Back to Journals » Clinical Ophthalmology » Volume 12
Herpesviridae prevalence in aqueous humor using PCR
Authors Keorochana N , Intaraprasong W, Choontanom R
Received 19 May 2018
Accepted for publication 3 July 2018
Published 7 September 2018 Volume 2018:12 Pages 1707—1711
DOI https://doi.org/10.2147/OPTH.S174694
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Narumon Keorochana, Wasamon Intaraprasong, Raveewan Choontanom
Department of Ophthalmology, Phramongkutklao hospital, Phramongkutklao College of Medicine, Bangkok, Thailand
Objective: The aim of this study was to determine the prevalence of Herpesviridae family in aqueous humor and the prevalence of antibodies against Herpesviridae family in serum.
Methods: Participants undergoing cataract surgery were included in the study. Serum for viral serology including herpes simplex virus (HSV), varicella zoster virus (VZV), Epstein–Barr virus (EBV), and cytomegalovirus (CMV) was collected. Aqueous humor specimen was tapped for PCR analysis.
Results: Ninety-two participants were included with a mean age of 67.67 years (SD ±12.51). The prevalence of positive serology tests was 83.3% for HSV, 94.0% for VZV, 98.8% for EBV, and 97.6% for CMV. A total of 66 aqueous humor specimens were negative for PCR of Herpesviridae family.
Conclusion: This study showed previous HSV, VZV, EBV and CMV infections in >90% of the Thai population, while no viral presence was detected in aqueous humor. Thus, the serology test is unrelated to the presence of virus in the eye. We suggest that PCR is a valuable tool to diagnose intraocular viral infection and detect virus presenting active infection.
Keywords: Herpesviridae, prevalence, aqueous humor, PCR, serology, uveitis
Introduction
Herpesviridae is a family of DNA viruses, which are known to be common causes of infections in immunocompetent and immunocompromised individuals. Of all Herpesviridae, only eight types use humans as their primary host, called human herpes virus (HHV) 1–8. The prevalence of seropositive for herpes viruses increases with age and varies geographically. HHV can be latent or lytic. Many affected people are unaware because they do not exhibit any symptoms.
Five of these eight types are common and are as follows: herpes simplex viruses (HSV) 1 and 2, varicella zoster virus (VZV), Epstein–Barr virus (EBV), and cytomegalovirus. They can affect various ocular tissues. HSV1 and VZV2 are by far the most common ocular pathogens. Each has an incidence of ~10–20/100,000 person-years.
One consequence of becoming infected is the possibility of uveitis. Uveitis can be in either one eye or both eyes and also involves other parts of the eye. In Thailand, the most common identified cause of infectious uveitis was herpetic uveitis (17.2%).3 Uveitis is considered as an ophthalmic emergency that needs to be examined and treated properly to control the inflammation. Leaving the condition untreated can lead to blindness.4
Therefore, an accurate diagnosis is critical because some of the disease’s signs are difficult to identify. To provide effective treatment, the virus must be specified exactly and precisely. For example, it remains unclear that EBV is the pathogenesis of uveitis. Consequently, treatment using acyclovir is also uncertain, even though many studies report the effectiveness of acyclovir in EBV infection.5–7
The first aim of the research was to determine the prevalence of the Herpesviridae family in the intraocular fluid of noninflammatory eye to verify whether the virus could live in this circumstance without being a cause of pathogenesis. Second, the study aimed to review their serum IgG antibody results. This research used PCR, which is rapid, sensitive, and accurate to detect viral DNA. The result of the study is an approach that can be used to develop further effective treatment.
Materials and methods
The study used a cross-sectional design. Participants were enrolled between June 2017 and September 2017 at the Department of Ophthalmology, Phramongkutklao Hospital, Bangkok, Thailand. These participants were undergoing elective cataract surgery. Inclusion criteria included 1) no active intraocular inflammation on biomicroscopic examination and 2) normal fundus examination preoperative. Exclusion criteria included 1) any evidence of previous intraocular and ocular surface inflammation, 2) abnormal fundus examination postoperative (when the cataract precluded a good view of the fundus preoperative), 3) HIV-positive status or being on immunosuppressive drugs, 4) previous ocular surgery or intraocular injection, and 5) receiving antiviral drugs for herpes treatment 6 months preoperative.
Demographic data (age, sex, and underlying disease), ocular history (ocular disease, history of previous ophthalmic surgery, and history of topical eye medication), and ocular examination were recorded.
Blood serum specimens were obtained after written informed consent was obtained. The sera were sent to Department of Immunology, Phramongkutklao College of Medicine. The presence of anti-HSV2 IgG, anti-VZV IgG, anti-cytomegalovirus (CMV) IgG, and anti-EBV-EA (Epstein–Barr early antigen) IgG was detected by qualitative analysis using commercially available ELISA kits (Chorus Trio; Diesse Diagnostic Senese Spa, Milano, Italy), and the results were interpreted according to the manufacturer’s instructions. Samples were recorded as seropositive, seronegative, or borderline according to the levels of IgG being >1.2 IU/mL, <0.8 IU/mL, or between 0.8 and 1.2 IU/mL, respectively. Sensitivity and specification were ~100%.
During the intraoperative step, participants were cleaned with a povidone-iodine 5% solution and draped as per routine strict sterile technique. Initial entry into the anterior chamber was made through the limbus as a part of the cataract surgery followed by passing blunt-tip needle on a 1 mL syringe and withdrawing 0.1 mL of aqueous humor fluid. Aqueous humor specimens were placed in a sterile tube. The rest of the cataract surgery was performed following a standard operating procedure. All specimens were stored on ice until they could be transported to the laboratory and processed using the PCR analysis to detect viruses (HSV1, HSV2, VZV, EBV, and CMV).
All samples were processed using the qualitative assay, that is, the Multiplex real-time PCR to detect pathogen genes, using four tubes of the Fast Track Diagnostics Kit (Fast Track Diagnostics Ltd., Esch-sur-Alzette, Luxembourg), TaqMan® probe (Premier Biosoft International, CA, USA), and automated extraction platform for total nucleic acid (DNA and RNA) purification from primary sample tubes by EMAG® (BioMérieux, Craponne, France). Limits of detection were as low as 102 copies/mL.
Statistical analyses
Demographic data were presented in terms of percentage, mean, and SD. The prevalence rate of serum for viral serology and that of aqueous humor for PCR of the Herpesviridae family were calculated.
The formula used in this study to calculate the sample size for the prevalence rate8 of 26.36% or 0.2636 at 95% confidence interval (α=0.05) was N=z2×p×q/d2 (Z=1.96, P is the prevalence, Q=[1-P], and d is the error margin [±10%]). The sample size needed for this study was at least 75 participants. The data were analyzed using the statistical software package (STATA/MP12).
Ethics approval and patient consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study protocol received approval from the Institutional Review Board of the Royal Thai Army Medical Department. Written informed consent was obtained from each participant who received a detailed explanation of all the procedures involved as a part of the study.
Results
A total of 92 participants were enrolled in the study. From all the participants, 84 serum and 66 aqueous humor specimens were analyzed. Regarding the samples, only 58 participants were analyzed for both serum and aqueous humor. No complications were noted during specimen collection in any of the medical records. The baseline characteristics are shown in Table 1. The mean age of the participants was 67.7 (±12.5) years (18–88 years); 55 (60%) participants were males. A total of 85 participants presented a chronic disease (92.4%), 58 participants had no other eye diseases (63%), and 82 participants had no topical eye medication (89.1%).
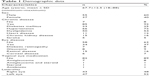  | Table 1 Demographic data |
A total of 84 serum specimens and 66 aqueous humor specimens were collected. The overall prevalence of viral serology and viral DNA from aqueous humor PCR analysis are presented in Tables 2 and 3, respectively. All participants presented negative PCR results to all pathogens analyzed in this study.
Discussion
This study evaluated the prevalence of HSV, VZV, EBV, and CMV in serum and aqueous humor in a normal population undergoing routine cataract surgery at Phramongkutklao Hospital.
The estimated prevalence of HSV1, HSV2, VZV, EBV, and CMV previously documented for the general population was 67%, 11% (WHO, 2017),32 >90%,9–12 >90%,13,14 and >50% in adults (The Center of Disease Control [CDC], 2018),33 respectively. The finding was similar in this study. Approximately >90% of the normal Thai population had previous HSV2, VZV, EBV, and CMV infections without presenting any symptoms. Unfortunately, this study could not show the prevalence of HSV1 due to laboratory technical problem.
In addition, no evidence of these viruses was detected in intraocular fluid. Our study suggests that herpes viruses were not detected in aqueous humor from a noninflammatory eye despite being seropositive to these herpes viruses. These results are consistent with a related study in which no herpes viruses DNA was detected in aqueous humor from normal cataract patients15 or HIV-negative patients.16 We were unable to prove a significant correlation in detecting the Herpesviridae family between aqueous humor and serum. Therefore, the serology test proved unreliable in diagnosing viral uveitis because of the high number of false positives. However, we could not determine whether negative results indicated a noninfectious cause. Clinical evaluation and further investigation must be conducted to rule out other infectious origins.
Related literature17,18 has reported that some viruses such as HSV1, EBV, and HHV6 may be detected in intraocular fluid including the retinal epithelium without being the cause of the retinal infection. However, many related studies19–23 have described cases of “ocular EBV infection” such as Keorochana24 who reported EBV vasculitis by PCR from vitreous biopsy in immunocompetent patients, which was successfully treated with intravenous acyclovir. Our study supports the idea that HSV1, HSV2, VZV, EBV, and CMV might play a latent and nonpathogenic role in serum; however, detecting them in intraocular areas would imply a real active disease. Further investigation is required using quantitative PCR25 and Goldman-Witmer coefficient26–28 to provide additional valuable data to determine the pathogenic value of the virus load and local antibodies’ production from local samples.
PCR technique from aqueous tapping could help to confirm the early diagnosis of suspected viral uveitis in patients instead. Not only anterior uveitis but one related study also revealed a high frequency of positive results without complications from aqueous analysis among patients with posterior uveitis. The aqueous tap with subsequent laboratory investigation is strongly recommended to accurately diagnose and treat patients with suspected infectious uveitis located in the posterior segment of the eye.29,30 The sensitivity was 91.3%, and the specificity was 98.8%.31
This study was only conducted among elderly and immunocompetent patients; therefore, generalizing to different age and immune status groups might be limited. Additionally, because we determined the prevalence of many types of herpes viruses, a larger sample size is needed.
Conclusion
This study showed that previous HSV, VZV, EBV, and CMV infections were more than 90% in the Thai population. In addition, no viruses were detected in aqueous humor. Our findings disclosed that the aqueous humor in a noninflammatory eye was unlikely to detect herpes virus. Detecting intraocular infection of the Herpesviridae family may imply a pathologic clue of active infection. Furthermore, we suggest that PCR is a valuable tool for early diagnosis of intraocular viral infection instead of the serology test, which is unreliable because of the high number of false positives.
Acknowledgments
The authors would like to thank Dr Wallop Iemsomboon, MD, Department of Ophthalmology, Phramongkutklao Hospital for his advice. This study was financially supported by the Office of Research Development, Phramongkutklao College of Medicine and Phramongkutklao Hospital, Bangkok, Thailand. This study was funded by Phramongkutklao College of Medicine.
Author contributions
NK contributed to the study conception and design. NK and WI contributed to the acquisition of data. NK, WI, and RC contributed to the analysis and interpretation of data. NK and WI contributed to the drafting of manuscript. NK and RC contributed to the critical revision. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18(2):127–143. | ||
Liesegang TJ. Varicella-zoster virus eye disease. Cornea. 1999;18(5):511–531. | ||
Sukavatcharin S, Kijdaoroong O, Lekhanont K, Arj-Ong Vallipakorn S. Pattern of Uveitis in a Tertiary Ophthalmology Center in Thailand. Ocul Immunol Inflamm. 2017;25(sup 1):S94–S99. | ||
National Eye Institute. Facts About Uveitis [Internet]. Bethesda, MD: National Eye Institute; 2011. Updated 2011 Aug. https://nei.nih.gov/health/uveitis/uveitis. Accessed August 28, 2018. | ||
Pagano JS, Sixbey JW, Lin JC. Acyclovir and Epstein–Barr virus infec- tion. J Antimicrob Chemother. 1983;12(suppl B):113–121. | ||
Andersson J, Sköldenberg B, Ernberg I, Britton S, Henle W, Andersson U. Acyclovir treatment in primary Epstein-Barr virus infection. A double-blind placebo-controlled study. Scand J Infect Dis Suppl. 1985;47:107–115. | ||
Andersson J, Ernberg I. Management of Epstein-Barr virus infections. Am J Med. 1988;85(2A):107–115. | ||
Laaks D, Smit DP, Harvey J. Polymerase chain reaction to search for Herpes viruses in uveitic and healthy eyes: a South African perspective. Afr Health Sci. 2015;15(3):748–754. | ||
Heininger U, Braun-Fahrländer C, Desgrandchamps D, et al. Seroprevalence of varicella-zoster virus immunoglobulin G antibodies in Swiss adolescents and risk factor analysis for seronegativity. Pediatr Infect Dis J. 2001;20(8):775–778. | ||
Wutzler P, Färber I, Wagenpfeil S, Bisanz H, Tischer A, et al. Seroprevalence of varicella-zoster virus in the German population. Vaccine. 2001;20(1–2):121–124. | ||
Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70 Suppl 1:S111–S118. | ||
Kudesia G, Partridge S, Farrington CP, Soltanpoor N. Changes in age related seroprevalence of antibody to varicella zoster virus: impact on vaccine strategy. J Clin Pathol. 2002;55(2):154–155. | ||
Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343(7):481–492. | ||
Suntornlohanakul R, Wanlapakorn N, Vongpunsawad S, Thongmee T, Chansaenroj J, Poovorawan Y. Seroprevalence of Anti-EBV IgG among Various Age Groups from Khon Kaen Province, Thailand. Asian Pac J Cancer Prev. 2015;16(17):7583–7587. | ||
Yamamoto S, Pavan-Langston D. Kinoshita S, Nishida K, Shimomura Y, Tano Y. Detecting herpes virus DNA in uveitis using the polymerase chain reaction. Br J Ophthalmol. 1996;80:465–468. | ||
Laaks D, Smit DP, Harvey J. Polymerase chain reaction to search for Herpes viruses in uveitic and healthy eyes: a South African perspective. Afr Health Sci. 2015;15(3):748–754. | ||
Usui N. Detection of herpes virus DNA in intraocular tissue. J JpnOphthalmol Soc. 1994;98:443–448. | ||
Mitchell SM, Fox JD, Tedder RS, Gazzard BG, Lightman S. Vitreous fluid sampling and viral genome detection for the diagnosis of viral retinitis in patients with AIDS. J Med Virol. 1994;43(4):336–340. | ||
Kim SJ, Barañano DE, Grossniklaus HE, Martin DF. Epstein-barr infection of the retina: case report and review of the literature. Retin Cases Brief Rep. 2011;5(1):1–5. | ||
Sugita S, Shimizu N, Watanabe K, et al. Use of multiplex PCR and real-time PCR to detect human herpes virus genome in ocular fluids of patients with uveitis. Br J Ophthalmol. 2008;92(7):928–932. | ||
Yamamoto M, Ohga S, Ohnishi Y, Inomata H. Optic disk vasculitis associated with chronic active Epstein-Barr virus infection. Ophthalmologica. 2002;216(3):221–225. | ||
Matoba AY. Ocular disease associated with Epstein-Barr virus infection. Surv Ophthalmol. 1990;35(2):145–150. | ||
Schaal S, Kagan A, Wang Y, Chan CC, Kaplan HJ. Acute retinal necrosis associated with Epstein-Barr virus: immunohistopathologic confirmation. JAMA Ophthalmol. 2014;132(7):881–882. | ||
Keorochana N. A case report of Epstein–Barr virus-associated retinal vasculitis: successful treatment using only acyclovir therapy. International Medical Case Reports Journal. 2017:213–218. | ||
Yamamoto S, Sugita S, Sugamoto Y, et al. Quantitative PCR for the detection of genomic DNA of Epstein-Barr virus in ocular fluids of patients with uveitis. Jpn J Ophthalmol. 2008;52(6):463–467. | ||
Dussaix E, Cerqueti PM, Pontet F, Bloch-Michel E, Bloch ME. New approaches to the detection of locally produced antiviral antibodies in the aqueous of patients with endogenous uveitis. Ophthalmologica. 1987;194(2–3):145–149. | ||
Pleyer U, Chee SP. Current aspects on the management of viral uveitis in immunocompetent individuals. Clin Ophthalmol. 2015;9:1017–1028. | ||
Kongyai N, Sirirungsi W, Pathanapitoon K, et al. Viral causes of unexplained anterior uveitis in Thailand. Eye. 2012;26(4):529–534. | ||
Rothova A, de Boer JH, Ten Dam-van Loon NH, et al. Usefulness of aqueous humor analysis for the diagnosis of posterior uveitis. Ophthalmology. 2008;115(2):306–311. | ||
Westeneng AC, Rothova A, de Boer JH, de Groot-Mijnes JD. Infectious uveitis in immunocompromised patients and the diagnostic value of polymerase chain reaction and Goldmann-Witmer coefficient in aqueous analysis. Am J Ophthalmol. 2007;144(5):781–785. | ||
Sugita S, Ogawa M, Shimizu N, et al. Use of a comprehensive polymerase chain reaction system for diagnosis of ocular infectious diseases. Ophthalmology. 2013;120(9):1761–1768. | ||
World Health Organization [webpage on the internet]. Herpes simple virus; 2017 [updated January 31, 2017]. Available from http://www.who.int/en/news-room/fact-sheets/detail/herpes-simplex-virus. Accessed August 28, 2018. | ||
The Centre of Disease Control [webpage on the internet]. Cytomegalovirus (CMV) and Congenital CMV infection; 2018 [updated June 6, 2018]. Available from https://www.cdc.gov/cmv/index.html. Accessed August 28, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


