Back to Journals » Clinical and Experimental Gastroenterology » Volume 13
Identification of Novel Therapeutic Molecular Targets in Inflammatory Bowel Disease by Using Genetic Databases
Authors Mohan S , Mok S , Judge T
Received 22 June 2020
Accepted for publication 9 September 2020
Published 19 October 2020 Volume 2020:13 Pages 467—473
DOI https://doi.org/10.2147/CEG.S264812
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Koulaouzidis
Sachin Mohan,1– 3 Shaffer Mok,4 Thomas Judge5
1Department of Gastroenterology and Hepatology, University of Minnesota School of Medicine, St Paul, MN, USA; 2Regions Hospital, Department of Gastroenterology and Hepatology, St Paul, MN, USA; 3Health Partners Digestive Care Center, St Paul, MN, 55130, USA; 4Division of Gastroenterology and Hepatology, University Hospital Digestive Health Institute, Westlake, OH 44145, USA; 5Division of Gastroenterology and Liver Diseases, Cooper University Hospital, Mount Laurel, NJ 08054, USA
Correspondence: Sachin Mohan
Regions Hospital, Department of Gastroenterology and Hepatology, 435 Phalen Blvd, St Paul, MN 55130, USA
Tel +1 651-254-8680
Fax +1 651-254-8656
Email [email protected]
Purpose: Utilization of genetic databases to identify genes involved in ulcerative colitis (UC), Crohn’s disease (CD), and their extra-intestinal manifestations.
Methods: Protein coding genes involved in ulcerative colitis (3783 genes), Crohn’s disease (3980 genes), uveitis (1043 genes), arthritis (5583 genes), primary sclerosing cholangitis (PSC) (1313 genes), and pyoderma gangrenosum (119 genes) were categorized using four genetic databases. These include Genecards: The Human Gene Database (www.genecards.org), DisGeNET (https://www.disgenet.org/), The Comparative Toxicogenomics Database (http://ctdbase.org/) and the Universal Protein Resource (https://www.uniprot.org/). NDex, Network Data Exchange (http://www.ndexbio.org/), was then utilized for mapping a unique signal pathway from the identified shared genes involved in the above disease processes.
Results: We have detected a unique array of 20 genes with the highest probability of overlay in UC, CD, uveitis, arthritis, pyoderma gangrenosum, and PSC. Figure 1 represents the interactome of these 20 protein coding genes. Of note, unique immune modulators in different disease processes are also noted. Interleukin-25 (IL-25) and monensin-resistant homolog 2 (MON-2) are only noted in UC, CD, pyoderma gangrenosum, and arthritis. Arachidonate 5-lipoxygenase (ALOX5) is involved in UC, CD, and arthritis. SLCO1B3 is exclusively involved with pyoderma gangrenosum, UC, and CD. As expected, TNF involvement is noted in CD, UC, PSC, and arthritis. Table 1 depicts the detailed result.
Conclusion: Our work has identified a distinctive set of genes involved in IBD and its associated extra-intestinal disease processes. These genes play crucial roles in mechanisms of immune response, inflammation, and apoptosis and further our understanding of this complex disease process. We postulate that these genes play a critical role at intersecting pathways involved in inflammatory bowel disease, and these novel molecules, their upstream and downstream effectors, are potential targets for future therapeutic agents.
Keywords: inflammatory bowel diseases, IBD, ulcerative colitis, UC, Crohn’s disease, CD, arthritis, primary sclerosing cholangitis, PSC, uveitis, pyoderma gangrenosum
Plain Language Summary
In the era of translational and personalized medicine, Inflammatory Bowel disease (IBD) remains a complex disease. This complicated disease presents with a wide array of symptoms that arise from underlying pathophysiological alterations in the patient’s mucosal immune system, the intestinal microbiome, the patient’s genome, and environmental factors. While the predominant disease manifests with gastrointestinal symptoms, involvement of other organs, including but not limited to the skin, eyes, and bones that present as arthritis, uveitis, and pyoderma gangrenosum, is not uncommon. Though the last decade has identified possible genetic factors that confer susceptibility to IBD, the signaling pathways involved in these extra-intestinal manifestations still remain poorly understood. In this study, we use genetic databases to identify an exclusive set of genes that are involved in the extra-intestinal manifestations of IBD and propose these molecules as potential drug targets.
Introduction
Inflammatory Bowel Diseases, which primarily include Ulcerative Colitis and Crohn’s disease, are now increasingly recognized as a complex, multi-factorial constellation of diseases whose incidence has been increasing globally.1,2 While the disease is predominantly described as a gastrointestinal disease, symptoms involving other organ systems are increasingly recognized.3,4 In the past decade, studies conducted in both animals and human models have suggested that several genetic factors are involved in the pathogenesis of IBD.5–10 Animal studies have argued for an immune mediated role for IBD. These studies have utilized techniques like gene deletion, gene mutation, chemical induction, and genetic engineering, where manipulation of several genes, specifically distinct immune regulators increase the genetic susceptibility for IBD; possibly by alteration of host defense mechanisms and modulation of the individual’s microbiome.6,7,10 The human studies on the other hand posit that there is a multi-factorial basis, where genetic alterations in combination with environmental factors have been implicated. This data is derived from studies of inheritance patterns of Crohn’s in monozygotic twins, where a higher incidenceof Crohn’s has been noted.7,8,11 Further, association studies of genomewide databases have postulated that more than 230 IBD loci are implicated in the pathogenesis of IBD.12–14 However, understanding of the mechanistic basis of extra-intestinal manifestations of IBD still remains limited. Few of the signaling pathways known have been proposed to be shared with host response to various bacteria and other immune mediated disorders which comprise extra-intestinal manifestations. This study aims to identify genes involved in IBD and its’ extra-intestinal manifestations, ie, PSC, pyoderma gangrenosum, arthritis, and uveitis. We hypothesize a network of signaling cascades exist that form the etiological backbone of these diseases, their diverse manifestations, and can offer potential pharmaceutical targets.
Methods
We utilized four genetic databases, including the Genecards: The Human Gene Database (www.genecards.org), DisGeNET (https://www.disgenet.org/), The Comparative Toxicogenomics Database (http://ctdbase.org/) and the Universal Protein Resource (https://www.uniprot.org/), to identify genes implicated in UC, CD, uveitis, arthritis, PSC, and pyoderma gangrenosum. Subsequently, using these genes as possible nodes (aka the common genes involved), and the underlying common signaling cascade in these disease processes, we queried NDex, Network Data Exchange (http://www.ndexbio.org/), an open source bioinformatic platform to predict signaling networks.15,16 Of note, these databases provide unique scores to each gene, based on information of curated databases and the likelihood of protein–protein interactions.
Results
Our initial analysis from the Genecards: The Human Gene Database, DisGeNET, The Comparative Toxicogenomics Database and the Universal Protein Resource, identified 3783 genes in UC, 3980 genes in CD, 1043 genes in uveitis, 5583 genes in arthritis, 1313 genes in PSC, and 119 genes in pyoderma gangrenosum. Of note, genes identified from these databases do not directly implicate casualty, but are a summation of altered genes in the respective disease state, from studies of animal models, tissue culture, and human databases. We then identified a unique array of 20 genes, that had the highest probability of involvement in UC, CD, uveitis, arthritis, pyoderma gangrenosum, and PSC. A signaling network or interactome (Figure 1) was then formulated using the NDex, Network Data Exchange.15,16 Of note, this genetic array had a strong emphasis on immune modulators, further arguing for an immune basis in the extra-intestinal presentations of IBD (Table 1 and Figure 1). As expected, we noted Tumor necrosis factor (TNF) involvement in CD, UC, PSC, and arthritis. Further, C-C motif chemokine ligand 2 (CCL2), a chemokine essential for recruitment of monocytes, memory T-cells, and dendritic cells, and an important agent in Protein kinase B (AKT) signaling and Pigment epithelium derived factor (PEDF) pathways, was also involved in CD, UC, arthritis and uveitis.17 Interestingly, unique modulators were also identified as common nodes in distinctive disease processes. We noted that two immune modulators (a) Interleukin-17A (IL-17A), a proinflammatory cytokine, and (b) Interleukin-21 (IL-21), a regulator of Natural Killer (NK) cells and cytotoxic T cells, are both implicated in CD, UC, uveitis, PSC, and arthritis.18,19 Arachidonate 5-lipoxygenase (ALOX5), an important member of the lipoxygenase gene family, was exclusively involved in UC, CD, and arthritis.20 Fas Ligand (FASLG), an important player in apoptosis and death receptor, and CCR5, a known HIV receptor, were detected in UC, CD, PSC, uveitis, and arthritis, but not in pyoderma gangrenosum.21,22 MON2, a regulator of endosome to golgi trafficking and IL-25, a mediator in IL-17 and PEDF signaling, were only noted in CD, UC, pyoderma gangrenosum, and arthritis.23,24 In contrast, SLCO1B3, which encodes for organic anion transporter, was exclusively involved in Pyoderma gangrenosum, UC and CD.25 (Table 1)
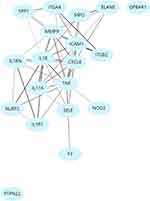 |
Figure 1 Signaling map of genes (interactome) overlapping in inflammatory bowel disease (UC and CD) and its extra-intestinal manifestations. |
Discussion
IBD is a complex group of heterogeneous disorders, with an underlying multifactorial pathophysiological basis. A dysregulation between environmental, underlying human microbiome, and genetic susceptibility factors, is hypothesized to play a crucial role in varying manifestation of IBD.1,–2,–5–13 In our study, we have used genetic data sets to look for overlapping genes involved in IBD and its’ extra-intestinal disease forms (like arthritis, uveitis, and pyoderma gangrenosum). Our study supports the theory that immune modulators are critical mediators in extra-intestinal manifestations of IBD. These genes allude towards cross-sectional nodes, that could further implicate to involvement of different signal transduction molecule cascades in different manifestations of IBD.
We hypothesize that these overlapping molecules act as focal points of intersecting signal transduction pathways and are involved in distinctive clinical presentations of IBD and hence are potential therapeutic targets. While our in silico analysis is limited by a lack of wet-lab experiments, it highlights interesting candidate molecules and possible network pathways, which can be utilized in future more focused experimental designs. We believe our analysis consolidates existing information and lays the groundwork for future in vitro and in vivo studies, dedicated towards understanding the pathophysiology of this complex disease. In vitro tissue culture experiments looking at overexpression, underexpression, and protein–protein interactions are key in elucidating the specific function of these molecules; which in turn would offer more insights towards in vivo studies, either in animal models or towards clinical trials in patients with IBD.
Of particular interest in this regard, and noteworthy here is IL-17A, which was identified as a potential target in our analysis.29,30 Secukinumab, an anti-IL-17A monoclonal antibody, was found to be safe and efficacious in psoriasis and rheumatoid arthritis.29,30 However, higher adverse events were noted in patients with moderate-to-severe CD.30 This highlights an inherent limitation of in silico database analyses and in vitro studies, where there is an inability to re-create the unique human intestinal immunological environment and the complex interactions of the host immune system with the gut microbes, which is perhaps critical in the pathophysiology of IBD. This could, in turn, be a limitation in direct translation of data retrieved from in silico analyses to animal model studies and then in therapeutic clinical trials.
Lastly, databases are ever changing, and comprise information from both animal and human tissues. Thus, we anticipate advancement in cell–cell networking, genomewide association studies to keep evolving over time. Though these ever-growing database repositories do offer the advantage of high throughput screening, we acknowledge that this information still has to be used in conjunction with wet lab data to advance our understanding of IBD.31–33
Conclusion
Our work identifies a unique set of 20 genes involved in IBD and associated extra-intestinal diseases. These genes are involved in various aspects of cellular processes and signal transduction like processes of apoptosis, inflammation, and immune response. We propose that bioinformatics and system immunology is a potent tool to dissect the complex signaling networks in IBD, and further exploration of upstream and downstream effectors of these candidate genes may help in greater understanding of IBD and its extra-intestinal manifestations.
Disclosure
The authors declare that they have no personal nor financial or non-financial conflicts of interest for this work.
The preliminary findings of this paper were presented at the Gastroenterology Conference(s), American College of Gastroenterology, ACG 2014, and DDW 2015, as poster presentation(s) with preliminary results. The poster’s abstract was published in “Poster Abstracts” in American Journal of Gastroenterology, October 2014, Volume 109, p S503 and in Journal Gastroenterology, DOI: https://doi.org/10.1016/S0016-5085(15)32974-7 (https://journals.lww.com/ajg/Fulltext/2014/10002/Using_Genetic_Databases_to_Explore_Biological.1702.aspx https://www.gastrojournal.org/article/S0016-5085(15)32974-7/abstract).
References
1. Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140:1704–1712.
2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 e42. doi:10.1053/j.gastro.2011.10.001
3. Ribaldone DG, Pellicano R, Actis GC. The gut and the inflammatory bowel diseases inside-out: extra-intestinal manifestations. Minerva Gastroenterol Dietol. 2019;65(4):309–318. doi:10.23736/S1121-421X.19.02577-7
4. Garber A, Regueiro M. Extraintestinal manifestations of inflammatory bowel disease: epidemiology, etiopathogenesis, and management. Curr Gastroenterol Rep. 2019;21(7):31. doi:10.1007/s11894-019-0698-1
5. Orholm M, Binder V, Sorensen TI, Rasmussen LP, Kyvik KO. Concordance of inflammatory bowel disease among Danish twins. Results of a nationwide study. Scand J Gastroenterol. 2000;35:1075–1081.
6. Saleh M, Elson CO. Experimental inflammatory bowel disease: insights into the host-microbiota dialog. Immunity. 2011;34(3):293–302. doi:10.1016/j.immuni.2011.03.008
7. Satsangi J, Jewell DP, Bell JI. The genetics of inflammatory bowel disease. Gut. 1997;40(5):572–574. doi:10.1136/gut.40.5.572
8. Thompson NP, Driscoll R, Pounder RE, Wakefield AJ. Genetics versus environment in inflammatory bowel disease: results of a British twin study. BMJ. 1996;312(7023):95–96. doi:10.1136/bmj.312.7023.95
9. Tysk C, Lindberg E, Jarnerot G, Floderus-Myrhed B. Ulcerative colitis and Crohn’s disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988;29(7):990–996. doi:10.1136/gut.29.7.990
10. Mizoguchi A, Mizoguchi E. Animal models of IBD: linkage to human disease. Curr Opin Pharmacol. 2010;10(5):578–587. doi:10.1016/j.coph.2010.05.007
11. Halfvarson J, Bodin L, Tysk C, Lindberg E, Jarnerot G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology. 2003;124(7):1767–1773. doi:10.1016/S0016-5085(03)00385-8
12. Cleynen I, Boucher G, Jostins L, et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet. 2015.
13. Ferguson LR, Shelling AN, Browning BL, Huebner C, Petermann I. Genes, diet and inflammatory bowel disease. Mutat Res. 2007;622(1–2):70–83. doi:10.1016/j.mrfmmm.2007.05.011
14. Actis GC, Pellicano R, Fagoonee S, Ribaldone DG. History of inflammatory bowel diseases. J Clin Med. 2019;8(11):1970. doi:10.3390/jcm8111970
15. Pratt D, Chen J, Welker D, et al. NDEx, the network data exchange. Cell Syst. 2015;1(4):302–305. doi:10.1016/j.cels.2015.10.001
16. Szklarczyk D, Morris JH, Cook H, et al. The STRING database in 2017: quality-controlled protein-protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45(D1):D362–D368. doi:10.1093/nar/gkw937
17. Lim SY, Yuzhalin AE, Gordon-Weeks AN, Muschel RJ. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget. 2016;7(19):
18. Abusleme L, Moutsopoulos NM. IL-17: overview and role in oral immunity and microbiome. Oral Dis. 2017;23(7):
19. Davis MR, Zhu Z, Hansen DM, Bai Q, Fang Y. The role of IL-21 in immunity and cancer. Cancer Lett. 2015;358(2):
20. Costea I, Mack DR, Israel D, et al. Genes involved in the metabolism of poly-unsaturated fatty-acids (PUFA) and risk for Crohn’s disease in children & young adults. PLoS One. 2010;5(12):e15672. doi:10.1371/journal.pone.0015672
21. Rieux-Laucat F, Magérus-Chatinet A, Neven B. The autoimmune lymphoproliferative syndrome with defective FAS or FAS-ligand functions. J Clin Immunol. 2018;38(5):
22. Ellwanger JH, Kulmann-Leal B, Kaminski VL, Rodrigues AG, de Souza Bragatte MA, Chies JAB. Beyond HIV infection: neglected and varied impacts of CCR5 and CCR5Δ32 on viral diseases. Virus Res. 2020;286:198040. doi:10.1016/j.virusres.2020.198040
23. Su J, Chen T, Ji XY, et al. IL-25 downregulates Th1/Th17 immune response in an IL-10-dependent manner in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(4):720–728. doi:10.1097/MIB.0b013e3182802a76
24. Mahajan D, Tie HC, Chen B, Lu L. Dopey1-Mon2 complex binds to dual-lipids and recruits kinesin-1 for membrane trafficking. Nat Commun. 2019;10(1):3218. doi:10.1038/s41467-019-11056-5
25. Malagnino V, Hussner J, Issa A, Midzic A, Meyer Zu Schwabedissen HE. OATP1B3-1B7, a novel organic anion transporting polypeptide, is modulated by FXR ligands and transports bile acids. Am J Physiol Gastrointest Liver Physiol. 2019;317(6):G751–G762. doi:10.1152/ajpgi.00330.2018
26. Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi:10.1093/nar/28.1.27
27. UniProt Consortium.UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2019;47(D1):D506–D515. doi:10.1093/nar/gky1049
28. Letovsky SI, Cottingham RW, Porter CJ, Li PW. GDB: the Human Genome Database. Nucleic Acids Res. 1998;26(1):94–99. doi:10.1093/nar/26.1.94
29. Ruiz de Morales JMG, Puig L, Daudén E. Critical role of interleukin (IL)-17 in inflammatory and immune disorders: an updated review of the evidence focusing in controversies. Autoimmun Rev. 2020;19(1):102429. doi:10.1016/j.autrev.2019.102429
30. Hueber W, Sands BE, Lewitzky S, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700. doi:10.1136/gutjnl-2011-301668
31. Smillie CS, Biton M, Ordovas-Montanes J, et al. Intra- and inter-cellular rewiring of the human colon during ulcerative colitis. Cell. 2019;178(3):714–730.e22. doi:10.1016/j.cell.2019.06.029
32. Perez-Jeldres T, Alvarez-Lobos M, Rivera Nieves J, Villablanca EJ. The cell circuitry of ulcerative colitis, a new view for a highly complex disease. Gastroenterology. 2020;158(5):1506–1508. doi:10.1053/j.gastro.2020.02.019
33. Shi H, Yan KK, Ding L, Qian C, Chi H, Yu J. Network approaches for dissecting the immune system. iScience. 2020;23(8):101354. doi:10.1016/j.isci.2020.101354
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

