and screening process.
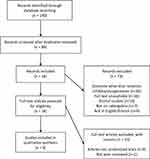 |
Figure 1 PRISMA flow diagram. |
Data Analysis
Studies were characterized according to their year of publication, research methodologies, participant attributes, interventions and types of outcome measured. Given the heterogeneity of study designs amongst studies and the limited number of studies on this subject, a statistical meta-analysis was not possible. We, therefore, conducted a narrative synthesis review. Risk of bias was assessed for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions.10
Results
We have identified 140 articles from our primary search, of which 51 duplicates were removed. The remaining 89 articles were then subjected to abstract review and 16 of which were selected for full-text review. Finally, there were 6 RCT-based articles selected for data extraction and final discussion in this article. The results of these included studies are presented in Tables 1 and 2.
 |
Table 1 Study Demographics and Methodologies |
 |
Table 2 Study Outcomes |
Overview of Available Literature
The 6 RCTs were mainly conducted in industrialized countries, including one large multicentre study conducted in Europe.11 Studies were published between 1987 and 2009.
Participant Attributes
Between all studies, a total of 693 participants had been recruited. Exclusion criteria were specified for few studies including women with a history of hypersensitivity to ergot alkaloids, essential hypertension, pre-eclampsia, hepatic or renal disorder, history of agalactia or hypogalactia and patients who underwent treatments of another disorder that may interfere with prolactin secretion.11,12 The most common reason for lactation inhibition was “personal reasons.” One study had recruited participants who were unable to breastfeed due to stillbirth or neonatal death.13 The European Multicentre study also recruited women who had medical contraindications to breastfeeding but did not elaborate beyond.11
Interventions
Four studies have compared cabergoline with placebo;12,14–16 one study has compared cabergoline with bromocriptine14 and one study compared cabergoline with estrogen–androgen combination.13 Cabergoline was administered orally as a one-time dose in all of the studies for lactation inhibition with doses ranging between 0.4 and 1 mg. Nisha et al had additionally used a 0.25 mg twice a day for 2 days as dosing regimen for women with already-established lactation.13 Majority of the studies have commenced treatments shortly after delivery within 24 hrs, the longest being 50 hrs postdelivery.14
Outcome Assessment Methods
Assessment of outcomes was variable amongst the studies. Two studies utilized daily self-administered questionnaires during in-hospital stay11,15 regarding symptoms of lactation, while four studies utilized a combination of interview and daily breast examinations conducted by healthcare providers.12–14,16 The major parameters of breast symptoms sought for include milk secretion, breast pain and engorgement. Treatment success was determined on various end observation periods with some on day 311 on day 4,15 on day 1412,14,16 as well as one chosen the endpoint to be “no milk expression even on pressing of the breasts”13 The definition for “successful treatment” also varied, some studies allow mild spontaneous milk secretion,11 while others require a complete absence of any breast symptoms including secretion, tenderness or engorgement.12,14
Efficacy Outcome Measures
Cabergoline vs Placebo
Three studies have demonstrated a linear dose–response relationship for cabergoline on lactation inhibition, with the lowest effective dose being 600 mcg (see Table 2).12,15,16 Caballero-Gordo et al randomized 140 healthy puerperal women to 0.5 mg, 0.75 mg, 1 mg of cabergoline or placebo given within 24 hrs of delivery. The percentages of participants with no breast symptoms up to postpartum day 14 were 90% (n=36), 62.5% (n=28), 45% (n=18) and 20% (n=4), respectively, for 1 mg, 0.75 mg, 0.5 mg cabergoline and placebo.12 The differences were statistically significant with regard to each group.12 Melis et al randomized 17 healthy puerperal women to 400 mcg cabergoline, 600 mcg cabergoline or placebo given within 24–48 hrs of delivery.15 By day 2 after treatment, all five women in the placebo group had evident engorgement and were subsequently given bromocriptine and excluded from the study thereafter.15 Forty-three percent (n=3) and 100% (n=5) of those received 400 mcg and 600 mcg cabergoline, respectively, had no signs of lactation or breast engorgement throughout the observation period.15 Melis et al, in another study, randomized 32 healthy postpartum women to 0.4 mg, 0.6 mg, 0.8 mg of cabergoline or placebo given within 24 hrs of delivery and found that lactation was completely prevented in subjects receiving 600 mcg and 800 mcg of cabergoline. Half of the subjects receiving 400 mcg cabergoline had complete success. In the placebo group, only one woman showed no signs of lactation during the 14-day observation period.16
Cabergoline vs Bromocriptine
The European multicenter study and Giorda et al have both reached the same conclusion that 1 mg of cabergoline given as a single oral dose is non-inferior to 5 mg daily for 14 days of bromocriptine in preventing puerperal lactation.11,14 The European multicenter study demonstrated that if a difference does exist between the two compounds, it is unlikely to be more than 10% (p<0.001).11 Complete success where there is absence of any breast signs other than mild spontaneous milk secretion on day 14 was achieved in 78% (n=106) of participants receiving cabergoline versus that of 69% (n=94) for bromocriptine.11 Giorda et al reported a slightly higher efficacy for both groups where 94% (n=17) and 89% (n=16) of participants in the cabergoline and bromocriptine group, respectively; had absence of secretion, engorgement or tenderness on day 14.14
Time to Effect and Duration of Effect
The time to lactation inhibition in those reported was less than 1 day. For those with established lactation, cabergoline on average required 3.29 days to stop.13 In Caballero-Gordo et al, where participants who failed an initial dose of cabergoline 1 mg and then received an additional 1 mg dose, symptoms disappeared completely within 48 hrs.12 The European multicenter study was the only one that had examined rebound lactation as one of the outcomes and found significantly fewer (p<0.0001) participants with rebound breast symptoms on day 21 in the cabergoline (5%, n=5) versus bromocriptine group (24%, n=23).11
Lactation Inhibition vs Suppression
Nisha et al was the only study that had made a clear distinction between inhibition (i.e., preventing the onset of lactation) and suppression (i.e., stopping established lactation). They have used different dosing regimens for the two purposes. The authors found that the mean number of days required for inhibition of lactation was fewer in the cabergoline group compared to those receiving the estrogen–androgen combination (0.73±0.963 days vs 1.81±1.81 days, p=0.001).13 The mean number of days required for lactation suppression between cabergoline and estrogen–androgen combination was not statistically significant (3.29±2.59 days vs 3.96±2.81 days, p=0.244).13 Preventive efficacy of cabergoline vs estrogen–androgen combination was 100% vs 98%, respectively (p=0.286).13 Suppressive efficacy was 96% vs 79%, respectively (p=0.017).13
Safety Outcome Measures
Overall Safety of Cabergoline
Safety outcomes were reported in all but one of the six studies,13 and results are presented in Table 2. Three studies have reported <10% of the participants experienced side effects (0%,16 3%,12 8%15). More specifically, Caballero-Gordo et al reported that 3% (n=4) of participants had dizziness and headaches that were mild and self-limiting.12 No significant differences were reported between different doses (0.5 mg, 0.75 mg, 1 mg) of cabergoline and placebo in systolic and diastolic blood pressures of the participants.12 In the study by Melis et al who examined 17 healthy postpartum women, one individual had asymptomatic orthostatic hypotension 3–6 hrs after the administration of cabergoline with a drop of 15 and 5 in SBP and DBP, respectively.15 None of the individuals had symptomatic hypotension and also no major side effects were found in this study.15 Melis et al, in a separate study, had found that of the 32 healthy puerperal women, no side effects were observed in participants that received cabergoline.16 More specifically no nausea, vomiting, hypotension or nasal stuffiness were reported.16
Two studies have reported >10% of participants experienced side effects (16%11 and 28%14). The European multicenter study has reported adverse effects in 16% (n=22) of cabergoline-treated individuals in contrast to 26% (n=36) of bromocriptine-treated individuals.11 Majority of the side effects occurred early after drug administration and tapered down after the first 3–4 days.11 Interestingly, this study found 2 individuals in the cabergoline group had unexplained side effects including epistaxis and transient hemianopia, and 4 individuals in the bromocriptine group experienced facial paralysis, precordial pain, fever and vaginal hemorrhage.11 Whether or not these symptoms were related to the 2 compounds is unknown as there were no such prior events described.11 Giorda et al enrolled 36 healthy postpartum women who underwent cesarean section and randomized them equally to receive either cabergoline or bromocriptine for lactation inhibition.14 He had reported 28% (n=5) and 78% (n=14) of individuals received cabergoline and bromocriptine, respectively, had developed adverse effects.14 More specifically, 3 individuals of the cabergoline group experienced dizziness after administration, 1 with headache and 1 with vomiting.14 The authors believed the individual with vomiting may have been due to laparotomy rather than cabergoline. All of the reported adverse events resolved spontaneously with the exception of one individual with headache that required analgesic.14 This is in contrast to the bromocriptine treatment group where 1 individual experienced transient amaurosis of unknown origin, 1 had dizziness, 9 had nausea, and 4 had headaches.14 The majority of these also spontaneously resolved however some required dosage adjustment. The reported duration of side effects was between 5 mins to 5 days and 3 mins to 14 days in the cabergoline and bromocriptine groups, respectively.14
Overall, these results consistently indicate that cabergoline, given as a single dose for lactation inhibition, is safe and is associated with only minor, self-limiting side effects. Cabergoline has a more favorable safety profile than bromocriptine.
Potential Risk of Bias
We have utilized Cochrane Collaboration’s tool for assessing the risk of bias.10 See Figure 2 for summary and Table 3 for details of each study. With regard to the risk of selection bias, only two studies11,14 have clearly stated the use of a random number table, with all other studies simply stating that the participants were “randomized,” but no methods of sequence generation were described.12,13,15,16 Three studies have reported methods of allocation concealment with the use of “double-dummy technique” in the European study to make up the differences in treatment lengths in different arms,11 and the use of identical vials containing lyophilized powder of cabergoline and placebo in the Melis 1988 study16 and use of sealed envelope containing instructions for different treatment arms in Nisha et al.13 With regard to blinding, three studies had used a double-blind approach and therefore considered low risk.11,12,16 Two studies (Giorda et al and Melis et al) were conducted in a single-blind fashion.14,15 and one study (Nisha et al) was not blinded.13 Overall, we considered all 6 trials to be at low risk of attrition bias. The European study had 5% non-adherence to the protocol, some were due to intolerance, lost to follow up and other non-specified reasons.11 Furthermore, the same had 34 total participants out of 272 that had received concomitant treatment that may interfere with lactation such as ergot derivatives and oral contraceptives.11 For the three studies with reported exclusion criteria, one study had excluded those undergoing treatment that may interfere with prolactin secretion,12 another one had excluded those with concomitant acute disease.11 Overall, given the ease of administration of cabergoline, the protocol adherence rate was high across the studies. In terms of selective reporting, we considered all but one trial to be at low risk. Melis et al 1988 had reported “no nausea, vomiting, hypotension or nasal stuffiness reported by the subjects who received cabergoline,” specific symptoms sought for were not prespecified in the “Methods” section.16 Three studies appear free of other bias.13,14,16
 |  |  |
Table 3 Risk of Bias Assessments |
 |
Figure 2 “Risk of bias” summary. |
Discussion
Narrative Review Summary
This narrative review is the first study to focus solely on cabergoline’s efficacy and safety in lactation inhibition. It can be given as 1 mg within 24 hrs postpartum and has a rapid onset of action of 1 day. The most common reported side effects include dizziness, headaches and nausea that occurs mainly in the first 3 days post-treatment and tapers afterward. Cochrane has published a review in 2012 that consists of 62 controlled trials (6428 mothers) on treatments of lactation suppression.4 Of those, 30 trials investigated bromocriptine compared to only 5 trials investigated cabergoline. Two studies included in this paper were not part of that review.13,15 The Cochrane review has concluded weak evidence that some pharmacologic treatments (i.e., bromocriptine and estrogen preparations) are better than no treatment in lactation suppression.4 No specific conclusions about cabergoline were drawn in that review. The Royal College of Obstetricians and Gynaecologist (RCOG) in UK published a green-top guideline that suggest for women experienced late intrauterine fetal death and stillbirth women should be advised that dopamine agonists successfully suppress lactation in a very high proportion of women and are well tolerated by a very large majority; cabergoline is superior to bromocriptine.9 Our findings in this review are in support of that recommendation.
Limitations of Current Studies and Future Direction
One major limitation of this review is that the studies included mostly consist of small trials. Larger randomized controlled trials would be required to detect clinically important side effects between treatment groups; however, given the infrequency with which inhibition of lactation is desirable, designing a study with an adequate sample-size would be challenging. There are also large variations in dosages, methods of data collection and outcome assessments. Perhaps utilizing both questionnaires and breast exams conducted by healthcare providers would reduce bias. A more consistent definition of lactation inhibition and suppression as well as treatment success would be helpful.
Furthermore, choices regarding infant feeding are very personal subjects. Often the reasons for not breastfeeding may be related to stillbirth, neonatal death or maternal illness, but the choice of not breastfeeding can be related to more personal reasons. Therefore, the underlying emotional aspect deserves attention as well. For instance, in cultures such as in Malawi, engorged and dropping breasts were seen as social taboos as it is thought to bring future illness to mother and child.18 None of the studies have assessed patient satisfaction as an outcome, more studies should consider the acceptability of the treatment to be one of the outcome measures.
Although studies included in this review did not assess or report the incidence of psychosis post-cabergoline administration, there have been a few case reports of psychosis and mania associated with its use.17 However, these were in non-pregnant population of which cabergoline was used for treatment of pituitary prolactinomas. The time taken for onset or worsening of psychiatric symptoms was greater than 1 month in the majority, and that for improvements was 1 week to 1 month post-cessation of cabergoline.17 Nevertheless, prescribers need to be aware of this rare potential adverse effect and increased mental health monitoring during the early postpartum periods may be helpful.
Conclusion
Non-pharmacologic methods for lactation inhibition lack evidence in efficacy. Currently available pharmacologic agents such as bromocriptine are not ideal given its lengthy administration and potential serious side effects. Postpartum women who are unable to breastfeed can suffer pain from engorgement as well as emotional distress. This review evaluated the effectiveness and safety of cabergoline in puerperal lactation inhibition and has demonstrated that a single dose of 1 mg cabergoline given soon after delivery is effective and generally safe in postpartum women. However, careful consideration of risk is necessary particularly in the setting of psychiatric population. Vigilance is still needed in prescribing cabergoline for lactation inhibition.
Acknowledgments
We wish to thank Tania Gottschalk (University of Manitoba Health Sciences librarian) for her efforts in selecting the appropriate MeSH terms and guiding us through the study retrieval process.
Disclosure
Dr Isabelle Boucoiran reports personal fees from FRQS during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Spitz AM, Lee NC, Peterson HB. Treatment for lactation suppression Little progress in one hundred years. Am J Obstet Gynecol. 1998;179(6):1485–1490. doi:10.1016/S0002-9378(98)70013-4
2. Gionet L. Health at a Glance - Breastfeeding trends in Canada. November 2015.
3. Schanler RJ, Potak DC. Physiology of lactation. UpToDate.
4. Oladapo OT, Fawole B. Treatments for suppression of lactation. Cochrane Database Syst Rev. 2012;(9):
5. Snellen M, Power J, Blankley G, Galbally M. Pharmacological lactation suppression with D2 receptor agonists and risk of postpartum psychosis: A systematic review. Aust N Z J Obstet Gynaecol. 2016;56:336–340. doi:10.1111/ajo.12479
6. Money D, Tulloch K, Boucoiran I, Caddy S. Guidelines for the care of pregnant women living with HIV and interventions to reduce perinatal transmission: executive summary. J Obstet Gynaecol Can. 2014;36(8):721–734. doi:10.1016/S1701-2163(15)30515-6
7. Centers for Disease Control and Prevention. HIV among pregnant women, infants, and children. Available from: https://www.cdc.gov/hiv/group/gender/pregnantwomen/index.html.
8. Gilleece Y. British HIV Association guidelines for the management of HIV infection in pregnant women 2018. HIV Med. 2012;13:87–157. doi:10.1111/j.1468-1293.2012.01030.x
9. ROG. Late Intrauterine Fetal Death and Stillbirth Green-top Guideline No. 55.
10. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [Updated March 2011]. The Cochrane Collaboration; 2011. Available from: www.handbook.cochrane.org.
11. European Multicentre Study Group for Cabergoline in Lactation Inhibition. Single dose cabergoline versus bromocriptine in inhibition of puerperal lactation: randomised, double blind, multicentre study. BMJ. 1991;302:1367–1371.
12. Caballero-Gordo A, Lopez-Nazareno N, Calderay M, Caballero J, Mancheño E, Sghedoni D. Oral cabergoline. Single-dose inhibition of puerperal lactation. J Reprod Med. 1991;36:717–721.
13. Nisha SU, Sachan V, Sankhwar P. Role of newer drug cabergoline in lactation suppression as compared to estrogen-androgen combination. J Obstet Gynaecol India. 2009;152–155.
14. Giorda G, Vincentiis S, Motta T, Casazza S, Fadin M, D’Alberton A. Cabergoline vs bromocriptine in suppression of lactation after Cesarean delivery. Gynecol Obstet Invest. 1991;31:93–96. doi:10.1159/000293109
15. Melis GB, Gambacciani M, Paoletti AM. Dose-related prolactin inhibitory effect of the new long-acting dopamine receptor agonist cabergoline in normal cycling, puerperal, and hyperprolactinemic women. J Clin Endocrinol Metab. 1987;65:541–545. doi:10.1210/jcem-65-3-541
16. Melis G, Mais V, Paoletti A, Beneventi F, Gambacciani M, Fioretti P. Prevention of puerperal lactation by a single oral administration of the new prolactin-inhibiting drug, cabergoline. Obstet Gynecol. 1988;71:311–314.
17. Gupta P, Tundup T, Singh J. Treatment complexities in psychosis associated with cabergoline treatment in patients having pituitary prolactinomas. Asian J Psychiatry. 2018;31(1):129–132. doi:10.1016/j.ajp.2018.01.011
18. Buhendwa L, Zachariah R, Teck R, et al. Cabergoline for suppression of puerperal lactation in a prevention of mother-to-child HIV-transmission programme in rural Malawi. Trop Doct. 2008;38:30–32. doi:10.1258/td.2007.060091
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
