Back to Journals » Clinical Ophthalmology » Volume 14
How Can We Improve Toric Intraocular Lens Calculation Methods? Current Insights
Authors Ferreira TB , Ribeiro F
Received 3 April 2020
Accepted for publication 29 May 2020
Published 6 July 2020 Volume 2020:14 Pages 1899—1908
DOI https://doi.org/10.2147/OPTH.S238686
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Tiago B Ferreira,1 Filomena Ribeiro1– 3
1Hospital Da Luz Lisboa, Lisbon, Portugal; 2Faculdade De Medicina Da Universidade De Lisboa, Lisbon, Portugal; 3Visual Sciences Research Centre, University of Lisbon, Lisbon, Portugal
Correspondence: Tiago B Ferreira
Department of Ophthalmology, Hospital Da Luz Lisboa, Av. Lusíada, 100, Lisbon 1500-650, Portugal
Tel +351 217 104 400
Fax +351 217 104 409
Email [email protected]
Abstract: In this paper, we review current strategies for calculating toric intraocular lenses (IOLs). We discuss the prevalence and clinical relevance of astigmatism and the assessment of toric IOL candidates. We detail recommendations for evaluating astigmatism and current biometry and IOL power calculation techniques. Finally, error sources and results of current toric IOL calculators are discussed.
Keywords: astigmatism, toric intraocular lens, biometry, surgically induced astigmatism
The Relevance of Astigmatism
With the recent trend towards cataract surgery becoming a refractive procedure, the accuracy of spherical refractive result increased by the use of optical biometry combined with new generation intraocular lens (IOL) power calculation formulas.1 With the increased importance of a perfect refractive outcome, management of astigmatism became an integral part of any ophthalmic surgery. This importance was recently recognized in a study from the European Registry of Quality Outcomes database for cataract and refractive surgery, which resulted in a recommendation for increasing the use of toric IOLs in order to improve outcomes.2 It is known that correction of astigmatism of more than 0.5 D improves the visual outcomes of cataract surgery3 With the combination of optical biometry and last generation formulas, such as the Barrett Universal II or the Hill-radial basis function, it is now possible to obtain a spherical refractive result within ±0.50 D of the target in 72 to 80% of the eyes.4,5 On the other hand, with classical toric IOL calculation only 26 to 35% of the eyes achieve a result within ±0.50 D of the targeted astigmatism.1,6 In most studies, after toric IOL implantation, the mean refractive astigmatism ranges between −0.72 ±0.43 D and −1.03 ± 0.79.7,8 These results evidence the need of improving the cylindrical calculation of these IOLs. Furthermore, studies showed that some IOLs, particularly multifocal or aspheric designs, are ineffective if residual astigmatism is present after surgery.7 Nevertheless, toric IOL are the most effective method of correcting astigmatism during cataract surgery.9–11
Implanting a toric IOL is a complex process, with multiple steps that require optimization in order to obtain accurate results. (Figure 1).
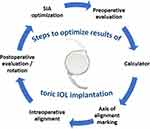 |
Figure 1 Steps involved in the pre-, intra- and postoperative study for implanting a toric IOL. |
Firstly, knowledge of ocular biometric parameters is essential. It is known that ocular biometric parameters vary with individual characteristics and in different populations.
We recently studied ocular biometric parameters with an optical biometer (Lenstar LS900, Haag-Streit AG, Köniz, Switzerland) in a population of 6506 eyes of 6506 candidates for cataract surgery.12
Figure 2 shows the distribution of corneal astigmatism in the studied population. While the mean corneal astigmatism was 1.08 ± 0.84 D (range 0–7.58), it is important to note that 1415 (43.5%) eyes showed a corneal astigmatism ≥ 1 D. The most frequent group showed astigmatism between 1.00 and 1.59 D and 461 eyes (3%) showed a corneal astigmatism over 3.00 D.
 |
Figure 2 Distribution of corneal astigmatism in the studied sample.Note: Reproduced from Ferreira TB, Hoffer KJ, Ribeiro F, Ribeiro P, O’Neill JG. Ocular biometric measurements in cataract surgery candidates in Portugal. PLoS One. 2017;12(10):e0184837. Creative Commons license and disclaimer available from: https://creativecommons.org/licenses/by/4.0/legalcode.12 |
Preoperative Evaluation for Toric Intraocular Lens Implantation
Candidate Assessment
As with any other ophthalmic surgery, careful ophthalmic examination is mandatory before toric IOL implantation. Any relevant medical history must be noted. Insufficient mydriasis may be a relative contraindication for toric IOLs, as it may hamper the visualization of the alignment marks in the IOL periphery.13–15 Other anterior segment conditions, namely corneal dystrophies, narrow anterior chambers, zonular instability, capsular bag size or the presence of abnormal anterior segment configurations that may influence toric IOL rotational stability are equally important. Anterior segment imaging with anterior segment optical coherence tomography or ultrasound biomicroscopy may be considered in these cases.
Corneal topography or tomography is mandatory in the preoperative study of these patients. Not only the keratometric values are important, ideally taken from different devices for comparison, but also contraindications for toric IOLs must be ruled-out. Although classically these include irregular astigmatism or corneal ectasias, it is important to note that, even though regular astigmatism is most suitable for toric IOL implantation,15 good results have been reported in eyes with irregular astigmatism.16 In these cases, not only patient expectations must be properly managed but stability of corneal disease must be documented.
The importance of corneal surface in cataract surgery, particularly when premium IOLs are considered, has been increasingly recognized. Not only visual quality but also accuracy of preoperative examinations is correlated with ocular surface integrity. Different tests may be used for evaluating dry eye. The tear meniscus height may be evaluated on the slit lamp and Meibomian gland disease (MGD) should be ruled-out. Also, on the slit lamp, tear film break-up time may be quantified.17,18 Schirmer’s test was considered the gold standard for many years.19 Other more complex tests, including tear film osmolarity, levels of lactoferrin or lysozyme, or the use of imaging techniques or impression cytology are seldom used in everyday clinical practice.
Besides general risks of cataract surgery, patients should be warned of a possible refractive surprise. In the case of a toric IOL, it may be caused by IOL misalignment, which, if associated with decreased visual acuity, may justify a secondary procedure.
Biometry and Intraocular Lens Power Calculation
Precise IOL power calculation is imperative to achieve a good outcome in cataract surgery. Optical biometry is nowadays the gold standard. Optical biometers are based on one of three principles: partial coherence interferometry (PCI), optical low-coherence refractometry (OLCR) or optical coherence tomography (OCT). The main advantage of optical over ultrasound biometry, is increased accuracy, while avoiding artifacts secondary to corneal compression, which may lead to an overestimation of the IOL power.20,21 While immersion ultrasound may also yield accurate results, it is a time-consuming technique dependent on an experienced operator.22,23 Optical biometry is less dependent on the operator.24,25 Furthermore, the risk of infection is eliminated and most optical biometers evaluate other anterior segment parameters essential for last generation formulas, such as corneal diameter or lens thickness. In all cataract or clear lens exchange surgeries, the surgeon must consider the targeted astigmatic correction. If surgery is planned in only one eye, the refractive status of the fellow eye should also be taken into account. Studies show that visual acuity (for distance and near) is superior when residual astigmatism is ATR than when it is WTR or oblique.26,27 Regardless of the axis, myopic astigmatism results in inferior distance visual acuity than hyperopic astigmatism.28 Knowledge of these outcomes may guide adjustment of a target to aid near visual acuity in cases where a presbyopia-correcting IOL is not primarily used for this purpose.
In cases of multifocal toric IOLs, minimal residual astigmatism is even more important, as studies show that astigmatism over 0.75 D not only deteriorates visual acuity but is also one of the main causes of dissatisfaction after surgery with this kind of IOLs.8,29,30
Evaluation of Corneal Astigmatism
Measurement of keratometry is an important variable in biometry, especially considering that the standard deviation of corneal topography can represent an error of 1 D in IOL power calculation. Also, corneal dioptric power is, for most IOL calculation formulas, an integral part of ELP estimation and, thus, of the resultant residual refractive error.
Several factors influence the accuracy of corneal astigmatism measurements. As previously mentioned, a stable tear film and the absence of corneal epitheliopathy are essential for precise measurements.31–34 Contact lenses users deserve special attention. These patients should interrupt contact lens use before astigmatism evaluation. The time for contact lens use interruption and for the cornea to attain a steady state is variable. The wear of soft contact lenses should be stopped for at least two weeks before topography.35 Rigid contact lens (RPG) wear discontinuation and evaluation of serial corneal topography and refraction are critical in the differential diagnosis of corneal warpage. The time of removal of RPG lenses before corneal topography is controversial.36 After RPG wear suspension for 7 days, studies report earlier changes on the anterior corneal surface but no changes in the posterior corneal surface.
Multiple instruments exist for the evaluation of corneal astigmatism. These include manual and automated keratometers, topographers based on the Placido principle, scanning-slit based tomographers, instruments based on the Scheimpflug principle, devices based on color point-source light-emitting diodes (LED) and anterior-segment optical coherence tomography (AS-OCT) devices. When comparing these devices, measurements of the anterior surface of the cornea and total corneal astigmatism by color-LED topography are more precise than those of automated keratometry or slit-scanning tomography. Furthermore, they are highly repeatable.37–39
Calculation of the Cylindrical Power of the Toric IOL
Despite the increased importance of a precise correction of astigmatism in cataract or clear lens exchange surgery, several sources of error in the calculation of the cylindrical power of toric IOLs still exist.
Firstly, the variable distance between the cornea and the IOL planes, implies that for, each cylindrical power at the IOL plane, a different magnitude of astigmatism is corrected at the corneal plane. Classical toric IOL calculators40 assume a fixed ratio between the cylindrical power of the IOL at the corneal and IOL planes. This results in undercorrection of astigmatism in eyes with long axial length (AL) and overcorrection in eyes with short AL. This effect is particularly important in eyes with high ametropias41,42 (eg in an eye with an AL of 20.0 mm the real ratio is 1.29 and in an eye with an AL of 30.0 mm the real ratio is 1.86).41,42 Different strategies were described to overcome this limitation, including considering the pachymetry and anterior chamber depth in the calculation of the IOL power.43,44
A distinct limitation is not considering the IOL spherical power, which influences the cylindrical power at the corneal plane due to the different vergence of the rays. This induces particularly important errors in IOLs with high cylindrical powers and, in particular, when the refractive error is associated with high hyperopia. For example, considering the same effective lens position (5.2 mm for Alcon), an Acrysof Toric SN60T3 IOL (1.50 D of cylinder at the IOL plane and 1.03 at the corneal plane, according to the manufacturer) has a true cylindrical power of 1.32 D at the corneal plane if the spherical power of the IOL is 17.0 D and 1.22 D if the spherical power is 28.0 D. In the case of a SN60T9, the error induced by not considering the IOL spherical power, may be over 1 D.45
Finally, the most important source of error in toric IOL power calculation is not considering the astigmatism of the posterior corneal surface46 This results in undercorrection of astigmatism in eyes with ATR astigmatism and overcorrection in eyes with WTR astigmatism.47
In an unpublished study, we investigated the characteristics of posterior and total corneal astigmatism in 755 eyes from 410 patients by color-LED topography.
All eyes were measured using a Color-LED topographer (Cassini I-Optics, Den Haag, The Netherlands, software v2.4.1). The Cassini has approximately 700 red, yellow, and green LEDs arranged in a specific pattern to ensure a 1-to-1 correspondence between the source and image points. Using forward ray tracing, the anterior corneal surface is reconstructed. This information, combined with the second Purkinje reflex of 7 additional infrared LEDs to study the posterior surface of the cornea (again, using forward ray tracing), allows the Cassini to measure total corneal astigmatism.
The following parameters were evaluated by the Cassini:
1. SimK corneal astigmatism (1.3375):
Corneal astigmatism from simulated keratometry (K) over the 0.0 mm to 3.0 mm zone. The magnitude of CA SimK is the difference between steep simulated K and flat simulated K, and the meridian is the steep simulated K meridian. This value is calculated based on the anterior corneal measurement only, using the effective corneal refractive index of 1.3375.
2. Anterior corneal astigmatism (1.376):
Corneal astigmatism from the anterior corneal surface, which is uniquely measured instantaneously over the 0.0mm to 3.0 mm zone. The magnitude of CA Anterior is calculated from the measured radius of curvature (RoC) by (1.376–1.0)/Roc, in which the refractive index of air is 1.0, the refractive index of the cornea is 1.376 and the Radius of Curvature is expressed in meters. The CA Anterior power is calculated for both the steep and flat meridian. The difference represents corneal astigmatism of the anterior corneal surface.
3. Posterior corneal astigmatism (1.336):
Corneal astigmatism from the posterior corneal surface, measured at a ring with a diameter of approximately 3.8 mm using an instantaneous recording of the second Purkinje images. A raytracing model is used to convert the measurement points into the posterior corneal radius of curvature for the steep and flat meridian. Posterior corneal power for both meridians is calculated through (1.336–1.376)/Roc, in which the refractive index of the cornea is 1.376, the refractive index of the aqueous humor is 1.336 and the Radius of Curvature is expressed in meters.
3. Total corneal astigmatism:
Corneal astigmatism from total corneal astigmatism (TCA) displayed on the device. The CA TCA is measured by parallel ray tracing through the anterior and posterior corneal surfaces using the Snell law over the central 0.0 mm to 3.0 mm zone. This calculation of total corneal astigmatism combines the contributions of the anterior and posterior corneal surfaces independently of the effect of corneal thickness.
The internal quality verification tests included in the device were utilized to validate the quality of the obtained measurement for acceptance in the study. The selected measurements of the Cassini device were those in which all quality indicators (Quality Factor Centration (Lateral), Quality Factor Focus (Axial), Quality Factor Corneal Coverage, Quality Factor Stability Value and Quality Factor Posterior Value) were 85% or more (green on the display).
We found that WTR orientation was the most common for posterior corneal astigmatism, in 70% of cases. This vertical orientation was the most common, irrespective of anterior meridian location. The location of the posterior steep meridian according to that of the anterior steep meridian is shown in Figure 3.
 |
Figure 3 Location of the posterior steep corneal meridian according to the location of the anterior steep corneal meridian. |
In 81% of eyes, total corneal astigmatism magnitude was 0.5 D or more. Posterior corneal astigmatism magnitude was 0.5 D or more and in 22% of eyes. The distribution of posterior and total corneal astigmatism is shown in Figure 4.
 |
Figure 4 Distribution of posterior and total corneal astigmatism. |
Given the limited accuracy and the frequent inability to directly evaluate the posterior corneal surface, several nomograms and mathematical models were developed to account for the effect of posterior corneal astigmatism when in is not directly measured.
The Baylor nomogram was the first to be published.48 Later, Goggin et al developed coefficients of adjustment to adjust the anterior keratometric power while also considering the spherical power of the IOL.2 Abulafia et al published the Abulafia-Koch formula. It consists of a mathematical regression to estimate the effect of the posterior corneal surface.49 Several other calculators were recently developed, accounting for ELP, spherical power of the IOL and/or total corneal astigmatism. One of these is the Holladay toric calculator (available in the software “Holladay IOL consultant & surgical outcomes assessment”), which takes the predicted ELP into account. The Barrett toric calculator50 considers the ELP, while also adjusting the cylindrical power the IOL axis of alignment. The calculator is based on a mathematical model and a regression formula for estimating the effect of the posterior corneal surface.51 It was recently updated to allow the introduction of keratometry values from different instruments and calculating of mean or median keratometry values to be used in the calculation. This “Median K” value further improves the results of this calculator.52 Furthermore, the Barrett toric calculator now allows using real measurements of the posterior corneal surface from an AS-OCT or a Scheimpflug device. This improves the outcomes in normal eyes.53 In addition, it is important to remember that, with this calculator, a customized method is available for patients previously submitted to refractive surgery, through the “Barrett True K Toric Calculator”, also available in the calculator.
Ray tracing is a distinct strategy for calculation the IOL power. It overcomes problems related with simplifications by using measured instead of presumed geometry, and not including keratometric indices. This avoids the overestimation of corneal power caused by using the common 1.3375 refractive index and the variability caused by the use of different keratometric indices in different devices. To consider all the potential applications of raytracing for IOL power calculation, the development of new pseudophakic eye models is warranted.54
Other recent toric calculators include the Emmetropia Verifying Optical (EVO) toric formula and the Panacea software, yet untested in accuracy to the best of our knowledge.
Recently, we compared the mean absolute error (MAE) in residual astigmatism predicted by the available new toric IOL calculation methods detailed in Table 1.
 |
Table 1 Toric IOL Calculation Methods Compared in the Study |
The lowest MAE and centroid error in predicted residual astigmatism was obtained by the Barrett Toric Calculator (both in the whole sample and in subgroups of eyes with WTR and ATR astigmatism). The Abulafia-Koch formula in conjunction with a strategy to estimate the ELP did not show statistically significant differences for the Barrett Toric Calculator.55
In a consecutive study, we showed that the prediction of the posterior corneal surface power with mathematical models is superior to its direct measurement with a Scheimpflug camera (Figure 5).
 |
Figure 5 Percentage of eyes within 0.25, 0.50, 0.75, and 1.00 diopters D of absolute astigmatic prediction error with each calculation method.Note: Republished with permission of SLACK Inc., Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of Methodologies Using Estimated or Measured Values of Total Corneal Astigmatism for Toric Intraocular Lens Power Calculation. 33;(12):794-800. J Refract Surg. Copyright 2017. permission conveyed through Copyright Clearance Center, Inc.59 |
The Importance of Surgically Induced Astigmatism in Toric IOL Calculation
Considering surgically induced astigmatism (SIA) is an integral part of toric IOL calculation. It is important to remember that the postoperative corneal power is the vectorial combination of preoperative corneal astigmatism with that generated by the creation of a clear corneal incision (CCI).
Even with small incisions and fixed meridians, SIA is highly variable, particularly in more curved corneas.
The femtosecond laser is a new technology for cataract surgery that allows the execution of several surgical steps, including the construction of CCIs.56 In a recent study,57 we compared the SIA in patients submitted to cataract surgery with CCIs created manually or by the femtosecond laser (300 eyes in each group) at superior oblique and temporal locations. The results are presented on Table 2.
 |
Table 2 SIA Vector, Flattening Effect, and Torque in the Femtosecond Laser and Manual CCI Groups |
Despite SIA, flattening effect (FE), torque, and the summated vector mean (SVM) for SIA being slightly lower in the group with CCIs created by the femtosecond laser, the differences for the manual group were not statistically significant.
For toric IOL calculation, the FE calculated at the corneal meridian of the incision should be used.58 In our study, for temporal incisions, the FE was −0.11 D in the femtosecond laser group and −0.13 D in the manual group. For superior oblique incisions, the FE was −0.21 D in the femtosecond laser group and −0.34 D in the manual group.
The results of the correlations between SIA and individual features are shown in Table 3.
 |
Table 3 Correlation Between Surgically Induced Astigmatism Magnitude and Individual Parameters |
These results support the unpredictability of SIA. However, it is interesting to note that, for the femtosecond laser group, a moderate to high significant positive correlation was found between preoperative corneal astigmatism magnitude and SIA. Thus, in these patients, the potential for a higher SIA to be generated during surgery should be considered.
Conclusions
A careful candidate selection, preoperative evaluation of astigmatism, usage of a last-generation calculator and accurate consideration of SIA are mandatory in order to improve clinical results with toric IOLs.
Acknowledgment
Part of this paper was presented at the 24th ESCRS Winter Meeting as a presentation/conference talk with interim findings.
Disclosure
The authors have no proprietary or financial interests in any of the products or devices used in this study and report no conflicts of interest in this work.
References
1. Koch DD, Hill W, Abulafia A, Wang L. Pursuing perfection in intraocular lens calculations: I. Logical approach for classifying IOL calculation formulas. J Cataract Refract Surg. 2017;43(6):717–718. doi:10.1016/j.jcrs.2017.06.006
2. Lundström M, Dickman M, Henry Y, et al. Risk factors for refractive error after cataract surgery: analysis of 282 811 cataract extractions reported to the European registry of quality outcomes for cataract and refractive surgery. J Cataract Refract Surg. 2018;44(4):45–447. doi:10.1016/j.jcrs.2018.01.031
3. Villegas EA, Alcon E, Artal P. Minimum amount of astigmatism that should be corrected. J Cataract Refract Surg. 2014;40(1):13–19. doi:10.1016/j.jcrs.2013.09.010
4. Melles RB, Holladay JT, Chang WJ. Accuracy of intraocular lens calculation formulas. Ophthalmology. 2018;125(2):169–178. doi:10.1016/j.ophtha.2017.08.027
5. Kane JX, Van Heerden A, Atik A, Petsoglou C. Accuracy of 3 new methods for intraocular lens power selection. J Cataract Refract Surg. 2017;43(3):333–339. doi:10.1016/j.jcrs.2016.12.021
6. Abulafia A, Barrett GD, Kleinmann G, et al. Prediction of refractive outcomes with toric intraocular lens implantation. J Cataract Refract Surg. 2015;41(5):936–944. doi:10.1016/j.jcrs.2014.08.036
7. Teus MA, Arruabarrena C, Hernández-Verdejo JL, Sales-Sanz A, Sales-Sanz M. Correlation between keratometric and refractive astigmatism in pseudophakic eyes. J Cataract Refract Surg. 2010;36(10):1671–1675. doi:10.1016/j.jcrs.2010.05.010
8. Hayashi K, Hayashi H, Nakao F, Hayashi F. Influence of astigmatism on multifocal and monofocal intraocular lenses. Am J Ophthalmol. 2000;130:477–482. doi:10.1016/S0002-9394(00)00526-2
9. Swampillai AJ, Khanan Kaabneh A, Habib NE, et al. Efficacy of toric intraocular lens implantation with high corneal astigmatism within the United Kingdom’s National Health Service. Eye. 2019. doi:10.1038/s41433-019-0744-0
10. Qammar A, Mullaney P. Paired opposite clear corneal incisions to correct preexisting astigmatism in cataract patients. J Cataract Refract Surg. 2005;31:1167–1170. doi:10.1016/j.jcrs.2004.11.053
11. Medicute J, Irigoyen C, Ruiz M, Illarramendi I, Ferrer-Blasco T, Montes-Mico R. Toric intraocular lenses versus opposite clear corneal incisions to correct astigmatism in eyes having cataract surgery. J Cataract Refract Surg. 2009;35:451–458. doi:10.1016/j.jcrs.2008.11.043
12. Ferreira TB, Hoffer KJ, Ribeiro F, Ribeiro P, O’Neill JG. Ocular biometric measurements in cataract surgery candidates in Portugal. PLoS One. 2017;12(10):e0184837. doi:10.1371/journal.pone.0184837.
13. Kohnen T, Kook D, Auffarth GU, Derhartunian V. Einsatzmoglichkeiten intraokularer Multifokallinsen und Kriterien der Patientenselektion [Use of multifocal intraocular lenses and criteria for patient selection]. Ophthalmologe. 2008;105:527–532. doi:10.1007/s00347-008-1745-8
14. Slade SG. Patient selection and education. In: Chang DF, editor.Mastering Refractive IOLs; the Art and Science. 331–431. Thorofare, NJ: Slack; 2008.
15. Visser N, Bauer N, Nuijts R. Toric intraocular lenses: historical overview, patient selection, IOL calculation, surgical techniques, clinical outcomes, and complications. J Cataract Refract Surg. 2013;39:624–637. doi:10.1016/j.jcrs.2013.02.020
16. Visser N, Gast STJM, Bauer NJC, Nuijts RMMA. Cataract surgery with toric intraocular lens implantation in keratoconus: a case report. Cornea. 2011;30:720–723. doi:10.1097/ICO.0b013e31820009d4
17. Chuang J, Shih KC, Chan TC, Wan KH, Jhanji V, Tong L. Preoperative optimization of ocular surface disease before cataract surgery. J Cataract Refract Surg. 2017;43(12):
18. Yu Y, Hua H, Wu M, et al. Evaluation of dry eye after femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2015;41:2614–2623. doi:10.1016/j.jcrs.2015.06.036
19. Lemp MA, Foulks GN. The definition and classification of dry eye disease: report of the definition and classification subcommittee of the international dry eye workShop (2007). Ocul Surf. 2007;5:
20. Weisenthal RW, Afshari NA, Bouchard CS, et al. Examination techniques for the external eye and cornea. In: external disease and cornea, basic and clinical science course. Sec. 2013;8:
21. Binkhorst RD. The accuracy of ultrasonic measurement of the axial length of the eye. Ophthalmic Surg. 1981;12:363–365.
22. Seres A, Németh J, Süveges I. Unexpected ametropia after intraocular lens implantation: the role of different factors of ultrasound biometry and surgery. Doc Ophthalmol Proc Ser. 1997;61:415–420.
23. Olsen T, Nielsen PJ. Immersion versus contact technique in the measurement of axial length by ultrasound. Acta Ophthalmol. 1989;67:101–102. doi:10.1111/j.1755-3768.1989.tb00732.x
24. Haigis W, Lege B, Miller N, Schneider B. Comparison of immersion ultrasound biometry and partial coherence interferometry for intraocular lens calculation according to Haigis. Graefes Arch Clin Exp Ophthalmol. 2000;238:765–773. doi:10.1007/s004170000188
25. Drexler W, Findl O, Menapace R, et al. Partial coherence interferometry: a novel approach to biometry in cataract surgery [letter]. Am J Ophthalmol. 1998;126:524–534. doi:10.1016/S0002-9394(98)00113-5
26. Kiss B, Findl O, Menapace R, et al. Refractive outcome of cataract surgery using partial coherence interferometry and ultrasound biometry. Clinical feasibility study of a commercial prototype II. J Cataract Refract Surg. 2002;28:230–234. doi:10.1016/S0886-3350(01)01274-3
27. Nagpal KM, Desai C, Trivedi RH, et al. Is pseudophakic astigmatism a desirable goal? Indian J Ophthalmol. 2000;48:213–216.
28. Nanavaty MA, Vasavada AR, Patel AS, et al. Analysis of patients with good uncorrected distance and near vision after monofocal intraocular lens implantation. J Cataract Refract Surg. 2006;32:1091–1097. doi:10.1016/j.jcrs.2006.03.021
29. Singh A, Pesala V, Garg P, Bharadwaj SR. Relation between uncorrected astigmatism and visual acuity in pseudophakia. Optom Vis Sci. 2013;90:378–384. doi:10.1097/OPX.0b013e318288afb5
30. Zheleznyak L, Kim MJ, MacRae S, Yoon G. Impact of corneal aberrations on through-focus image quality of presbyopia-correcting intraocular lenses using an adaptive optics bench system. J Cataract Refract Surg. 2012;38:1724–1733. doi:10.1016/j.jcrs.2012.05.032
31. de Vries NE, Webers CAB, Touwslager WRH, et al. Dissatisfaction after implantation of multifocal intraocular lenses. J Cataract Refract Surg. 2011;37:859–865. doi:10.1016/j.jcrs.2010.11.032
32. Nemeth J, Erdelyi B, Csakany B. Corneal topography changes after a 15 second pause in blinking. J Cataract Refract Surg. 2001;27:589–592. doi:10.1016/S0886-3350(01)00768-4
33. Dursun D, Monroy D, Knighton R, et al. The effects of experimental tear film removal on corneal surface regularity and barrier function. Ophthalmology. 2000;107:1754–1760. doi:10.1016/S0161-6420(00)00273-6
34. Dursun D, Piniella AM, Pflugfelder SC. Pseudokeratoconus caused by rosacea. Cornea. 2001;20:668–669. doi:10.1097/00003226-200108000-00024
35. McKernan L, O’Dwyer A, Simo Mannion L. The influence of soft contact lens wear and two weeks lens cessation on corneal curvature. Contact Lens Anterior Eye. 2014;37(1):31–37. doi:10.1016/j.clae.2013.07.014
36. Jinabhai A, Radhakrishnan H, O’Donnell C. Corneal changes after suspending 282 contact lens wear in early pellucid marginal corneal degeneration and moderate 283 keratoconus. Eye Contact Lens. 2011;37:99–105. doi:10.1097/ICL.0b013e31820592b1
37. Piñero DP, Camps VJ, de Fez D, García C, Caballero MT. Validation of posterior corneal curvature measurements with color light-emitting diode topography. Eur J Ophthalmol. 2019;21:1120672119870738.
38. Ferreira TB, Ribeiro FJ. A novel color-LED corneal topographer to assess astigmatism in pseudophakic eyes. Clin Ophthalmol. 2016;10:1521–1529. doi:10.2147/OPTH.S113027
39. Ferreira TB, Ribeiro FJ. Comparability and repeatability of different methods of corneal astigmatism assessment. Clin Ophthalmol. 2018;12:29–34. doi:10.2147/OPTH.S146730
40. http://acrysoftoriccalculator.com.
41. Savini G, Hoffer KJ, Ducoli P. A new slant on toric intraocular lens power calculation. J Refract Surg. 2013;29(5):348–354. doi:10.3928/1081597X-20130415-06
42. Savini G, Hoffer KJ, Carbonelli M, Ducoli P, Barboni P. Influence of axial length and corneal power on the astigmatic power of toric intraocular lenses. J Cataract Refract Surg. 2013;39:1900–1903. doi:10.1016/j.jcrs.2013.04.047
43. Savini G, Naeser K. An analysis of the factors influencing the residual refractive astigmatism after cataract surgery with toric intraocular lenses. Invest Ophthalmol Vis Sci. 2015;56:827–835. doi:10.1167/iovs.14-15903
44. Fam HB, Lim KL. Meridional analysis for calculating the expected spherocylindrical refraction in eyes with toric intraocular lenses. J Cataract Refract Surg. 2007;33:2072–2076. doi:10.1016/j.jcrs.2007.07.034
45. Goggin M, Moore S, Easterman A. Outcome of toric intraocular lens implantation after adjusting for anterior chamber depth and intraocular lens sphere equivalent power effects. Arch Ophthalmol. 2011;129:
46. Ninomiya Y, Minami K, Miyata K, et al. Toric intraocular lenses in eyes with with-the-rule, against-the-rule, and oblique astigmatism: one-year results. J Cataract Refract Surg. 2016;42:1431–1440. doi:10.1016/j.jcrs.2016.07.034
47. Koch DD, Jenkins RB, Weikert MP, et al. Correction astigmatism with toric intraocular lenses: effect of posterior corneal astigmatism. J Cataract Refract Surg. 2013;39(12):1803–1809. doi:10.1016/j.jcrs.2013.06.027
48. Goggin M, Zamora-Alejo K, Esterman A, van Zyl L. Adjustment of anterior corneal astigmatism values to incorporate the likely effect of posterior corneal curvature for toric intraocular lens calculation. J Refract Surg. 2015;31:98–102. doi:10.3928/1081597X-20150122-04
49. Abulafia A, Koch DD, Wang L, et al. New regression formula for toric intraocular lens calculations. J Cataract Refract Surg. 2016;42:663–671. doi:10.1016/j.jcrs.2016.02.038
50. https://www.apacrs.org/toric_calculator20/Toric%20Calculator.aspx.
51. Barrett GD. An improved universal theoretical formula for intraocular lens power prediction. J Cataract Refract Surgery. 1993;19:713–720. doi:10.1016/S0886-3350(13)80339-2
52. Barret G, Lipsky L. Integrated K to improve toric IOL prediction. ASCRS. 2018.
53. Skrzypecki J, Sanghvi PM, Suh LH. Performance of the barrett toric calculator with and without measurements of posterior corneal curvature. Eye. 2019;33(11):1762–1767. doi:10.1038/s41433-019-0489-9.
54. Ribeiro FJ, Castanheira-Dinis A, Dias JM. Personalized pseudophakic model for refractive assessment. Bui BV, editor. PLoS One. 2012. 7. 10. e46780. doi:10.1371/journal.pone.0046780.
55. Gundersen KG, Potvin R. Clinical outcomes with toric intraocular lenses planned using an optical low coherence reflectometry ocular biometer with a new toric calculator. Clin Ophthalmol. 2016;10:2141–2147. doi:10.2147/OPTH.S120414
56. Nagy ZZ. New technology update: femtosecond laser in cataract surgery. Clin Ophthalmol. 2014;8:1157–1167. doi:10.2147/OPTH.S36040
57. Ferreira TB, Ribeiro FJ, Pinheiro J, Ribeiro FJ, O’Neill JG. Comparison of surgically induced astigmatism and morphologic features resulting from femtosecond laser and manual clear corneal incisions for cataract surgery. J Refract Surgery. 2018;34(5):322–329. doi:10.3928/1081597X-20180301-01
58. Alpins N, Ong JKY, Stamatelatos G. Asymmetric corneal flattening effect after small incision cataract surgery. J Refract Surg. 2016;32(9):598–603. doi:10.3928/1081597X-20160608-01
59. Ferreira TB, Ribeiro P, Ribeiro FJ, O’Neill JG. Comparison of Methodologies Using Estimated or Measured Values of Total Corneal Astigmatism for Toric Intraocular Lens Power Calculation. J Refract Surg. 2017;33(12):794–800.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
