Back to Journals » Clinical Ophthalmology » Volume 14
Impact of Ocular Surface Disease Treatment in Patients with Glaucoma
Authors Mylla Boso AL, Gasperi E, Fernandes L, Costa VP, Alves M
Received 4 September 2019
Accepted for publication 4 November 2019
Published 14 January 2020 Volume 2020:14 Pages 103—111
DOI https://doi.org/10.2147/OPTH.S229815
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ana Luiza Mylla Boso, Erica Gasperi, Leticia Fernandes, Vital Paulino Costa, Monica Alves
Department of Ophthalmology and Otorhinolaryngology, Faculty of Medical Sciences, University of Campinas (UNICAMP), Campinas, Brazil
Correspondence: Ana Luiza Mylla Boso
Department of Ophthalmology and Otorhinolaryngology, Faculty of Medical Sciences, University of Campinas, Cidade Universitária Zeferino Vaz - Barão Geraldo, Campinas, SP 13083-970, Brazil
Tel/Fax +55-19 3521 7396
Email [email protected]
Purpose: Chronic topical treatment for glaucoma may lead to Ocular Surface Disease (OSD). This study aimed to evaluate: (1) the prevalence of OSD in glaucoma patients under topical treatment, quantifying symptoms and objective ocular surface parameters and (2) the impact of ocular surface treatment on OSD and IOP control.
Methods: Patients with primary open angle or primary angle closure glaucoma under topical treatment for at least 6 months were enrolled in the study. Patients underwent symptom screening with the ocular surface disease index (OSDI) questionnaire, assessment of objective ocular surface parameters, ocular surface staining and Schirmer test. A treatment for OSD with eyelid hygiene, fluorometholone acetate 0.1%, preservative-free lubricants, free-acid supplementation and oral tetracyclin derivate was started, and the same evaluation was performed.
Results: In our sample (n=19), 73.68% of the patients reported severe symptoms of dry eye disease, with OSDI scores higher than 33 at baseline. Tear film instability was found in 50% of patients, while 23.53% had severe meibomian gland abnormalities. Fluorescein and lissamine green stainings were abnormal in 88.24% and 82.35% of patients, respectively. After ocular surface treatment, statistically significant improvement was found in best-corrected visual acuity (p=0.0003), OSDI score (p< 0.0001), bulbar redness (p=0.0196) and fluorescein staining (p< 0.0001.) Mean IOP following OSD treatment reduced − 1.59 mmHg from baseline in the left eye (p=0.0510).
Conclusion: The prevalence of OSD signs and symptoms was high in glaucoma patients under medical treatment. Short-term OSD treatment may improve ocular surface disease and IOP control, with no need to discontinue glaucoma medications.
Keywords: ocular surface disease, glaucoma, preservative, keratograph, benzalkonium chloride
Introduction
Glaucoma is the leading cause of irreversible blindness globally (World Health Organization, 2018). While lowering the intraocular pressure (IOP) is the most important measure to prevent further damage to the optic disc, long-term topical treatment represents a continuous hazard to the ocular surface homeostasis. Long-term topical glaucoma therapy has been associated with reduced density of goblet cells and squamous metaplasia of the conjunctival epithelium,1 dysfunction of meibomian glands, conjunctival and corneal desquamation,2 and overexpression of proinflammatory cytokines.3 Significative loss of goblet cells, which can cause dry eye, inflammation, and fibrosis, was observed in animal and human models.1 As a consequence of the inflammatory changes, chronic use of IOP-lowering medications can also affect bleb scarring in filtration surgery, since it is a risk factor for conjunctival fibrosis which can ultimately result in failure of trabeculectomy.4
Some studies have investigated the coexistence of glaucoma and ocular surface disease (OSD).5–7 The prevalence of OSD is estimated to vary between 5% and 30% in the general population,8,9 but may increase up to 50% in glaucoma patients under medical treatment.10,11 Several risk factors such as aging, hormone imbalance, systemic comorbidities, systemic medications and environmental exposure are frequently associated with the chronic use of IOP-lowering eyedrops, triggering proinflammatory responses and ocular surface dysfunction in patients with glaucoma.12,13 In this context, OSD symptoms may affect compliance among these patients. In fact, compliance has been reported to be less than 50% in glaucoma patients14,15 and local side effects are reported to be one of the reasons.16–18 OSD signs and symptoms may vary enormously among glaucoma patients,19,20 and available tests are not well standardized and can be biased by subjective interpretations. Recent equipments such as the Keratograph 5M (Oculus, Wetzlar, Germany) corneal topographer are now available to assess signs of OSD with proper documentation and objective classification, but studies are still scarce.21
OSD management in glaucoma patients has been previously investigated, demonstrating clinical and symptomatic improvement along with better IOP control in a sample of four patients evaluated by subjective parameters.22
This study aims to evaluate the prevalence of OSD in glaucoma patients under topical treatment, quantifying symptoms and measuring objective ocular surface parameters. Furthermore, this study evaluates the impact of OSD treatment on IOP control, improvement of ocular surface parameters and symptoms.
Materials and Methods
This prospective interventional study was carried out with the approval of the Institutional Research Board of University of Campinas (approval number 53127515.7.0000.5404) and was conducted in accordance with the tenets of the Declaration of Helsinki and current legislation on clinical research. Written informed consent was obtained from all subjects after explanation of the procedures and study requirements. Participants were diagnosed with either primary open-angle or primary angle-closure glaucoma and had been using at least one topical hypotensive drug for at least 6 months, with no previous diagnosis of ocular surface disease.
First, a comprehensive anamnesis was obtained to access general data such as age, gender, time since glaucoma diagnosis, number and types of drops used, previous ocular surgeries and ocular and systemic comorbidities followed by a detailed ocular examination. The patients were asked to answer a quick compliance questionnaire with 3 questions: (1) “In the past week, did you miss any of the antiglaucoma drops?” (2) “If yes, how many doses have you missed?”, and (3) “What was the reason?”. The best corrected visual acuity was registered in logMAR scale.
OSD symptoms were evaluated using the ocular surface disease index (OSDI) questionnaire, which is the most frequently used survey instrument for assessment of ocular surface disease severity.23 It consists of 12 items that evaluate symptoms, functional limitations and environmental factors, scored from 0 (symptoms none of the time) to 4 (symptoms all the time). The total score, which ranges from 0 to 100, is calculated through the formula: (sum of the score for all the questions answered) x 100/number of questions answered x 4. A score between 0 and 12 was considered normal, 13 to 22 indicated mild dry eye disease, 23 to 32 moderate dry eye disease, and 33 or above severe dry eye disease.24
Ocular surface parameters were evaluated using Keratograph 5M, a non-invasive equipment developed to assess the tear film and the ocular surface. Tear meniscus height (TMH), bulbar redness, non-invasive tear break-up time (NITBUT) and meibography were photo-documented and analyzed objectively. All procedures were sequentially performed by the same examiner, in accordance with specific guidelines and regulations, as described below.24
Tear film volume was assessed through the TMH, measured perpendicularly to the inferior eyelid margin, centered on the pupil, in millimeters (mm). The TMH was also classified as normal (if greater than 0.3 mm) or reduced (mild reduction from 0.19 mm to 0.3 mm, moderate from 0.1 mm to 0.2 mm and severe if under 0.1 mm).
Bulbar redness, a suggestive sign of ocular surface inflammation, was evaluated using digital imaging and automatically scored by the equipment software.
NITBUT assesses the tear film stability without the influence of fluorescein, and the Keratograph software provides measurements of the first breakup spot of the tear film in seconds (NIKBUT first), its average time (NIKBUT avg), and a classification in different levels of severity.
Meibography allows observation of the morphology of the meibomian glands through infrared images. Photographic documentation of everted upper and lower eyelids was obtained. All images were then analyzed and graded according to the meiboscore scale as: (0) if the lid had no missing glands, (1) if partial or absent glands were found in <33% of the lid area, (2) if partial or missing glands were found in 33–66% of the lid area or (3) if absent or missing glands were found in >66% of the lid area.25
Fluorescein staining of the ocular surface using an image registration was graded according to the National Eye Institute/Industry Workshop guidelines (NEI).26 This scale divides the cornea into five areas and grades the staining from 0 (absent) to 3 (severe) in each of them, with a total score varying from 0 to 15. The sum was then ranked in different levels of severity: 0 if the total score was between 0 and 1; 1 if the total score was between 2 and 4; 2 if the total score was between 5 and 9; and 3 if the total score was between 10 and 15.
Fluorescein tear breakup time (FBUT), a subjective assessment of tear film stability, was also measured in seconds (s) after asking the patients to blink naturally 3 times. Values of 8–15 s were categorized as being normal; 7–5 s indicated mild instability; 4–1 s moderate instability; immediate breakup as severe instability.
Lissamine green stains ocular surface epithelial cells that are unprotected by mucin or glycocalyx, as well as damaged cells. Dye was instilled and the image was evaluated according to the Oxford grading scale.26 It was also categorized into four different grades of severity from 0 to 3.
The Schirmer test, without instillation of anesthetic drops, was done by folding a Schirmer paper strip, placed between the tarsal and bulbar conjunctiva of the lower lid margin, in its temporal one-third. After 5 minutes, the strips were examined and the length of wetting in millimeters was documented. The results were then ranked as (0) if the length was >10 mm; (1) if the length was 5–10 mm; (2) if the length was 1–5 mm; (3) if the length was <1mm.
Both eyes were assessed in all patients, unless one of the eyes was not on any antiglaucoma drop.
Finally, an anesthetic drop was instilled and IOP was measured with a Goldmann applanation tonometer.
The same operator performed all the examinations before and after treatment. The pictures taken with keratograph which required subjective evaluation were analyzed and classified by a second investigator who was blind for the patient’s status.
Table 1 summarizes the OSD classification according to each parameter evaluated in this study.
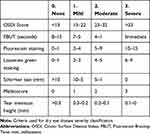 |
Table 1 OSD Classification According to Each Parameter Studied |
All patients underwent a complete OSD treatment, consisting of eyelid hygiene using a gel twice a day, fluorometholone acetate 0.1% one drop at night, preservative-free lubricant every 2 hrs, oral free-acid supplementation (omega 3 and flaxseed oil capsule 2g a day) and oral tetracyclin derivate (doxycycline hydrochloride 100 milligram per day, during 30 days). Anti-glaucoma treatment was not modified, and a second appointment was scheduled for the same evaluation 1 to 3 months after treatment.
Exploratory data analysis was performed through summary measures (mean, standard deviation, minimum, median, maximum, frequency and percentage). Comparison of pre- and post-treatment parameters was performed using the Wilcoxon test. The level of significance was 5%. The analyses were performed using the computer program The SAS System for Windows (Statistical Analysis System), version 9.4. (SAS Institute Inc, Cary, NC, USA).
Results
Thirty-two glaucoma patients under topical treatment were enrolled in this study. Of these, nineteen returned after treatment. Eyes not on topical glaucoma treatment during the study enrollment were not included, resulting in thirty-six eyes with pre- and post-treatment data. Eleven patients (57.89%) were female and 8 (42.10%) were male, with a mean age of 66.74 ± 9.79 years (range from 48–82 years).
Patients had a diagnosis of glaucoma for 9.82 ± 7.92 years and had been on antiglaucoma topical medication since then. The mean number of IOP-lowering drugs used by the patients was 3.05 ± 0.91, among which 2.58 ±. 1.17 were BAK-preserved drops. The mean number of instilled drops was 5.21 ±1.90 per day. Most patients (82.35%) said they have not missed any doses of the prescribed antiglaucoma drops in the previous week. Of the remaining 17.64%, 11.77% recalled missing only one dose in the past 7 days due to forgetfulness. Only one patient (5.88%) reported missing 3 or more doses in the previous week, due to ocular discomfort symptoms.
In our sample, OSD varied according to each parameter. At the initial evaluation, all patients met one or more criteria to the diagnosis of OSD, considering the parameters previously summarized in Table 1. Only one patient scored less than 13 points in the OSDI questionnaire. Four out of 19 (21.05%) patients had an OSDI score between 23–31 (moderate symptoms), and 14 (73.68%) scored higher than 33, corresponding to severe symptoms.
The FBUT was graded as level 0 (normal), in 12.50% of the eyes. Abnormal tear quality was found in 87.50% of the eyes, graded as level 1 in 37.50%, and as level 2 in 50% of the examined eyes. The NITBUT was classified as grade 0 in 29.41% of the eyes, grade 1 in 41.18%, grade 2 in 5.88%, and grade 3 (with immediate tear film breakup) in 23.53%.
Tear production was evaluated through Schirmer test and considered normal (with readings >10 mm) in 64.71% of the eyes. Mild and moderate tear deficiency were both found in 17.65% of the eyes, while none had severe deficiency. TMH was considered normal in 41.18% of the eyes, whereas readings consistent with mild and moderate tear deficiency were found in 17.65% and 35.29% of the eyes, respectively. Readings of less than 0.1mm were seen in 5.88% of the eyes.
Regarding the meiboscore, 88.24% of the patients exhibited dysfunctions in meibomian gland morphology. Among the 32 eyes, 47.06% had mild abnormalities, 17.65% moderate, and 23.53% were graded as level 3, with severe abnormalities.
Staining of the ocular surface both with fluorescein and lissamine green were abnormal in the majority of the eyes, with figures of 88.24% and 82.35%, respectively. Fluorescein staining was mild in 23.53% of the eyes, moderate in 47.06%, and severe in 17.65%. With lissamine green, mild staining was found in 35.29% of the eyes, while moderate and severe staining were observed in 41.18% and 5.88%, respectively.
In summary, OSDI score, meibography, FBUT and ocular surface staining were found to be the most altered parameters, with 80% or more of the examined eyes graded as a level > zero. On the other hand, the tear production, evaluated by the Schirmer test and the tear meniscus height, was abnormal in less than 60% of the patients.
All parameters were evaluated and compared to a sex- and age-matched control group. Comparisons were made to better understand OSD in glaucoma patients compared to healthy individuals, as demonstrated in Table 2. Results demonstrated profound changes in most parameters related to the ocular surface and in symptoms. Only Schirmer test and TMH did not differ statistically between the groups, possibly pointing a compensatory moment of reflex tearing.
 |
Table 2 Comparison Between the Evaluated Parameters in the Study Group Before Ocular Surface Treatment and Healthy Subjects (Control Group) |
Figure 1 displays the distribution of the severity of each parameter.
 |
Figure 1 Dispersion diagram displaying data distribution of OSD severity at baseline according to different parameters. |
Mean duration of ocular surface treatment was 60.84 ± 26.27 days. After treatment, an improvement of symptoms and ocular surface parameters was observed, as illustrated in Figure 2 and Table 3. Figure 3 demonstrates the improvement observed in bulbar redness through photo documentation. Statistically significant improvements were found in best-corrected visual acuity in both eyes (p=0.0031 and p=0.0003), OSDI score (p<0.0001), bulbar redness (p=0.0414 and p=0.0196) and fluorescein staining of the ocular surface (p<0.0001 for both eyes). After treatment, mean IOP reduced 1.4 mmHg and 1.6 mmHg from baseline in OD and OS, respectively (p= 0.9471 and p=0.0510, respectively). Of note, an IOP reduction ≥2 mmHg was observed in 58% of the eyes after treatment.
 |
Table 3 Descriptive Variables Pre and Post Treatment |
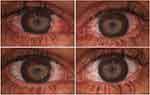 |
Figure 3 Pre-treatment (upper) and post-treatment (lower) images of both eyes of one of the patients enrolled in the study, showing significative improvement in bulbar redness. |
Discussion
Ocular surface disease signs and symptoms were prevalent among glaucoma patients under topical treatment in our sample, with all patients presenting at least one abnormal test. OSDI questionnaire scores showed that more than 70% of the patients had severe symptoms, which is a high prevalence rate compared to other studies.10,27 This may be explained by the fact that our series included patients followed at a public tertiary hospital for over 10 years. OSD treatment reduced 43.26 points on OSDI score and 84% experienced at least a one-level reduction in the severity of OSDI (from severe to moderate, moderate to mild, or mild to none). Only 10% presented severe OSD after treatment, compared to 70% pre-treatment.
Ocular surface damage evaluated through staining was positive in 88% of our sample. Other authors previously found higher prevalence of corneal staining in patients under IOP-lowering medications compared to healthy subjects.20,21 Corneal epithelial barrier disruption caused by preservatives (specially BAK) in eyedrops has been demonstrated both in vitro28,29 and in vivo.30 Histopathology has demonstrated cell injury, apoptosis and desquamation. The improvement of the corneal epithelium integrity after treatment, confirmed in our study by the reduction of fluorescein corneal staining, probably explains the significant improvement in visual acuity. OSD may cause visual disturbance reported as glare or blurred vision31 that can impact on daily activities and overall quality of life,32 but can often be overlooked.
Surface inflammation is a hallmark of OSD and topical treatment toxicity.33,34 Bulbar redness had a significant improvement after OSD treatment in both eyes. Although several antiglaucoma medications lead to conjunctival hyperemia,29,35,36 the significant reduction in bulbar redness occurred despite the maintenance of all glaucoma eyedrops previously in use.
Interestingly, Schirmer test results were normal in most of the patients and tear meniscus height leaned towards reduction after treatment. This finding may be explained by the fact that there is a reflex stimulus to tear production as a compensatory mechanism in OSD process. When the ocular surface homeostasis is recovered, there may be less reflective lacrimation and an increase in lipid layer,37 reducing TMH and Schirmer test measurements.
There was a trend towards lowering IOP following OSD treatment; furthermore, 58% of the eyes had an IOP reduction of at least 2 mmHg after OSD treatment. These findings can be explained either by the minimization of inflammatory stimuli that could affect the trabecular meshwork,22,29 or by a significant increase in compliance due to the reduced discomfort. Although only one patient reported having missed more than one antiglaucoma drop in a week through our questionnaire, self-reported compliance is questionable and often overestimated.38,39 Henry et al have observed statistically significant IOP reduction in glaucoma patients who switched from BAK-preserved prostaglandin to preservative-free travoprost.34 In a similar study, but with a very small sample (n=4), Batra et al suggested that severe OSD could exacerbate glaucoma due to inflammation and scarring of the trabecular meshwork and demonstrated that an intensive OSD treatment including preservative-free lubricants, preservative-free antiglaucoma medication and oral doxycycline improved IOP control.22 Their study was a retrospective review of the files of four patients with uncontrolled primary open angle glaucoma. However, this study lacks on describing ocular surface findings and OSD characterization in glaucoma patients. It also lacks statistical analysis and the small sample included only patients with uncontrolled IOP, not emphasizing that even when there is adequate IOP control, patients may experience OSD symptoms and ocular surface inflammation.
Previous studies that evaluated the impact of switching from BAK-preserved drops to preservative-free IOP-lowering medications demonstrated a significant improvement of OSD symptoms and bulbar redness. However, in some countries there are limited preservative-free options for treating glaucoma and/or their cost can be prohibitive for some patients. To our knowledge, this is the first study to investigate the impact of an intensive OSD treatment in a larger sample of glaucoma patients under long-term topical treatment using subjective and objective measurements of ocular surface parameters.
Nevertheless, our study has some limitations. Our follow-up time is short, and the period of treatment was not the same for all patients, so factors that can affect ocular surface parameters such as humidity and season were not controlled. However, the study design proposed complex changes in the patient routine, including lid hygiene, addition of another eyedrop and 2 oral medications. Long-term OSD treatment may be complicated to patients, resulting in lower compliance. Yet, our results demonstrated that even a short-term OSD treatment is able to positively impact the ocular surface of glaucoma patients. The mean IOP reduction observed in our patients was not statistically significant, probably because the sample size is small. Also, the effect of IOP diurnal variation was not considered. We cannot rule out the possibility that the observed IOP reduction in our patients was the result of increased compliance, not because the discomfort was reduced, but simply because they were participating in a study. In a future study, a group of glaucoma patients followed without OSD treatment could serve as control. Regarding sample size, since no previous study has been conducted using ocular surface parameters and OSD treatment, we performed a post hoc calculation of the power of our sample for some of the parameters. Thus, the sample of 19 patients can substantially support our conclusions once it reached the power of 87%, 94% and 100% for conjunctival hyperemia, fluorescein staining and OSDI scores, respectively, with a P value of 0.05. Finally, as this was a prospective, longitudinal study, patients who had more symptoms were possibly more prone to participate in the study and return for follow-up after treatment.
We had a rate 40% loss at follow-up, which can be explained by multiple factors. Many of the patients seen in our hospital live in distant towns and have a poor financial situation. Also, most of the patients enrolled were elderly and needed an escort. Having to travel to the hospital for a second visit could be a burden for the families since no financial compensation was offered to the participants.
Glaucoma affects millions around the world and topical glaucoma drugs may impact negatively on patients’ ocular surface, symptoms and ultimately the quality of life and vision. Glaucoma experts may not be entirely familiar neither with potential damage signs on tear film and ocular surface nor with all therapeutic strategies to restore ocular surface homeostasis, increase tear film protective function and overall reduce important irritative symptoms frequently reported by patients. Indeed, unfortunately, preservative-free medications are not available in many countries, placing patients into a long-term hazardous situation. As a chronic and sight-threatening disease, glaucoma treatment demands lifetime management and strict compliance. We have demonstrated that intensive ocular surface treatment of glaucoma patients under topical treatment may improve clinical parameters and symptoms, including IOP. In addition to the strategies we employed, and since preservatives have been shown to harm the ocular surface, avoiding or minimizing preservative exposure may result in further improvement of the ocular surface. This study showed a detailed characterization of OSD related to glaucoma eyedrops and the positive impact of a comprehensive ocular surface treatment, raising concerns and important awareness for both glaucoma and ocular surface practices.
Disclosure
Vital Paulino Costa consults for and reports grants, personal fees from Novartis, Genom, Alcon, Allergan, Aerie and Novartis, and is on the speaker’s bureau for Allergan, Alcon, Novartis, and Iridex. Monica Alves consults and is on speaker’s bureau for Alcon and Uniao Quimica and reports grants from FAPESP, during the conduct of the study; and personal fees from Alcon, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Di Staso S, Agnifili L, Ciancaglini M, et al. In vivo scanning laser confocal microscopy of conjunctival goblet cells in medically-controlled glaucoma. In Vivo (Brooklyn). 2018;32:437–443.
2. Di Staso S, Agnifili L, Cecannecchia S, et al. In vivo analysis of prostaglandins-induced ocular surface and periocular adnexa modifications in patients with glaucoma. In vivo. 2018; 32(2):211–220. doi:10.21873/invivo.11227
3. Mastropasqua L, Agnifili L, Mastropasqua R, et al. Conjunctival modifications induced by medical and surgical therapies in patients with glaucoma. Curr Opin Pharmacol. 2013. doi:10.1016/j.coph.2012.10.002
4. Mastropasqua R, Fasanella V, Brescia L, et al. In vivo confocal imaging of the conjunctiva as a predictive tool for the glaucoma filtration surgery outcome. Invest Ophthalmol Vis Sci. 2017;58:BIO114–BIO120. doi:10.1167/iovs.17-21795
5. Servat JJ, Bernardino CR. Effects of common topical antiglaucoma medications on the ocular surface, eyelids and periorbital tissue. Drugs and Aging. 2011;28:267–282. doi:10.2165/11588830-000000000-00000
6. Costa VP, Marcon IM, Filho RPG, Malta RFS. The prevalence of ocular surface complaints in brazilian patients with glaucoma or ocular hypertension. Arq Bras Oftalmol. 2013;76:221–225. doi:10.1590/S0004-27492013000400006
7. Costa VP, Da Silva RS, Renato A. The need for artificial tears in glaucoma patients: a comparative, retrospective study. Arq Bras Oftalmol. 2013;76:6–9. doi:10.1590/S0004-27492013000100003
8. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–283. doi:10.1016/j.jtos.2017.05.008
9. Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol (Chicago, Ill. 1960). 2000;118:1264–1268.
10. Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17:350–355. doi:10.1097/IJG.0b013e31815c5f4f
11. Fechtner RD, Godfrey DG, Budenz D, et al. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010;29:618–621. doi:10.1097/ICO.0b013e3181c325b2
12. Baudouin C, Labbé A, Liang H, et al. Preservatives in eyedrops: the good, the bad and the ugly. Prog Retin Eye Res. 2010;29:312–334. doi:10.1016/j.preteyeres.2010.03.001
13. Broadway D, Grierson I, Hitchings R. Adverse effects of topical antiglaucomatous medications on the conjunctiva. Br J Ophthalmol. 1993;77:590–596. doi:10.1136/bjo.77.9.590
14. Stryker JE, Beck AD, Primo SA, et al. An exploratory study of factors influencing glaucoma treatment adherence. J Glaucoma. 2010;19:66–72. doi:10.1097/IJG.0b013e31819c4679
15. Schwartz GF, Quigley HA. Adherence and persistence with glaucoma therapy. Surv Ophthalmol. 2008;53(Suppl1):S57–68. doi:10.1016/j.survophthal.2008.08.002
16. Tsai JC, McClure CA, Ramos SE, et al. Compliance barriers in glaucoma: a systematic classification. J Glaucoma. 2003;12:393–398. doi:10.1097/00061198-200310000-00001
17. Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma results from the Glaucoma Adherence and Persistency Study. Ophthalmology. 2008;115:
18. Sleath B, Robin AL, Covert D, et al. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113:431–436. doi:10.1016/j.ophtha.2005.10.034
19. Kamath AP, Satyanarayana S, Rodrigues F. Ocular surface changes in primary open angle glaucoma with long term topical anti glaucoma medication. Med J Armed Forces India. 2007;63:341–345. doi:10.1016/S0377-1237(07)80011-6
20. Mathews PM, Ramulu PY, Friedman DS, et al. K. Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology. 2013;120:2241–2248. doi:10.1016/j.ophtha.2013.03.045
21. Portela RC, Fares NT, Machado LF, et al. Evaluation of ocular surface disease in patients with glaucoma: clinical parameters, self-report assessment, and keratograph analysis. J Glaucoma. 2018;27:794–801. doi:10.1097/IJG.0000000000001007
22. Batra R, Tailor R, Mohamed S. Ocular surface disease exacerbated glaucoma: optimizing the ocular surface improves intraocular pressure control. J Glaucoma. 2014;23:56–60. doi:10.1097/IJG.0b013e318264cd68
23. Schiffman RM, Christianson MD, Jacobsen G, et al. Reliability and validity of the ocular surface disease index. Arch Ophthalmol (Chicago, Ill. 1960). 2000;118:615–621. doi:10.1001/archopht.118.5.615
24. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574. doi:10.1016/j.jtos.2017.05.001
25. Arita R, Itoh K, Inoue K, et al. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology. 2008;115:911–915. doi:10.1016/j.ophtha.2007.06.031
26. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22:640–650. doi:10.1097/00003226-200310000-00008
27. Garcia-Feijoo J, Sampaolesi JR. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441–446. doi:10.2147/OPTH.S29158
28. Cha S-H, Lee J-S, Oum B-S, et al. Corneal epithelial cellular dysfunction from benzalkonium chloride (BAC) in vitro. Clin Experiment Ophthalmol. 2004;32:180–184.
29. Baudouin C, Pisella PJ, Fillacier K, et al. Ocular surface inflammatory changes induced by topical antiglaucoma drugs: human and animal studies. Ophthalmology. 1999;106:556–563. doi:10.1016/S0161-6420(99)90116-1
30. Ichijima H, Petroll WM, Jester J, et al. Confocal microscopic studies of living rabbit cornea treated with benzalkonium chloride. Cornea. 1992;11:221–225. doi:10.1097/00003226-199211030-00006
31. Begley CG, Chalmers RL, Abetz L, et al. The relationship between habitual patient-reported symptoms and clinical signs among patients with dry eye of varying severity. Investig Ophthalmol Vis Sci. 2003;44:4753–4761. doi:10.1167/iovs.03-0270
32. Schiffman RM, Walt JG, Jacobsen G, et al. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110:1412–1419. doi:10.1016/S0161-6420(03)00462-7
33. Yee RW. The effect of drop vehicle on the efficacy and side effects of topical glaucoma therapy: a review. Curr Opin Ophthalmol. 2007;18:134–139. doi:10.1097/ICU.0b013e328089f1c8
34. Henry JC, Peace JH, Stewart JA, et al. Efficacy, safety, and improved tolerability of travoprost BAK-free ophthalmic solution compared with prior prostaglandin therapy. Clin Ophthalmol. 2008;2:613–621. doi:10.2147/opth.s3881
35. Kobayashi H, Kobayashi K. A correlation between latanoprost-induced conjunctival hyperemia and intraocular pressure-lowering effect. J Glaucoma. 2011;20:3–6. doi:10.1097/IJG.0b013e3181d26024
36. Rosin LM, Bell NP. Preservative toxicity in glaucoma medication: clinical evaluation of benzalkonium chloride-free 0.5% timolol eye drops. Clin Ophthalmol. 2013;7:2131–2135. doi:10.2147/OPTH.S41358
37. Arita R, Morishige N, Fujii T, et al. Tear interferometric patterns reflect clinical tear dynamics in dry eye patients. Investig Ophthalmol Vis Sci. 2016;57:3928–3934.
38. Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83:711–716. doi:10.2105/AJPH.83.5.711
39. Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012;21:234–240. doi:10.1097/IJG.0b013e31821dac86
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

