Back to Journals » Hepatic Medicine: Evidence and Research » Volume 12
Hepatic Differentiation of Marmoset Embryonic Stem Cells and Functional Characterization of ESC-Derived Hepatocyte-Like Cells
Authors Aravalli RN , Collins DP , Hapke JH, Crane AT , Steer CJ
Received 21 December 2019
Accepted for publication 29 January 2020
Published 13 February 2020 Volume 2020:12 Pages 15—27
DOI https://doi.org/10.2147/HMER.S243277
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Rajagopal N Aravalli,1 Daniel P Collins,2 Joel H Hapke,2 Andrew T Crane,3 Clifford J Steer4,5
1Department of Electrical and Computer Engineering, University of Minnesota, Minneapolis, MN 55455, USA; 2Cytomedical Design Group LLC, St. Paul, MN 55127, USA; 3Department of Neurosurgery, University of Minnesota Medical School, Minneapolis, MN 55455, USA; 4Department of Medicine, University of Minnesota Medical School, Minneapolis, MN 55455, USA; 5Department of Genetics, Cell Biology and Development, University of Minnesota, Minneapolis, MN 55455, USA
Correspondence: Rajagopal N Aravalli
Department of Electrical and Computer Engineering, University of Minnesota, 200 Union Street SE, Minneapolis, MN 55455, USA
Email [email protected]
Clifford J Steer
Department of Medicine, University of Minnesota, 420 Delaware Street SE, Minneapolis, MN 55455, USA
Email [email protected]
Background: Primary human hepatocytes (PHHs) are the ideal candidates for studying critical liver functions such as drug metabolism and toxicity. However, as they are isolated from discarded livers that are unsuitable for transplantation, they possess limited expansion ability in vitro and their enzymatic functions deteriorate rapidly because they are often of poor quality. Therefore, there is a compelling reason to find reliable alternative sources of hepatocytes.
Methods: In this study, we report on efficient and robust differentiation of embryonic stem cells (ESC) from the common marmoset Callithrix jacchus into functional hepatocyte-like cells (HLC) using a simple, and reproducible three-step procedure. ESC-derived HLCs were examined by morphological analysis and tested for their expression of hepatocyte-specific markers using a combination of immunohistochemistry, RT-PCR, and biochemical assays. Primary human hepatocytes were used as controls.
Results: ESC-derived HLCs expressed each of the hepatocyte-specific markers tested, including albumin; α-fetoprotein; asialoglycoprotein receptor 1; α-1 antitrypsin; hepatocyte nuclear factors 1α and 4; cytokeratin 18; hepatocyte growth factor receptor; transferrin; tyrosine aminotransferase; alkaline phosphatase; c-reactive protein; cytochrome P450 enzymes CYP1A2, CYP2E1 and CYP3A4; and coagulation factors FVII and FIX. They were functionally competent as demonstrated by biochemical assays in addition to producing urea.
Conclusion: Our data strongly suggest that marmoset HLCs possess characteristics similar to those of PHHs. They could, therefore, be invaluable for studies on drug metabolism and cell transplantation therapy for a variety of liver disorders. Because of the similarities in the anatomical and physiological features of the common marmoset to that of humans, Callithrix jacchus is an appropriate animal model to study human disease conditions and cellular functions.
Keywords: Callithrix jacchus, marmoset, hepatocyte, embryonic stem cell, differentiation, liver
Introduction
Limited availability of suitable donor tissue for human hepatocyte isolation and transplantation remains a major challenge in regenerative medicine. Only a small supply of human hepatocytes is currently available from organs determined to be inappropriate for transplantation.1 Moreover, mature human hepatocyte proliferation in culture is limited to few passages, and efforts to fully replicate their enzymatic functions in in vitro conditions have not been successful.2 Hence, the molecular mediators that regulate hepatocyte proliferation and could potentially promote their expansion in vitro are being actively studied. Alternative sources with greater reliability, proliferation, and differentiation into multiple liver cell types, such as stem cells, are under intense investigation. Stem cell-derived hepatocytes not only provide a continuous source of cells for transplantation but also are potentially useful for disease modelling, and investigations into drug function and metabolism.
In recent years, the common marmoset (Callithrix jacchus), a small New World monkey that is native to Brazil, has emerged as a non-human primate model for human diseases, including those of the liver.3–9 Adult marmosets have an average height of 20–30 cm and weigh about 350–400 g. Because of their small body size, shorter gestation period, ease of handling, and lower maintenance cost than other non-human primates, such as rhesus macaque and cynomolgus monkeys, they have received much attention for biomedical research.5 Marmosets have proven to be much closer to humans than rodents for pharmacokinetic and toxicological screening; and their cells effectively cross-react with human cytokines and hormones.4,7 In addition, the relative liver mass of marmosets is more similar to that of humans than mice, making it an ideal animal model to study common liver diseases, such as non-alcoholic fatty liver disease9 and hepatitis C virus infection.10 In this study, we report for the first time on hepatic differentiation of marmoset embryonic stem cells (ESC) into functional hepatocyte-like cells (HLC).
Materials and Methods
Marmoset Embryonic Stem Cells
The common marmoset (C. jacchus) embryonic stem cell line cj36711,12 established at the Wisconsin National Primate Research Center (kindly provided by Dr. T. G. Golos) was cultured in E8 medium (Cat# A15169-01; Thermo Fisher Scientific, Waltham, MA), with E8 supplement (1X; Cat# A15171-01; Thermo Fisher Scientific); GlutaMAX (1X; Cat# 35050-061; Life Technologies, Grand Island, NY); lipid concentrate (1:100; Cat# 11905-031; Life Technologies); recombinant human nodal (100 ng/mL; Cat# 3218-ND; R&D Systems, Minneapolis, MN); and glutathione (1.94 μg/mL; Cat# G4251; Sigma Aldrich, St. Louis, MO). Cells were grown on matrigel-coated plates (0.083 μg/well; Cat# 354230; BD Biosciences, San Jose, CA) and passaged using Accutase (Cat# 7920; Stem Cell Technologies, Vancouver, Canada) when reaching 80% confluency.
Primary Human Hepatocytes
Primary human hepatocytes (PHH) were purchased from Sekisui XenoTech (Kansas City, KS). They were grown either in collagen-coated 6-well plates (Cat# 356400, Corning Life Sciences, Tewksbury, MA) at a concentration of 1 x 106 cells/well or in 16-well collagen-coated slides at 5 x 104 cells per well according to supplier instructions. After plating for 16 hrs, PHHs were used in immunocytochemical studies and functional assays.
Karyotyping of Marmoset ESCs
Adherent marmoset ESCs were harvested after overnight colcemid arrest. The cells were treated with 0.75 M KCl hypotonic solution and fixed with 3:1 methanol:acetic acid. They were then spread onto glass slides according to standard cytogenetic protocols and stained with Wright-Giemsa stain. Twenty G-banded metaphases were analyzed using an Olympus BX61 microscope outfitted with 10x and 100x objectives. Metaphase chromosomes were imaged and karyotyped using Applied Spectral Imaging (ASI) software.
Hepatic Differentiation of Marmoset ESCs
Marmoset ESCs were grown in 6-well matrigel-coated plates until they reached ~80% confluency in E8 medium, as mentioned above. Following, the E8 medium was replaced with RPMI medium (Cat# 11875093, Thermo Fisher Scientific) enriched with B27 supplements (Cat# 17504001, Thermo Fisher Scientific). For hepatic differentiation, we used a modified three-step differentiation method, which we previously reported for induced pluripotent stem (iPS) cells with modifications.13 Briefly, in the first step, ESCs were cultured in RPMI medium containing B27 supplements and activin A (100 ng/mL; Cat# 338-AC) for 5 days at 37°C with 5% CO2 to induce the formation of definitive endoderm. Media was changed daily, and after 5 days, it was replaced with RPMI/B27 containing 10 ng/mL fibroblast growth factor-2 (FGF-2; Cat #233-FB), 10 ng/mL bone morphogenetic protein-4 (BMP-4; Cat# 314-BP) and 20 ng/mL hepatocyte growth factor (HGF; Cat# 294-HG) to generate hepatocyte-like cells (HLCs). Cultures were grown for 5 days and the medium was replaced with RPMI/B27 containing oncostatin M (Cat# 295-OM) to induce the maturation of the HLCs. After 5 days in this medium, HLCs were dissociated from plates with Accutase and were subjected to biochemical and morphological analyses, as described below. All reagents used in the differentiation protocol were purchased from R&D Systems.
Expansion and Maintenance of Marmoset HLCs
Marmoset ESC-derived HLCs were expanded and maintained in the hepatocyte phenotype by culturing in a specifically defined medium consisting of Williams Medium E (Cat# A1217601, Gibco, Grand Island, NY) supplemented with fatty acid-free BSA (Cat# A7030, Sigma); GlutaMAX; hydrocortisone-21-hemisuccinate (Cat# H2270, Sigma); ITS supplement (Cat# 17-838Z; Lonza, Walkerville, MD); epithelial growth factor (80 ng/mL; Cat# 236-EG, R&D Systems); FGF-4 (20 ng/mL; Cat 7460-F4, R&D Systems); HGF (40 ng/mL), stem cell factor (40 ng/mL; Cat# 255-SC, R&D Systems); oncostatin M (20 ng/mL); BMP-4 (20 ng/mL); and interleukin 1β (10 ng/mL; Cat# 201-LB, R&D Systems). Growth medium was changed 3 times per week, and HLCs were cultured for up to 60 days.
Immunohistochemistry
Marmoset HLCs were harvested from culture plates by dissociating them with TrypLE (Cat# 12605010, Thermo Fisher Scientific), enumerated and resuspended in the same medium at a density of 1x105 cells/mL. A 200 µL aliquot of cell suspension was added to each well of a type I collagen-coated (Cat# C3867, Sigma) 16-well glass chamber slide (Cat#178599, Thermo Fisher Scientific). Cells were cultured overnight to facilitate adherence to the slide. They were then fixed for 1 hr in 1% formalin and permeabilized using the PermaCyte Medium (CMDG, St. Paul, MN). Subsequently, HLCs were incubated with 100 ng of each antibody against alkaline phosphatase (Cat# MAB1448), α-fetoprotein (AFP) (Cat# MAB1369), albumin (Cat# MAB1456), C-reactive protein (Cat# MAB17071), HGF receptor (Cat# MAB3583), nestin (Cat# MAB1269), SOX17 (Cat# MAB1924), asialoglycoprotein receptor 1 (Cat# MAB4394), GATA4 (Cat# MAB2606) and α-1-antitrypsin (AAT) (Cat# MAB1268) (all from R&D Systems), coagulation factors VII (Cat# MA5-16932) and IX (Cat# HYB133-01-02) (both from Invitrogen, Rockford, IL), hepatocyte nuclear factor-4 (Cat# ABIN561308), CYP1A2 (Cat# ab151728) and CYP3A4 (Cat# ab124921) (all from Abcam, Cambridge, MA) for 40 mins. After washing once with PermaCyte medium, cells were stained either with Alexa 594-labeled goat-anti-mouse (Cat# A11005, Life Technologies) or Alexa 594-labeled goat-anti-rabbit (Cat# A11072, Life Technologies) secondary antibodies. Cells were also counterstained with the DAPI dye to visualize the nucleus of each cell. Positivity of the staining was confirmed by comparing them with isotype controls (Cat# QTC1000, CMDG, St. Paul, MN). HLCs were then analyzed using an Olympus Fluoview 1000 confocal microscope. Intracellular triglyceride content was assessed by staining with AdipoRedTM Assay Reagent (Cat# PT-709, Lonza, Walkserville, MD). Using the same preparation procedure as with antibody staining, 2 μl of AdipoRed reagent was added to a well and incubated for 15 minutes. After incubation, cells were washed with PermaCyte medium and visualized under the confocal microscope.
RT-PCR Analysis
To test for the expression hepatocyte-specific genes in HLCs, total RNA was extracted from cells using the UltraPure™ Phenol:Chloroform:Isoamyl Alcohol reagent (Cat# 15593031, Invitrogen). Platinum® Quantitative RT-PCR ThermoScript ™ One-Step System (Cat# 11731015, Invitrogen) was used to carry out RT-PCR reactions according to the manufacturer’s recommendations. Total RNA isolated from marmoset ESCs was used as the negative control. Marmoset-specific albumin PCR primers were designed using the GenBank sequence NC_013898.1 (Gene ID: 100391910), whereas the other marmoset-specific primer sequences for α-fetoprotein (AFP), cytokeratin 18 (CK18), transferrin (TF), α-1 antitrypsin (AAT), hepatocyte nuclear factor 1α (HNF1α), tyrosine aminotransferase (TAT), cytochrome P450 2E1 (CYP2E1), and the house-keeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were reported earlier (Table 1).14–16 All the primers were obtained from Integrated DNA Technologies (Coralville, IA). The conditions for PCR reactions were an initial denaturation at 94°C for 3 min followed by 30 cycles of denaturation at 94°C for 1 min, annealing for 1 min at 56°C, and elongation for 1 min at 72°C. PCR products were then resolved using a 1% agarose gel, and visualized under UV light.
 |
Table 1 RT-PCR Primers Used in This Study |
Urea Production
Urea synthesis in ESC-derived HLCs was determined with a colorimetric assay (Cat# K376-100, Biovision, Milpitas, CA). Briefly, cells were dissociated from the culture plate using the TrypLE reagent, counted with hemocytometer and centrifuged at 400 x g for 5 mins. The supernatant was discarded and the pelleted cells were re-suspended in 1 mL of WIF water (Cat# 4.86505.1000, EMD Chemicals, Gibbstown, NJ). Cells were then exposed to three repeated freeze-thaw cycles of freezing at −80°C and thawing at room temperature to rupture and release the intracellular contents into the fluid phase. Cells were then centrifuged at 1000 x g for 10 mins to pellet the cell debris. The supernatant was collected and analyzed by the urea assay, according to the manufacturer’s instructions. Results were standardized to 1x106 cells to enable comparison between cell culture samples with different cell numbers. Resultant samples were analyzed on the Emax microplate reader (Molecular Devices. San Jose, CA) and processed with SoftMax PRO 4.8 Analysis Software. Marmoset ESCs and PHHs were used as negative and positive controls, respectively.
Results
Differentiation of Marmoset ESCs into Hepatocyte-Like Cells
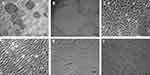
To determine whether marmoset ESCs are capable of differentiating into hepatocyte-like cells (HLC), we applied a modified three-step differentiation protocol that we previously developed for the hepatic differentiation of iPS cells.13 It involved the use of activin A to induce the definitive endoderm, followed by the propagation of cells with FGF-2, BMP-4 and HGF to differentiate them into the hepatic lineage. In the final step, cells were exposed to oncostatin M to induce their maturation into functional HLCs. In this protocol, each step was of 5-day duration and cells were maintained in matrigel-coated plates in RPMI medium with B27 supplements. After 15 days through this procedure, >90% of the cells displayed hepatocyte-specific morphology, and a large number of cells were clearly bi-nucleated indicating that the marmoset ESCs had been successfully differentiated into HLCs (Figure 1).
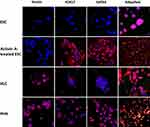
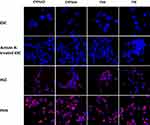
It has been well documented that activin A induces the differentiation of definitive endoderm in stem cells.17–20 A number of transcription factors, including GATA factors and SOX factors, are expressed during the differentiation of ESCs and in the DE.21–23 GATA4 plays a critical role in endoderm formation and GATA4-deficient ESCs were shown to be specifically defective in forming the visceral endoderm.24 In this study, we have carried out immunohistochemistry with activin A-treated ESCs to determine whether they had differentiated into DE. As shown in Figure 2, activin A-treated ESCs tested positive for both SOX17 and GATA4. However, the expression of these two proteins was found to be lower in HLCs than in PHHs. On the other hand, nestin which was associated with the progenitor cells was absent in ESC and activin A-treated ESCs but was expressed by HLCs and PHHs. This finding was not unexpected having been demonstrated that hepatocytes cultured in vitro express various biliary and extrahepatic progenitor markers, including nestin.25 In addition, activin A treatment did not alter the chromosomes of ESCs, as shown by karyotype analysis (Figure 3). Undifferentiated ESCs, activin A-treated ESCs and differentiated HLCs displayed normal female karyotype (46, XX), which was similar to published data on marmoset ESC cell lines.26,27 Taken together, these results support the finding that marmoset ESCs are capable of differentiating into definitive endoderm.17
 |
Figure 3 Karyotype analysis of marmoset ESCs (A), activin A-treated ESCs (B) and ESC-derived HLCs (C). |
Expression of Hepatocyte-Specific Markers by Marmoset HLCs
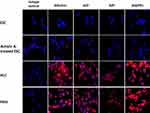
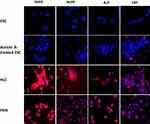
To study the expression of hepatocyte-specific markers in ESC-derived HLCs, we carried out immunohistochemical analyses using antibodies against albumin; AFP; AAT; ASGPR1; HNF4; HGFR; ALP; CRP; CYP1A2; CYP3A4; FVII; and FIX. As shown in Figures 4–6, differentiated HLCs expressed all these markers demonstrating that the differentiated HLCs possess hepatocyte-like characteristics. Throughout the studies, PHHs were used as positive control and an isotype control served as the negative control. While the expression of the majority of markers in HLCs was very similar to PHHs, the expression of inducible proteins CYP1A2, CYP3A4, FVII and FIX was lower. This result was in agreement with reports that the basal expression of certain CYP enzymes varies in culture conditions,28 possibly due to the discrepancy in the quality of the donor hepatocytes. Similarly, coagulation factors FVII and FIX were present in low levels in normal hepatocytes.29,30
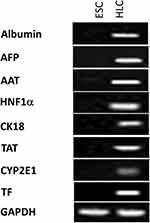
To further validate the hepatocyte marker expression in ESC-derived HLCs, RT-PCR was performed using marmoset-specific primers for albumin, AFP, AAT, HNF1α, cytokeratin 18, transferrin, CYP2E1, and tyrosine aminotransferase. GAPDH was chosen as the internal standard because it is the most stable in stem cells among many house-keeping genes that are used for reference of gene expression.31,32 As shown in Figure 7, HLCs expressed the litany of markers tested and ESCs were positive only for GAPDH. Collectively, these results demonstrated that HLCs express hepatocyte markers both at the RNA and protein levels, and are potentially capable of hepatocyte-specific functions.
Urea Production by ESC-Derived HLCs
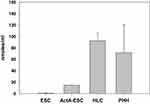
To further ascertain the functionality of ESC-derived HLCs, we tested their ability to produce urea, as a hallmark of primary hepatocytes. As stated above, HLC urea production was determined via a colorimetric assay using PHHs as positive control. As shown in Figure 8, HLCs synthesized urea at levels similar to that of PHHs confirming that these cells are metabolically active. Interestingly, however, activin A-treated ESCs that are committed to form definitive endoderm also produced small amounts of urea suggesting that the urea production could be an early metabolic step during the development of hepatocytes. Primary marmoset ESCs were included as negative controls for this study and they expressed non-detectable levels of urea under identical conditions.
Discussion
The inherent ability of ESCs to differentiate into any mammalian cell type has rendered them attractive for investigations on modelling human liver diseases, studies on drug metabolism and liver regeneration. To date, ESCs from human, rodent, porcine, and monkey species have been differentiated into HLCs.33–49 In this study, we have carried out hepatic differentiation of marmoset ESCs into HLCs and characterized them using a combination of immunohistochemistry, morphological analyses, RT-PCR, and biochemical assays. Activin A-treated marmoset ESCs and primary human hepatocytes (PHHs) were used as controls. Our results demonstrated that the marmoset ESC-derived HLCs possess functional characteristics similar to those of PHHs. Additionally, these HLCs displayed specific characteristics that define a typical “hepatocyte”, as determined by both qualitative assays and qualitative analysis.50
Significantly, our hepatic differentiation protocol is distinct from that of other reports of non-human primates, namely rhesus macaque ESCs42,43 and cynomolgus ESCs.48,49 We have used a modified three-step procedure that was previously developed in our laboratory to differentiate porcine iPS cells into HLCs.13 Each step of this protocol was for a 5-day period, in which the first step was the induction of definitive endoderm by treating marmoset ESCs with activin A, followed by hepatocyte differentiation with growth factors FGF-2 and BMP-4. The final step involved the treatment of HLCs with HGF and oncostatin M to induce their full maturation to hepatocyte-like status (Figure 1). In contrast, rhesus macaque ESC differentiation procedures were of 20-day42 and 30-day43 durations, while that of cynomolgus ESC was either a 27-day48 or 28-day49 procedure. Thus, our protocol is significantly shorter in duration and produced mature HLCs within 15 days. In addition, our procedure did not include any feeder cultures or co-cultures. Each of the marmoset cell types (ESC, activin A-treated ESC and ESC-derived HLC) examined by karyotyping had a chromosome number of 46 (Figure 3). No abnormalities such as fused chromosomes, marker chromosomes or extra chromosomes were found. The results confirmed that our differentiation procedure did not alter the karyotype of marmoset ESCs, and the differentiated HLCs exhibited a normal number, shape and structure of 46 chromosomes.
Here, we have studied the expression of 17 hepatocyte markers in HLCs. Most notable of these is the asialoglycoprotein receptor (ASGPR1), a liver-specific protein that is highly produced in well-differentiated hepatocytes. Its utility as a definitive marker of hepatocyte identity is well established, and it has been used to isolate stem cell-derived hepatocytes.51 As shown in Figure 4, ASGPR1 is expressed on the cell surface by HLCs at the comparable levels to that of PHHs. C-reactive protein, another marker specifically produced by hepatocytes,52 was also produced by HLCs (Figure 5). Similar to ASGPR1, the hepatocyte appears to be the only cell type that produces coagulator factor IX.29 Differentiated marmoset HLCs produced FIX (Figure 6) and showed the presence of triglycerides (Figure 2) at levels similar to PHHs further underscoring that marmoset HLCs have matured into hepatocytes.
Additionally, the expression of other common hepatocyte markers albumin, α-fetoprotein, hepatocyte nuclear factor 4, hepatocyte growth factor receptor demonstrated unequivocally that the marmoset ESC-derived HLCs fully differentiated and matured into HLCs (Figures 4–6). Among these, HNF4 is an orphan nuclear receptor that functions as a master regulator of hepatic differentiation. During liver development, it is expressed in primary and extra-embryonic visceral endoderm, and HNF4-null embryos exhibit severe defects in visceral endoderm development.53
We have also studied the expression of three class I cytochrome P450 (CYP) enzymes, CYP1A2, CYP2E1 and CYP3A4 in marmoset HLCs. Most notably, they are highly conserved, have no known important functional polymorphisms, and are active in the metabolism of pre-carcinogens and drugs.54 CYP1A2, which accounts for about 13% of all CYP450 enzymes in the liver, is a major enzyme that metabolizes both endogenous compounds such as melatonin, estradiol, bilirubin and arachidonic acid, as well as several clinical drugs, including analgesics and antipyretics (reviewed in55). Another major liver-specific cytochrome P450 enzyme is CYP3A4, which is involved in the metabolic oxidation of more than 50% of all drugs including acetaminophen.56 CYP2E1 metabolizes low molecular weight solvents such as alcohol, toxic chemicals like chloroform and carbon tetrachloride; and environmental contaminants such as benzene and acrylamide.57,58 Marmoset ESC-derived HLCs expressed all three CYP enzymes tested (Figures 6 and 7), albeit at lower levels than PHHs. This is in line with previous reports that the basal level expression of CYP450 isotypes is low in stem cell-derived HLCs.59–61
Conclusions
In summary, we have carried out efficient hepatic differentiation of marmoset ESCs and demonstrated that the HLCs generated from them possessed specific characteristics similar to those of primary human hepatocytes. To the best of our knowledge, this is the first report on hepatic differentiation of any marmoset stem cell type. Our simple, three-step in vitro differentiation protocol produced functional HLCs within 15 days in culture. HLCs generated with this procedure were propagated in culture for up to 60 days without altering their phenotype. Because of the cross-reactivity of marmoset proteins to human antibodies62–64 and the results from our studies, these marmoset HLCs might be ideal candidates to study modelling and cell therapy of human liver diseases; and for liver regeneration. Their expression of CYP genes involved in the breakdown of various toxic molecules and chemicals also make them suitable for investigations on drug metabolism.
Acknowledgments
We thank T. G. Golos (Wisconsin National Primate Research Center, University of Wisconsin, Madison, WI) for providing marmoset ESCs; Edward Zhai for the initial cell culture work; and Molly Lynch and LeAnn Oseth for the marmoset ESC karyotype analyses. This work was supported by funding from the Institute of Engineering in Medicine Seed Grant from the University of Minnesota to RNA and CJS; NIH R01 DK117286-01 to CJS; and BioE, LLC to DPC and JHH.
Disclosure
Dr Daniel Collins reports other funding from CMDG, LLC, outside of the submitted work. The authors declare no additional conflicts of interest.
References
1. Dhawan A, Puppi J, Hughes RD, Mitry RR. Human hepatocyte transplantation: current experience and future challenges. Nat Rev Gastroenterol Hepatol. 2010;7(5):288–298. doi:10.1038/nrgastro.2010.44
2. Forbes SJ, Gupta S, Dhawan A. Cell therapy for liver disease: from liver transplantation to cell factory. J Hepatol. 2015;62(1 Suppl):S157–S169. doi:10.1016/j.jhep.2015.02.040
3. Mansfield K. Marmoset models commonly used in biomedical research. Comp Med. 2003;53(4):383–392.
4. Carrion R
5. Okano H, Hikishima K, Iriki A, Sasaki E. The common marmoset as a novel animal model system for biomedical and neuroscience research applications. Semin Fetal Neonatal Med. 2012;17(6):336–340. doi:10.1016/j.siny.2012.07.002
6. `t Hart BA, Abbott DH, Nakamura K, Fuchs E. The marmoset monkey: a multi-purpose preclinical and translational model of human biology and disease. Drug Discov Today. 2012;17(21–22):1160–1165. doi:10.1016/j.drudis.2012.06.009
7. Uno Y, Uehara S, Yamazaki H. Utility of non-human primates in drug development: comparison of non-human primate and human drug-metabolizing cytochrome P450 enzymes. Biochem Pharmacol. 2016;121:1–7. doi:10.1016/j.bcp.2016.06.008
8. Havel PJ, Kievit P, Comuzzie AG, Bremer AA. Use and importance of nonhuman primates in metabolic disease research: current state of the field. ILAR J. 2017;58(2):251–258. doi:10.1093/ilar/ilx031
9. Kramer JA, Grindley J, Crowell AM, et al. The common marmoset as a model for the study of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Vet Pathol. 2015;52(2):404–413. doi:10.1177/0300985814537839
10. Li T, Zhu S, Shuai L, et al. Infection of common marmosets with hepatitis C virus/GB virus-B chimeras. Hepatology. 2014;59(3):789–802. doi:10.1002/hep.26750
11. Vermilyea SC, Meyer M, Smuga-Otto K, et al. Induced pluripotent stem cell-derived dopaminergic neurons from adult common marmoset fibroblasts. Stem Cells Dev. 2017;26(17):1225–1235. doi:10.1089/scd.2017.0069
12. Zhou B, Leung LC, Ward TR, et al. Haplotype-phased common marmoset embryonic stem cells for genome editing using CRISPR/Cas9. bioRxiv. 2018. doi:10.1101/373886
13. Aravalli RN, Cressman EN, Steer CJ. Hepatic differentiation of porcine induced pluripotent stem cells in vitro. Vet J 2012;194(3):369–374. doi:10.1016/j.tvjl.2012.05.013
14. Fujii Y, Kitaura K, Matsutani T, et al. Immune-related gene expression profile in laboratory common marmosets assessed by an accurate quantitative real-time PCR using selected reference genes. PLoS One. 2013;8(2):e56296. doi:10.1371/journal.pone.0056296
15. Sasaki E, Hanazawa K, Kurita R, et al. Establishment of novel embryonic stem cell lines derived from the common marmoset (Callithrix jacchus). Stem Cells. 2005;23(9):1304–1313. doi:10.1634/stemcells.2004-0366
16. Uehara S, Uno Y, Inoue T, et al. Activation and deactivation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine by cytochrome P450 enzymes and flavin-containing monooxygenases in common marmosets (Callithrix jacchus). Drug Metab Dispos. 2015;43(5):735–742. doi:10.1124/dmd.115.063594
17. Diekmann U, Naujok O, Blasczyk R, Müller T. Embryonic stem cells of the non-human primate Callithrix jacchus can be differentiated into definitive endoderm by Activin-A but not IDE-1/2. J Tissue Eng Regen Med. 2015;9(4):473–479. doi:10.1002/term.1709
18. Teo AK, Ali Y, Wong KY, et al. Activin and BMP4 synergistically promote formation of definitive endoderm in human embryonic stem cells. Stem Cells. 2012;30(4):631–642. doi:10.1002/stem.1022
19. Wang Z, Li W, Chen T, et al. Activin A can induce definitive endoderm differentiation from human parthenogenetic embryonic stem cells. Biotechnol Lett. 2015;37(8):1711–1717. doi:10.1007/s10529-015-1829-x
20. D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23(12):1534–1541. doi:10.1038/nbt1163
21. Shimoda M, Kanai-Azuma M, Hara K, et al. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J Cell Sci. 2007;120(Pt 21):3859–3869. doi:10.1242/jcs.007856
22. Mfopou J, Geeraerts M, Dejene R, et al. Efficient definitive endoderm induction from mouse embryonic stem cell adherent cultures: a rapid screening model for differentiation studies. Stem Cell Res. 2014;12(1):166–177. doi:10.1016/j.scr.2013.10.004
23. Niakan KK, Ji H, Maehr R, et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 2010;24(3):312–326. doi:10.1101/gad.1833510
24. Soudais C, Bielinska M, Heikinheimo M, et al. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121(11):3877–3888.
25. Koenig S, Krause P, Drabent B, et al. The expression of mesenchymal, neural and haematopoietic stem cell markers in adult hepatocytes proliferating in vitro. J Hepatol. 2006;44(6):1115–1124. doi:10.1016/j.jhep.2005.09.016
26. Debowski K, Drummer C, Lentes J, et al. The transcriptomes of novel marmoset monkey embryonic stem cell lines reflect distinct genomic features. Sci Rep. 2016;6:29122. doi:10.1038/srep29122
27. Trettner S, Findeisen A, Taube S, Horn PA, Sasaki E, Zur Nieden NI. Osteogenic induction from marmoset embryonic stem cells cultured in feeder-dependent and feeder-independent conditions. Osteoporos Int. 2014;25(4):1255–1266. doi:10.1007/s00198-013-2566-4
28. Olsavsky KM, Page JL, Johnson MC, Zarbl H, Strom SC, Omiecinski CJ. Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol Appl Pharmacol. 2007;222(1):42–56. doi:10.1016/j.taap.2007.03.032
29. Tatsumi K, Ohashi K, Mukobata S, et al. Hepatocyte is a sole cell type responsible for the production of coagulation factor IX in vivo. Cell Med. 2012;3(1–3):25–31. doi:10.3727/215517912X639496
30. Perry DJ. Factor VII deficiency. Br J Haematol. 2002;118(3):689–700.
31. Panina Y, Germond A, Masui S, Watanabe TM. Validation of common housekeeping genes as reference for qPCR gene expression analysis during iPS reprogramming process. Sci Rep. 2018;8(1):8716. doi:10.1038/s41598-018-26707-8
32. Murphy CL, Polak JM. Differentiating embryonic stem cells: GAPDH, but neither HPRT nor β-tubulin is suitable as an internal standard for measuring RNA levels. Tissue Eng. 2002;8(4):551–559. doi:10.1089/107632702760240472
33. Agarwal S, Holton KL, Lanza R. Efficient differentiation of functional hepatocytes from human embryonic stem cells. Stem Cells. 2008;26(5):1117–1127. doi:10.1634/stemcells.2007-1102
34. Baharvand H, Hashemi SM, Shahsavani M. Differentiation of human embryonic stem cells into functional hepatocyte-like cells in a serum-free adherent culture condition. Differentiation. 2008;76(5):465–477. doi:10.1111/j.1432-0436.2007.00252.x
35. Cai J, Zhao Y, Liu Y, et al. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45(5):1229–1239. doi:10.1002/(ISSN)1527-3350
36. Choi D, Oh HJ, Chang UJ, et al. In vivo differentiation of mouse embryonic stem cells into hepatocytes. Cell Transplant. 2002;11(4):359–368. doi:10.3727/000000002783985792
37. Duan Y, Catana A, Meng Y, et al. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells. 2007;25(12):3058–3068. doi:10.1634/stemcells.2007-0291
38. Duan Y, Ma X, Zou W, et al. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28(4):674–686. doi:10.1002/stem.315
39. Hay DC, Zhao D, Fletcher J, et al. Efficient differentiation of hepatocytes from human embryonic stem cells exhibiting markers recapitulating liver development in vivo. Stem Cells. 2008;26(4):894–902. doi:10.1634/stemcells.2007-0718
40. He ZY, Deng L, Li YF, et al. Murine embryonic stem cell-derived hepatocytes correct metabolic liver disease after serial liver repopulation. Int J Biochem Cell Biol. 2012;44(4):648–658. doi:10.1016/j.biocel.2012.01.002
41. Ishii T, Yasuchika K, Fujii H, et al. In vitro differentiation and maturation of mouse embryonic stem cells into hepatocytes. Exp Cell Res. 2005;309(1):68–77. doi:10.1016/j.yexcr.2005.05.028
42. Kuai XL, Shao N, Lu H, Xiao SD, Zheng Q. Differentiation of nonhuman primate embryonic stem cells into hepatocyte-like cells. J Dig Dis. 2014;15(1):27–34. doi:10.1111/1751-2980.12103
43. Ma X, Duan Y, Jung CJ, Wu J, VandeVoort CA, Zern MA. The differentiation of hepatocyte-like cells from monkey embryonic stem cells. Cloning Stem Cells. 2008;10(4):485–493. doi:10.1089/clo.2007.0012
44. Park KM, Hussein KH, Ghim JH, et al. Hepatic differentiation of porcine embryonic stem cells for translational research of hepatocyte transplantation. Transplant Proc. 2015;47(3):775–779. doi:10.1016/j.transproceed.2015.01.020
45. Touboul T, Hannan NR, Corbineau S, et al. Generation of functional hepatocytes from human embryonic stem cells under chemically defined conditions that recapitulate liver development. Hepatology. 2010;51(5):1754–1765. doi:10.1002/hep.23506
46. Yamada T, Yoshikawa M, Kanda S, et al. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20(2):146–154. doi:10.1634/stemcells.20-2-146
47. Yamamoto H, Quinn G, Asari A, et al. Differentiation of embryonic stem cells into hepatocytes: biological functions and therapeutic application. Hepatology. 2003;37(5):383–393. doi:10.1053/jhep.2003.50202
48. Maruyama J, Matsunaga T, Yamaori S, et al. Differentiation of monkey embryonic stem cells to hepatocytes by feeder-free dispersion culture and expression analyses of cytochrome p450 enzymes responsible for drug metabolism. Biol Pharm Bull. 2013;36(2):292–298. doi:10.1248/bpb.b12-00866
49. Saito K, Yoshikawa M, Ouji Y, et al. Promoted differentiation of cynomolgus monkey ES cells into hepatocyte-like cells by co-culture with mouse fetal liver-derived cells. World J Gastroenterol. 2006;12(42):6818–6827. doi:10.3748/wjg.v12.i42.6818
50. Hengstler J, Brulport M, Schormann W, et al. Generation of human hepatocytes by stem cell technology: definition of the hepatocyte. Exp Opin Drug Metabol Toxicol. 2005;1(1):61–74. doi:10.1517/17425255.1.1.61
51. Peters DT, Henderson CA, Warren CR, et al. Asialoglycoprotein receptor 1 is a specific cell-surface marker for isolating hepatocytes derived from human pluripotent stem cells. Development. 2016;143(9):1475–1481. doi:10.1242/dev.132209
52. Kushner I, Feldmann G. Control of the acute phase response. Demonstration of C-reactive protein synthesis and secretion by hepatocytes during acute inflammation in the rabbit. J Exp Med. 1978;148(2):466–477. doi:10.1084/jem.148.2.466
53. Chen WS, Manova K, Weinstein DC, et al. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8(20):2466–2477. doi:10.1101/gad.8.20.2466
54. Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25(11):1679–1691. doi:10.1038/sj.onc.1209377
55. Sridhar J, Goyal N, Liu J, Foroozesh M. Review of ligand specificity factors for CYP1A subfamily enzymes from molecular modeling studies reported to-date. Molecules. 2017;22(7):
56. Sevrioukova IF, Poulos TL. Current approaches for investigating and predicting cytochrome P450 3A4-ligand interactions. Adv Exp Med Biol. 2015;851:83–105.
57. Novak RF, Woodcroft KJ. The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch Pharm Res. 2000;23(4):267–282. doi:10.1007/BF02975435
58. Abdelmegeed MA, Ha SK, Choi Y, Akbar M, Song BJ. Role of CYP2E1 in mitochondrial dysfunction and hepatic injury by alcohol and non-alcoholic substances. Curr Mol Pharmacol. 2017;10(3):207–225. doi:10.2174/1874467208666150817111114
59. Tsuchiya H, Matsunaga T, Aikawa K, et al. Evaluation of human embryonic stem cell-derived hepatocyte-like cells for detection of CYP1A inducers. Drug Metab Pharmacokinet. 2012;27(6):598–604. doi:10.2133/dmpk.DMPK-12-RG-017
60. Sa-ngiamsuntorn K, Wongkajornsilp A, Kasetsinsombat K, et al. Upregulation of CYP 450s expression of immortalized hepatocyte-like cells derived from mesenchymal stem cells by enzyme inducers. BMC Biotechnol. 2011;11:89. doi:10.1186/1472-6750-11-89
61. Takayama K, Hagihara Y, Toba Y, Sekiguchi K, Sakurai F, Mizuguchi H. Enrichment of high-functioning human iPS cell-derived hepatocyte-like cells for pharmaceutical research. Biomaterials. 2018;161:24–32. doi:10.1016/j.biomaterials.2018.01.019
62. Neubert R, Foerster M, Nogueira AC, Helge H. Cross-reactivity of antihuman monoclonal antibodies with cell surface receptors in the common marmoset. Life Sci. 1996;58(4):317–324. doi:10.1016/0024-3205(95)02291-0
63. Kireta S, Zola H, Gilchrist RB, Coates PT. Cross-reactivity of anti-human chemokine receptor and anti-TNF family antibodies with common marmoset (Callithrix jacchus) leukocytes. Cell Immunol. 2005;236(1–2):115–122. doi:10.1016/j.cellimm.2005.08.017
64. Riecke K, Nogueira AC, Alexi-Meskishvili V, Stahlmann R. Cross-reactivity of antibodies on thymic epithelial cells from humans and marmosets by flow-cytometry. J Med Primatol. 2000;29(5):343–349. doi:10.1111/jmp.2000.29.issue-5
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.