Back to Journals » International Journal of Nanomedicine » Volume 12
High-intensity focused ultrasound-triggered nanoscale bubble-generating liposomes for efficient and safe tumor ablation under photoacoustic imaging monitoring
Authors Feng G, Hao L , Xu C, Ran H, Zheng Y, Li P , Cao Y, Wang Q, Xia J, Wang Z
Received 22 February 2017
Accepted for publication 19 May 2017
Published 28 June 2017 Volume 2017:12 Pages 4647—4659
DOI https://doi.org/10.2147/IJN.S135391
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Gang Feng,1 Lan Hao,1 Chunyan Xu,1 Haitao Ran,1 Yuanyi Zheng,1 Pan Li,1 Yang Cao,1 Qi Wang,2 Jizhu Xia,1 Zhigang Wang1
1Department of Ultrasound, Institute of Ultrasound Imaging, the Second Affiliated Hospital of Chongqing Medical University, Chongqing Key Laboratory of Ultrasound Molecular Imaging, 2State Key Laboratory of Ultrasound Engineering in Medicine Co-Founded by Chongqing and the Ministry of Science and Technology, Chongqing, People’s Republic of China
Abstract: High-intensity focused ultrasound (HIFU) is widely applied to tumors in clinical practice due to its minimally invasive approach. However, several issues lower therapeutic efficiency in some cases. Many synergists such as microbubbles and perfluorocarbon nanoparticles have recently been used to improve HIFU treatment efficiency, but none were determined to be effective and safe in vivo. In this study, nanoscale bubble-generating liposomes (liposomes containing ammonium bicarbonate [Lip-ABC]) were prepared by film hydration followed by sequential extrusion. Their stable nanoscale particle diameter was confirmed, and their bubble-generating capacity after HIFU triggering was demonstrated with ultrasound imaging. Lip-ABC had good stability in vivo and accumulated in the tumor interstitial space based on the enhanced permeability and retention effect evaluated by photoacoustic imaging. When used to synergize HIFU ablation to bovine liver in vitro and implanted breast tumors of BALB/c nude mice, Lip-ABC outperformed the control. Importantly, all mice survived HIFU treatment, suggesting that Lip-ABC is a safe HIFU synergist.
Keywords: HIFU, nanoscale bubble-generating liposomes, efficiency, synergize
Introduction
The first-line clinical treatments for tumors are surgery, chemotherapy, and radiotherapy. These therapies either create large wounds or have serious side effects, resulting in high pain levels. High-intensity focused ultrasound (HIFU) is a micro-invasive or noninvasive therapeutic modality that is effective due to the good penetrability and directivity of ultrasound in biological tissues.1 In HIFU, low-intensity ultrasonic beams released in all directions from an external header are aggregated to a focal point or region in tumor tissue to generate HIFU. Consequently, the acoustic intensity of HIFU at the focus is above 10,000 w/cm2 and is accompanied by very strong physical reactions such as heat, cavitation, and mechanical effects. The temperature of the target area is rapidly elevated to above 60°C, causing instant and irreversible coagulative necrosis. Due to the rapid decrease of ultrasound intensity, tissues surrounding the focal regions are relatively unaffected. Given these advantages, the clinical application of HIFU is becoming more widespread.
HIFU ablation can take up to several hours in clinical practice.2 Long HIFU treatment sessions may damage normal peritumoral tissues because of unwanted energy deposition. Therefore, there is a need to improve the ability of HIFU to efficiently ablate tumors and minimize damage to healthy tissue. A known effective method is the use of microbubbles (ultrasound contrast agent) to synergize HIFU ablation; they can increase the deposition of energy to the target area and improve the cavitation effect of ultrasound.3 Unfortunately, microbubbles have a large size and short circulation time in vivo. Since they are unable to access tumor interstitial spaces, they could shift the target area of HIFU ablation or increase the temperature and damage normal tissue.4 One group recently attempted to use perfluorocarbon nanoparticles to improve HIFU treatment and obtained good synergistic effects.5 The nanoparticles are very stable and can extravasate through leaky tumor vessels and accumulate in the tumor interstitial space, leading to an enhanced permeability and retention (EPR) effect that helps overcome the deficiencies of microbubbles. After HIFU triggering, perfluorocarbon in the nanoparticles will undergo a liquid–gas phase transition into microbubbles to enhance HIFU ablation. Following phase transition, the nanoparticles are dramatically enlarged, which may increase the risk of vascular or pulmonary embolism, so further research is needed.6
Recent studies described temperature-sensitive liposomes containing ammonium bicarbonate (Lip-ABC) solutions that were stable at 37°C with a slow decomposition rate.7,8 However, when the temperature exceeded 40°C, the ABC solutions in the liposomes rapidly decomposed and released a large amount of carbon dioxide (CO2) bubbles.7,8 No significant changes occurred in liposome diameter8 because CO2 bubbles are easily diffused out of the liposomes. Importantly, the liposomes were stable enough to cluster in the tumor interstitial space and decomposed to generate CO2 bubbles under the local heat increase (42°C).9 The use of liposomes to promote HIFU ablation has the potential to significantly reduce the danger of embolism. Several studies have examined the interaction between HIFU and liposomes, but they mainly focused on HIFU-triggering antitumor drug release from temperature-sensitive liposomes to enhance treatment effects.10,11 As far as using liposomes to synergize HIFU, only those containing fluorocarbon gas or liquid (lipid microbubbles or perfluorocarbon particles with lipid shell) have been studied; the synergistic effect of Lip-ABC on HIFU is unclear. We prepared Lip-ABC solutions, studied their characteristics, and investigated their effects and safety in HIFU therapy.
Materials and methods
Reagents
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] (DSPE-PEG2000), and liposome extruder were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Cholesterol was ordered from Bioengineering Co., Ltd. (Shanghai, People’s Republic of China). ABC and 5% glucose solution were provided by Kelong Chemical Reagent Factory (Chengdu, People’s Republic of China).
Lip-ABC preparation
Lip-ABC were prepared by film hydration followed by sequential extrusion. First, DPPC, DSPE-PEG2000, and cholesterol were dissolved into trichloromethane. Second, the residual organic solvent was removed by depressure evaporation with a rotary evaporator to develop an even layer of lipid film. Third, ABC solution (0.2 g/mL) was added to produce a milky white suspension by ultrasonic-assisted hydration of the lipid film at room temperature. Afterward, a semitranslucent emulsion was acquired by sequentially extruding the suspension with a liposome extruder at room temperature (eight times each for 800, 400, 200, and 100 nm filter films). Finally, the emulsion was dialyzed with 5% glucose solution to obtain Lip-ABC. The control liposomes containing phosphate-buffered saline (Lip-PBS) emulsion was prepared with PBS solution instead of ABC solution according to the same procedures.
Lip-ABC properties
The particle sizes before/after HIFU intervention and the surface electric potentials of Lip-ABC and Lip-PBS were detected with a Zetasizer instrument (Malvern Instruments, Malvern, UK). The ability of Lip-ABC or Lip-PBS to generate bubbles after temperature triggering was evaluated with an ultrasonic diagnostic apparatus (mylab9; Yum Medical Group, Genoa, Italy) at temperatures of 37°C, 42°C, and 50°C. A HIFU ultrasound monitoring system (Chongqing Haifu Medical Technology Co., Ltd., Chongqing, People’s Republic of China) was applied to assess the bubble-generating capacity of Lip-ABC or Lip-PBS by examining gray-scale changes after HIFU triggering at different powers.
Lip-ABC assisting HIFU to ablate bovine liver in vitro
Fresh bovine livers (provided by a slaughterhouse in Chongqing, People’s Republic of China) were flatwise placed in a plastic container with a piece of ultrasound-penetrable film at the bottom and soaked in degassed water. Then, three to five layers along the X-axis direction of the HIFU header and three to four points on each layer along the Y-axis direction were selected as HIFU ablation points. The ablation points were at least 1 cm apart and were not near blood vessels or bile ducts (observed by the ultrasound monitoring system). Next, 100 μL reagent (Lip-ABC, Lip-PBS, or PBS) was absorbed to inject into the ablation points before HIFU irradiation was performed. During the process, an assistant manually outlined “areas of interest” on ultrasound monitoring system and recorded average gray-scale differences and change areas. After ablation, the bovine livers were dissected layer by layer to determine coagulative necrosis position and measure their length and width for volume calculation (V = π/6× length × width × width, with mm3 as the measurement unit).
Tumor cell culture
MDA-MB-231 tumor cells are tumor cell lines obtained from the Basic Medical Research Institute of Chongqing Medical University and subcultured by the Institute of Ultrasound Imaging of Chongqing Medical University. These cells were cultured in culture flasks containing 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) together with Dulbecco’s Modified Eagle’s Medium (Thermo Fisher Scientific) and incubated at 37°C with 5% CO2 and saturated humidity.
Animal models
Forty-two BALB/c nude mice (20–24 g, 4–6 weeks old) were kept in the laboratory animal center of Chongqing Medical University (Chongqing, People’s Republic of China). All animal experiments were conducted according to the guidelines of the ethics committee of Chongqing Medical University, which approved the study. Human breast cancer MB231 cells (5×106) were administered by subcutaneous injection into the mouse back. After 2–4 weeks when the tumor diameter reached about 1 cm, the mice were randomly divided into Lip-ABC and PBS groups (n=21 each).
In vivo stability of Lip-ABC and selection of ablation time points
One milliliter of Lip-ABC or Lip-PBS was injected through caudal vein to three mice each in the Lip-ABC and Lip-PBS groups. Photoacoustic (PA) imaging (Vevo LAZR; VisualSonics Company, Toronto, ON, Canada) was performed to observe the tumors and record the PA values before and at different intervals after injection.
Lip-ABC assisting HIFU ablation of the implanted breast tumor
The remaining 18 mice in each group were treated with Lip-ABC or Lip-PBS. After 4 hours, they were anesthetized, fixed in an apparatus, and the tumors were exposed and soaked into degassed water. As previously described, spot irradiation ablation with HIFU was performed. The areas of interest on the ultrasound monitoring system were outlined, and the gray-scale difference and change area were recorded. Six mice were sacrificed at 1 hour, 3 days, and 7 days after HIFU treatment, respectively, and the tumors were dissected and stained with triphenyltetrazolium chloride (TTC). Next, hematoxylin–eosin (HE) staining, terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assays, and proliferating cell nuclear antigen (PCNA) examinations were performed to the margin samples around the coagulative necrosis areas at 3 and 7 days after HIFU treatment.
TTC staining
TTC staining was performed to observe the coagulative necrosis areas and calculate the necrosis volumes. Immediately after resection, the tumor was placed in 1% TTC solution (37°C) for 30 minutes. The formula for volume calculation was V = π/6× length × width × thickness, with mm3 as the measurement unit.
HE staining
Tumor tissues within 3 mm of the coagulative necrosis margin were fixed with 4% paraformaldehyde for routine HE staining.
TUNEL assay
Tumor tissues within 3 mm of the coagulative necrosis margin were detected with the TUNEL method according to the manufacturer’s instructions (Hoffman-La Roche Ltd., Basel, Switzerland).
PCNA
Tumor tissues within 3 mm of the coagulative necrosis margin were used. PCNA was detected with the streptavidin peroxidase method according to the manufacturer’s instructions (Santa Cruz Biotechnology Inc., Dallas, TX, USA).
Statistical analysis
All data are expressed as mean ± SD. For the in vitro experiments, one-way analysis of variance (ANOVA) was adopted when data were normally distributed; otherwise, a rank-sum test was performed. For the in vivo experiments, independent-sample t-tests were used to analyze normally distributed data, or rank-sum tests were performed. P<0.05 was considered statistically significant.
Results and discussion
Lip-ABC preparation and characteristics
ABC is a common chemical substance that decomposes to generate CO2 bubbles when heated to a high temperature12,13 and is widely applied as a leavening agent in the food industry.14 Among the decomposition products, NH3 is extremely easy to dissolve in water (at ambient temperatures and pressures, 700 vol units of NH3 can be dissolved in 1 vol unit of water) without any bubbles. However, CO2 solubility in water is weaker (under normal temperature and pressure conditions, 1 vol unit of water can dissolve equivalent volume of CO2), which may be why an ABC solution can generate numerous CO2 bubbles after being heated up.
ABC exists in ionic forms (NH4+ and HCO3−) once dissolved in water, which has a low permeability coefficient for lipid bilayers. The ABC solution is therefore incorporated and stably maintained in the internal aqueous phase of liposomes. In the experiments, Lip-ABC was prepared and consisted of DPPC, cholesterol, and DSPE-PEG2000 mixed at a ratio of 3:1:1 and ABC solution (0.2 g/mL). Liposomes formulated in the presence of aqueous PBS instead of ABC were used as the control (Lip-PBS). Using the method of phospholipid hydration followed by sequential extrusion and dialysis,15 it was not difficult to incorporate ABC solution into liposomes, measure the diameter with a liposome extrusion apparatus, and remove the excess ABC solution with dialysate. Both Lip-ABC and Lip-PBS had nanoscale particle diameters and good uniformity (Table 1; Figure 1A and B) and would be able to pass through the tumor vascular endothelial space. Their zeta potentials were also suitable to be maintained in a dispersed state not to gather, which is very important for in vivo applications (Table 1; Figure 1C and D).
  | Table 1 Lip-ABC and Lip-PBS formulation characteristics |
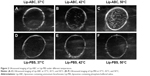
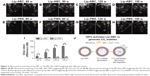
CO2 bubbles are hyperechogenic and can be used to enhance ultrasound imaging.16 We therefore evaluated the ability of Lip-ABC or Lip-PBS to generate CO2 bubbles after temperature changes or HIFU triggering. In the first experiment, bladders containing Lip-ABC or Lip-PBS were immersed in water at different temperatures and observed through an ultrasonic diagnostic apparatus. We found no significant high echoes in the bladder containing Lip-ABC at 37°C. However, high echoes markedly increased when the temperature reached 42°C and 50°C (Figure 2A–C). Conversely, no obvious dotted high echoes were noted in the control bladder with Lip-PBS at 37°C, 42°C, or 50°C (Figure 2D–F). These results showed that Lip-ABC exhibited slow decomposition at 37°C (normal body temperature) and accelerated decomposition after heating (above 42°C), which was consistent with previous studies.7,8 However, the temperature of the HIFU focal point can reach ≥60°C and is also expected to induce Lip-ABC decomposition and CO2 bubble production. To test the hypothesis, Lip-ABC and Lip-PBS solutions were exposed to HIFU ablation with different power levels. The Lip-ABC group had a greater average gray-scale difference (>30) after HIFU (90–150 w) triggering, whereas there were not significant differences (<30) in the Lip-PBS group (60–150 w) or Lip-ABC group (60 w). The differences were more obvious in the Lip-ABC group compared to the Lip-PBS group under all conditions, indicating that suitable HIFU could easily stimulate Lip-ABC to generate CO2 bubbles (Figure 3A–I). Apart from the HIFU thermal effect, this may be related to ultrasound mechanical effects. Vibration can reduce the dissolution of CO2 in water, with the successive decomposition reaction of ABC to generate CO2. Importantly, there were no significant changes in liposome diameters after HIFU (Table 2; Figure 4A and B). This could be due to the permeability of liposomes that permits small-molecule gases such as CO2 diffuse out easily and without enlarging liposomes volume (Figure 3J). This is different from perfluorocarbon nanoparticles that significantly expand during phase transition.
Lip-ABC synergizes HIFU ablation in vitro
HIFU is an excellent microinvasive or noninvasive treatment method that has been widely used to ablate tumors in many parts of the body such as the breast, liver, pancreas, kidney, prostate, ovary, and uterus. However, it has some inherent disadvantages. For example, HIFU ablation is performed from point to line, then to plane, and then to volume, which takes considerable time.2 In addition, its energy decreases for deeper tumors. As indicated in some studies, ultrasound energy exponentially attenuates with longer distance,17 so the energy can be significantly lower with notable decrease in HIFU treatment efficiency. Moreover, the acoustic channel areas for HIFU treatment are reduced if gas or bones block the path, which will limit the HIFU energy delivered to tumors and treatment effectiveness.18 When the tumor tissue density is even, the acoustic resistance will be weakened, making it difficult to deposit the HIFU energy and further decreasing treatment efficiency.19 In the abovementioned cases, HIFU treatment will require longer times and higher powers. This leads to unnecessary energy deposition in healthy tissues, which could cause temporary pains, skin burns, nerve injuries, and other physical damage.20 These problems underscore how HIFU delivery can be improved.
Many agents have been developed to synergize HIFU ablation such as iodinated oil, porphyrin, and microbubbles.21 Among these, microbubbles are the most promising.21 As ultrasound contrast agents, microbubbles show good ability to reflect ultrasound. When entering into biological tissues via blood circulation, microbubbles improve the acoustic impedance of the tissues, which will promote HIFU energy deposition in the target, enhancing the HIFU thermal effect.3 Moreover, microbubbles can degrade the cavitation threshold value and greatly improve the HIFU cavitation effect.3 This can foster the thermal effect to accelerate tissue heating.
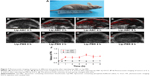
In addition to microbubbles, CO2 bubbles are also hyperechogenic and can theoretically synergize HIFU ablation. Lip-ABC generate CO2 bubbles after HIFU activation, which is expected to increase HIFU treatment efficiency. In this study, bovine livers were used in in vitro experiments to demonstrate the effect of Lip-ABC on HIFU. The HIFU ablation parameters (150 w, 5 s) were determined based on preliminary results. According to the common indexes to evaluate HIFU effect,5 the average gray-scale differences, gray-scale change areas, and coagulative necrosis volumes were compared among groups. There were no significant differences between the Lip-PBS and PBS groups, suggesting that the phospholipid shells of the liposomes did not exert a synergistic HIFU effect. However, the Lip-ABC group had significantly higher values of average gray-scale difference, gray-scale change area, and coagulative necrosis volume than the control groups (Figure 5A–I). Pathological examination revealed more evident vacuolar changes in the Lip-ABC group, indicating more intense cavitation (Figure 5J–L). These results revealed that Lip-ABC improved the ability of HIFU to ablate bovine liver. Presumably, the ABC solution inside the liposomes decomposed to generate CO2 bubbles after HIFU ablation, acting as a cavitation nucleus to reduce the cavitation threshold and significantly improving the effect of HIFU. On the other hand, the excellent reflection ability of CO2 bubbles to ultrasound promoted HIFU energy deposition, similar to microbubbles intensifying HIFU treatment.
Lip-ABC synergizing HIFU ablation in vivo
Although many studies have shown that microbubbles actively improve HIFU ablation, they are vulnerable to the impact of the blood stream, and their shells may fracture with dispersion of the perfluorocarbon gas at the core.22 For this reason, microbubbles only survive for ~10 minutes. In addition, their relative large size (micrometer diameter) prevents them from penetrating the tumor vascular endothelium gap (~380–780 nm). Microbubbles only exist in the blood circulation within tumor blood vessels and do not accumulate within the tumor interstitial, which may prevent HIFU from reaching targeted areas23 or induce damage of normal tissue. To overcome these problems, some researchers prepared liquid perfluorocarbon nanoparticles with a liquid core to make them stable in the blood circulation and accumulate in tumor interstitial space by the EPR effect.4,5 When HIFU ablated the tumor, these nanoparticles would turn into microbubbles through acoustic droplet vaporization, further enhancing the HIFU effect. However, not all the nanoparticles will collect in the tumor interstitial space; some nanoparticles will still circulate in the tumor vasculature. It is well known that when substances transfer from liquid to gaseous phase, the volume may increase by more than 1,000-fold. Moreover, perfluorocarbon does not easily diffuse out of the shell,24 so after acoustic droplet vaporization, the nanoparticles undergo a liquid–gas phase transition and transform to huge microbubbles, with the volume dramatically increasing by more than fivefold. If these huge microbubbles circulate in the blood, there is an increase in the risk of vascular and pulmonary embolism.25,26 Microbubbles used clinically are no more than 10 μm to avoid the risk of embolism. Due to these issues, the safety of HIFU synergists requires further study.
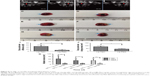
Lip-ABC did not obviously expand after HIFU triggering and also had good stability in previous studies.9,27 As depicted in Figure 6, we anticipated that Lip-ABC would safely synergize HIFU ablation. We therefore established animal tumor models (Figure 7A) and carried out in vivo experiments. PA signals were observed in the tumors of the Lip-ABC group, demonstrating that Lip-ABC could enhance imaging in vivo. It might be related to the continuous release of CO2 from Lip-ABC in the process of laser irradiation.28 Based on this phenomenon, PA imaging could be applied to monitor Lip-ABC accumulation in tumors. Lip-ABC stability in vivo was demonstrated, and the proper ablation time point was determined by PA imaging. The experimental results showed that the PA value gradually increased over time in the Lip-ABC group, reaching the highest point after 4 hours and then starting to decrease. This was in contrast with continuously weak signal in the control group (Figure 7B–J). The results indicate that Lip-ABC has excellent stability in vivo and can accumulate within tumor interstitial space, consistent with the EPR effect.29 Accordingly, the HIFU ablation time was selected as 4 hours after injection for subsequent experiments.
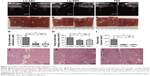
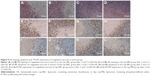
Since the Lip-PBS and PBS control groups showed no significant differences in the in vitro experiment, only Lip-PBS and Lip-ABC groups were used in the animal experiments. Four hours after solution injection, HIFU (150 w, 5 s) was applied to ablate the implanted breast cancer cell tumors of nude mice. Simultaneous ultrasound monitoring was performed to record the average gray-scale differences and gray-scale change areas of each group. The results revealed that both the average gray-scale difference and the gray-scale change area were significantly higher in the Lip-ABC experimental group (Figure 8A, B, I, and J). After HIFU treatment, the tumors were dissected to measure coagulative necrosis. Similar to the ultrasound monitoring results, the coagulative necrosis volume in the Lip-ABC group was much higher than that in the Lip-PBS control group at 1 hour, 3 days, and 7 days after HIFU treatment (Figure 8C–H and K). Macroscopic assessment revealed hemorrhages in both groups 1 hour after ablation, and it decreased with time at 3 and 7 days after HIFU. On the other hand, HE staining revealed that the cavitation phenomenon in coagulative necrosis was more obvious in the Lip-ABC group (Figure 9A–D). Furthermore, TUNEL experiments also showed more serious peripheral cell apoptosis in the Lip-ABC group (Figure 9E–H). PCNA expression was much lower in the Lip-ABC group than in the control group (Figure 9I–L). These results suggest that Lip-ABC enhanced the treatment effect, suggesting that it could be an excellent synergist of HIFU for tumor ablation. With regard to safety, the animals in the Lip-ABC and control groups all survived HIFU treatment. The liposome diameters did not significantly change after generating CO2 bubbles, so there was low risk of vascular or pulmonary embolization. Although there might be a considerable number of CO2 bubbles, they do not cause embolism because the bubbles break under the impact force of the blood stream. In fact, the first generation of ultrasound contrast agent was simple air bubbles that could not pass through pulmonary circulation due to their large size. These were safely intravenously injected into patients, owing to their poor stability, lasting only a few seconds in circulation.24 In addition, ABC in Lip-ABC decomposed to a small amount of ammonia that could be metabolized by liver without serious consequences.30 Intravenously injected Lip-ABC may be successful and safe for HIFU in vivo.
Lip-ABC had nanoscale size and good stability to collect in tumor tissues through the EPR effect without displacement of target areas and damage before HIFU ablation. In addition, Lip-ABC did not undergo any significant changes in particle size when generating bubbles, which could avoid vascular thrombosis caused by a significant volume increase. Given these advantages, Lip-ABC are worthy of further study.
Conclusion
Nanoscale bubble-generating liposomes (Lip-ABC) were successfully prepared. Their tiny diameter and good stability allow them to accumulate within tumor interstitial spaces by the EPR effect. Moreover, the liposomes can generate bubbles under the HIFU, increasing cavitation and energy depositions, thus enhancing the ablation effect. Liposomes are also characterized by their safety in the process of HIFU synergism due to their stable particle diameters after CO2 bubble generation.
Acknowledgments
The authors greatly appreciate Xiaoya Ding for providing HIFU technical support and Xiaorong Xia for her assistance with PA imaging. This work was supported by the National Nature Science Foundation of China (grant nos 81501481, 81401503, and 81371578).
Disclosure
The authors report no conflicts of interest in this work.
References
Zhou Y. High-intensity focused ultrasound treatment for advanced pancreatic cancer. Gastroenterol Res Pract. 2014;2014:205325. | ||
Luo W, Zhou X, Tian X, et al. Enhancement of ultrasound contrast agent in high-intensity focused ultrasound ablation. Adv Ther. 2006;23(6):861–868. | ||
Azmin M, Harfield C, Ahmad Z, Edirisinghe M, Stride E. How do microbubbles and ultrasound interact? Basic physical, dynamic and engineering principles. Curr Pharm Des. 2012;18(15):2118–2134. | ||
Moyer LC, Timbie KF, Sheeran PS, Price RJ, Miller GW, Dayton PA. High-intensity focused ultrasound ablation enhancement in vivo via phase-shift nanodroplets compared to microbubbles. J Ther Ultrasound. 2015;3:7. | ||
Zhou Y, Wang Z, Chen Y, et al. Microbubbles from gas-generating perfluorohexane nanoemulsions for targeted temperature-sensitive ultrasonography and synergistic HIFU ablation of tumors. Adv Mater. 2013;25(30):4123–4130. | ||
Shpak O, Verweij M, de Jong N, Versluis M. Droplets, bubbles and ultrasound interactions. Adv Exp Med Biol. 2016;880:157–174. | ||
Chung MF, Chen KJ, Liang HF. A liposomal system capable of generating CO2 bubbles to induce transient cavitation, lysosomal rupturing, and cell necrosis. Angew Chem Int Ed Engl. 2012;51(40):10089–10093. | ||
Chen KJ, Liang HF, Chen HL, et al. A thermoresponsive bubble-generating liposomal system for triggering localized extracellular drug delivery. ACS Nano. 2013;7(1):438–446. | ||
Chen KJ, Chaung EY, Wey SP, et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano. 2014;8(5):5105–5115. | ||
Deng Z, Xiao Y, Pan M, et al. Hyperthermia-triggered drug delivery from iRGD-modified temperature-sensitive liposomes enhances the anti-tumor efficacy using high intensity focused ultrasound. J Control Release. 2016;243:333–341. | ||
Liang X, Gao J, Jiang L, et al. Nanohybrid liposomal cerasomes with good physiological stability and rapid temperature responsiveness for high intensity focused ultrasound triggered local chemotherapy of cancer. ACS Nano. 2015;9(2):1280–1293. | ||
Boddien A, Gärtner F, Federsel C, et al. CO2-“neutral” hydrogen storage based on bicarbonates and formates. Angew Chem Int Ed Engl. 2011;50(28):6411–6414. | ||
Nowak P, Skrzypek J. The kinetics of chemical decom position of ammonium bicarbonate and carbonate in aqueous solutions. Chem Eng Sci. 1989;44:2375–2376. | ||
Min B, Bae IY, Lee HG, Yoo SH, Lee S. Utilization of pectin-enriched materials from apple pomace as a fat replacer in a model food system. Bioresour Technol. 2010;101(14):5414–5418. | ||
Boyer C, Zasadzinski JA. Multiple lipid compartments slow vesicle contents release in lipases and serum. ACS Nano. 2007;1(3):176–182. | ||
Kang E, Min HS, Lee J, et al. Nanobubbles from gas-generating polymeric nanoparticles: ultrasound imaging of living subjects. Angew Chem Int Ed Engl. 2010;49(3):524–528. | ||
Chen J, Hou GY, Marquet F, Han Y, Camarena F, Konofagou E. Radiation-force-based estimation of acoustic attenuation using harmonic motion imaging (HMI) in phantoms and in vitro livers before and after HIFU ablation. Phys Med Biol. 2015;60(19):7499–7512. | ||
Zhu H, Zhou K, Zhang L, et al. High intensity focused ultrasound (HIFU) therapy for local treatment of hepatocellular carcinoma: role of partial rib resection. Eur J Radiol. 2009;72(1):160–166. | ||
Cheng SQ, Zhou XD, Tang ZY, Yu Y, Bao SS, Qian DC. Iodized oil enhances the thermal effect of high-intensity focused ultrasound on ablating experimental liver cancer. J Cancer Res Clin Oncol. 1997;123(11–12):639–644. | ||
Poliachik S, Chandler W, Mourad P, et al. Effect of high-intensity focused ultrasound on whole blood with and without microbubble contrast agent. Ultrasound Med Biol. 1999;25(6):991–998. | ||
Clement GT. Perspectives in clinical uses of high-intensity focused ultrasound. Ultrasonics. 2004;42(10):1087–1093. | ||
Min HS, Kang E, Koo H, et al. Gas-generating polymeric microspheres for long-term and continuous in vivo ultrasound imaging. Biomaterials. 2012;33(3):936–944. | ||
Phillips LC, Puett C, Sheeran PS, Wilson Miller G, Matsunaga TO, Dayton PA. Phase-shift perfluorocarbon agents enhance high intensity focused ultrasound thermal delivery with reduced near-field heating. J Acoust Soc Am. 2013;134(2):1473–1482. | ||
Tinkov S, Bekeredjian R, Winter G, Coester C. Microbubbles as ultrasound triggered drug carriers. J Pharm Sci. 2009;98(6):1935–1961. | ||
Zhang M, Fabiilli ML, Haworth KJ, et al. Initial investigation of acoustic droplet vaporization for occlusion in canine kidney. Ultrasound Med Biol. 2010;36(10):1691–1703. | ||
Samuel S, Duprey A, Fabiilli ML, Bull JL, Fowlkes JB. In vivo microscopy of targeted vessel occlusion employing acoustic droplet vaporization. Microcirculation. 2012;19(6):501–509. | ||
Liao ZX, Chuang EY, Lin CC, et al. An AS1411 aptamer-conjugated liposomal system containing a bubble-generating agent for tumor-specific chemotherapy that overcomes multidrug resistance. J Control Release. 2015;208:42–51. | ||
Xia J, Feng G, Xia X, Hao L, Wang Z. NH4HCO3 gas-generating liposomal nanoparticle for photoacoustic imaging in breast cancer. Int J Nanomedicine. 2017;12:1803–1813. | ||
Koning GA, Eggermont AM, Lindner LH, ten Hagen TL. Hyperthermia and thermosensitive liposomes for improved delivery of chemotherapeutic drugs to solid tumors. Pharm Res. 2010;27(8):1750–1754. | ||
Lee HJ, Halliday N, Bray GP. Measurement and interpretation of plasma ammonia. Br J Hosp Med (Lond). 2014;75(3):C40–C43. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.