Back to Journals » Journal of Experimental Pharmacology » Volume 12
Gastroprotective Effect of DLBS2411 Bioactive Fraction from Cinnamomum burmannii Against Ethanol-Induced Gastric Damage in Rats
Authors Tjandrawinata RR , Nailufar F
Received 30 December 2019
Accepted for publication 4 March 2020
Published 20 March 2020 Volume 2020:12 Pages 87—95
DOI https://doi.org/10.2147/JEP.S244223
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bal Lokeshwar
Raymond R Tjandrawinata,1,2 Florensia Nailufar1
1Dexa Laboratories of Biomolecular Sciences, Dexa Medica, Cikarang, West Java 17550, Indonesia; 2Faculty of Biotechnology, Atma Jaya Catholic University of Indonesia, Tangerang 15345, Indonesia
Correspondence: Raymond R Tjandrawinata
Dexa Laboratories of Biomolecular Sciences, Dexa Medica, Industri Selatan V Block PP No. 7, Jababeka Industrial Estate II, Cikarang, West Java 17550, Indonesia
Tel +62 21 89841901
Fax +62 21 8984 1905
Email [email protected]
Background: The study was carried out to evaluate the anti-ulcerative and gastroprotective effect of DLBS2411, a bioactive fraction from Cinnamomum burmannii (Nees & T. Nees) Blume, in Wistar rats (Rattus norvegicus).
Methods: The rats were divided into five treatment groups, which were the Normal control group, Negative control group (ethanol-induced) and two treatment groups: DLBS2411 at the doses of 25 mg/kg body weight (BW) and 50 mg/kg BW, and the Positive control group treated with sucralfate at the dose of 100 mg/kg BW. Gastroprotective effect was measured by the ulcerative lesion index, ulcer surface area, percentage of lesion area, and cure ratio. Hematological and histopathological analyses were also conducted to gain additional data regarding the gastroprotective effect of DLBS2411 in the rats’ stomachs.
Results: DLBS2411 was found to contain not less than 15% of total phenolic compounds. Treatment with DLBS2411 at doses of 25 mg/kg BW and 50 mg/kg BW significantly reduced the percentage of ulcer area in rats. The percentage of ulcer area for the Negative control group and both doses in the DLBS2411 treatment group reached 22.64± 6.82%, 6.75± 4.41%, and 6.18± 4.63%, respectively. Ulcer surface area in the treatment groups and Positive control group also decreased. Histopathological data showed that gastric epithelial cells in the Negative control group were more severely ulcerated than in the treatment group of DLBS2411 and the Positive control group.
Conclusion: This study showed that DLBS2411 at the dose of 50 mg/kg BW was more effective in protecting the stomach lining than DLBS2411 at the dose of 25 mg/kg BW, as measured by percentage of ulceration inhibition and the ulcerative lesion index.
Keywords: gastroprotective, ethanol, Cinnamomum burmannii, DLBS2411, rat
Introduction
Ulceration results from the imbalance between gastroprotective and aggressive factors, such as acid–pepsin secretion, mucosal barriers, mucus secretion, blood flow, cellular regeneration, prostaglandins, and epidermal growth factors. The risk factors for gastric ulcer include consumption of non-steroidal anti–inflammatory drugs, stress, steroids, infection by Helicobacter pylori, and ethanol consumption.1–3 Furthermore, stress, smoking, and nutritional deficiencies have also been found to increase the incidence of gastric ulcer.2,3
The pathogenesis of gastric ulcer is believed to be a multifactorial process. In this process, chronic antral gastritis has been proven to be a major prerequisite in the pathogenesis of gastric and duodenal ulcer in humans.4 Considering several side effects of modern medicines (arrhythmia, gynecomastia, and hematopoietic changes), indigenous medicines possessing fewer and more tolerable side effects should be sought as an alternative for the treatment of gastric ulcer.
The mechanism of action and molecular activities of various herbs have been investigated in vivo and in vitro in our previous studies.5–12 In recent years, many studies have been carried out on isolation, determination of the phytochemical profile, and characterization of the pigments of Cinnamomum burmannii.13,14 It has been recommended for medicinal use because it displays antibacterial15 and antioxidant activities.16 The combination of Lagerstroemia speciosa and C. burmannii has been shown to increase glucose uptake in vitro and in vivo.6,17
DLBS2411, a standardized extract containing C. burmannii, was previously reported to have an effect on hydrogen/potassium adenosine triphosphate (H+/K+-ATPase) activity and to downregulate the expression of the gene encoding the enzyme. DLBS2411 was also reported to possess antioxidant activity and to show gastroprotective properties on acetic acid-induced gastric ulcer.18,19 However, the effect of DLBS2411 on ethanol-induced gastric ulcer remains unclear. In this study, DLBS2411 was studied further in an animal model of gastric ulcer. The aim of this study was to evaluate the anti-ulcerogenic and gastroprotective properties of DLBS2411.
Materials and Methods
Materials
Ethanol of analytical grade was obtained from Merck (Frankfurt, Germany). Sucralfate powder was obtained from Dexa Medica (Palembang, Indonesia). Xanthan gum was provided by Ferron Par Pharmaceutical (Cikarang, Indonesia). Parameters for hematological analysis were determined by a semi-automated hematology analyzer (MEK-6470K; Nihon Kohden, Japan) and cell pack diluents (Nihon Kohden, Japan).
Preparation of Bioactive Fraction DLBS2411
The dried C. burmannii bark was milled and macerated in water for 2 h. It was concentrated to approximately 1/10 of its initial volume and fractionated with methylene chloride to allow the aqueous phase to separate from the organic phase. The aqueous phase was then concentrated to eliminate any residual methylene chloride. The resulting concentrate was dried in an oven and the dried product was milled using Cone Mill (Comil underdrium 197; Quadro, Canada) and stored in a well-sealed container.
DLBS2411 is a red–brown aromatic powder with a bitter and astringent taste. It is practically insoluble in water or ethanol. Chemical identification of DLBS2411 was conducted using thin-layer chromatography plate Silica Gel 60 F254 as the stationary phase and 1-butanol-glacial acetic acid-water (5:2:2, v/v/v) as the mobile phase. The marker compound observed under UV light at 366 nm showed a fluorescent blue band at RF around 0.6, and under visible light after derivatization using 10% (v/v) H2SO4 showed a green band at RF around 0.5 and a yellow band at RF around 0.7. The total phenolic compounds (TPC) content of DLBS2411 was quantified using spectrophotometry and standardized to not less than 15.00% (w/w). In addition, DLBS2411 was designed to have a low coumarin content based on quantification by an HPLC isocratic elution system using acetonitrile–methanol (60:40, v/v) as the mobile phase, with a flow rate of 1.0 mL/min, C18 4.6× 150 mm 5 µm column as the stationary phase and UV detector set to 274 nm. The coumarin content of DLBS2411 was standardized to less than 0.025% (w/w).
Animals
This research was conducted in accordance with the Institutional Animal Care and Use Committee Guidebook (ARENA/OLAW), Guide for the Care and Use of Laboratory Animals (National Research Council [US] Committee) and Guidelines for the Euthanasia of Animals (AVMA). Wistar rats weighing 250–300 g were used in this study and caged in groups in a polysulfone filter top cage that was maintained under standard conditions (12 h light–12 h dark cycle, with room temperature at 23±2°C and relative humidity 30–70%) in the AAALAC International accredited facility. All procedures in this experiment have been reviewed and approved by the Institutional Animal Care and Use Committee of Dexa Laboratories of Biomolecular Sciences (DLBS), with protocol number DIS-DLBS-PROC-APC-036.
Experimental Procedures
The experiment was carried out according to the method of Morimoto et al, with slight modification.20 After 24 h of fasting, the rats were randomly divided into five groups, each group consisting of five animals. The Normal control and Negative control groups were given 1 mL/kg body weight (BW) of vehicle, and the Positive control group was treated with sucralfate at a dose of 100 mg/kg BW. The remaining groups received 25 and 50 mg/kg BW of DLBS2411 (Table 1).
 |
Table 1 Ethanol-Induced Gastric Ulcer Model; Treatment in Each Group |
All treatments were administered orogastrically. One hour after treatment, all groups except for the Normal control group received 99.5% ethanol 1 mL/kg BW to induce gastric ulcers. After 1 h, the rats were anesthetized using ketamine mixed with xylazine at doses of 80 mg/kg BW and 8 mg/kg BW, respectively, then killed with pentobarbital injection at a dose of 150 mg/kg BW.
Gastroprotective Analysis
The stomach of each rat was removed and opened along the greater curvature. Then, the stomach was gently rinsed with water to remove the gastric contents and blood clots, for subsequent scanning and measurement of the ulcerative lesion index (ULI).
The ulcers were classified as2
- level I, ulcer area <1 mm2
- level II, ulcer area 1–3 mm2
- level III, ulcer area >3 mm2
The classification of ulcer area was used for calculating the ULI, using the following equation:2
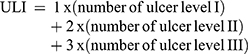
where the total area of lesions is in mm2.
- The percentage of lesion area in relation to the total stomach was calculated using the following equation:2
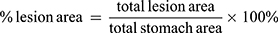
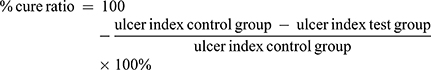
- The percentage of curative ratio was calculated using the following equation:2
Histopathological Examination
Gastric samples were collected, dipped in neutral buffer formaldehyde, and embedded in paraffin for histopathological examination. The sections (4–5 µm) were sliced using a microtome and stained with hematoxylin and eosin (H&E). The histopathological examination was performed by a histopathologist at Pusat Satwa Studi Primata (PSSP) (Bogor, West Java, Indonesia).
Hematological Analysis
Five-hundred-microliter blood samples from each rat were collected into MicrotainerTM blood tube before and after treatment with K2EDTA (Becton Dickinson, NJ, USA) for hematological analysis using a semi-automated hematology analyzer (MEK-6470K; Nihon Kohden, Japan) and cell pack diluents (Nihon Kohden, Japan). Parameters that were measured include red blood cells (RBC), white blood cells (WBC), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelets (PLT).
Statistical Analysis
Data for each group should be normally distributed and the variance should be homogeneous in order to be analyzed with one-way analysis of variance (ANOVA). The continuous data were expressed as mean ± SEM followed by post-hoc test for multiple comparisons (Tukey’s HSD or Games–Howell test), using SPSS® 23 Statistics software. Throughout the analysis, parametric variables were log transformed in order to meet the underlying distributional assumptions of the statistical models; otherwise, the corresponding non-parametric test results were used. All statistical tests were significant at the 5% significance level.
Results
Gastroprotective Analysis
The gastroprotective effect of DLBS2411 was measured by the ULI, percentage of ulceration, ulcerative surface area, and gastric acidity. Hematology and histopathology were also evaluated after treatment to gain additional data regarding the gastroprotective effect of DLBS2411 in the rats’ stomachs.
The ULI indicates the level of the lesion caused by ulcer-inducing material, which was absolute ethanol in this study. As shown in Table 2, the ULI score in the treatment groups of the DLBS2411 group (both doses) and Positive control group decreased about by 5.98%, 41.85%, and 76.09%, respectively, compared to the Negative control group.
 |
Table 2 Effects of DLBS2411 and Sucralfate on Ethanol-Induced Gastric Ulcers in Rats |
The percentage of reduction in ulcer surface area in the DLBS2411 treatment group, at both doses, and Positive control group was 75.46%, 75.59%, and 91.19% compared with the Negative control group, respectively. The same result was shown in the percentage of lesion area. The DLBS2411 treatment group (both doses) and Positive control group showed a decrease in the percentage of lesion area, by 70.19%, 71.64%, and 89.05%, respectively.
The percentage of ulcer area depends on the total gastric area and the surface area of ulceration. In this study, the highest percentage of ulcer area was found in the Negative control group. Both doses of DLBS2411 reduced the percentage of ethanol-induced ulcer area in the stomach of rats.
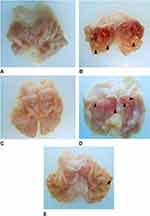
The level of ulcers formed as a result of ethanol induction was also determined from observations of the stomach (Figure 1). Ulcers were divided into three categories, which were ulcer levels I, II, and III, based on the area of the lesion. The number of ulcers in the treatment group DLBS2411 at a dose of 50 mg/kg BW and the Positive control group was less than in the Negative control group. The highest number of level I and level II ulcers was found in the treatment group with the bioactive fraction at a dose of 25 mg/kg BW, while the highest number of level III ulcers was found in the Negative control group (Figure 2).
 |
Figure 2 Classification of ulcer area. |
In addition, from these observations, it was found that the glandular area of stomach in the Negative control group became thinner than in the Normal control group. This was possibly due to the effect of ethanol ingestion. Treatment with DLBS2411 at doses of 25 and 50 mg/kg BW and sucralfate protected the stomach lining from the severe damage caused by ethanol.
Histopathological Analysis
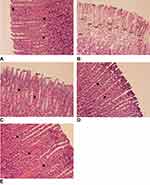
The stomachs of rats were fixed in neutral-buffered formalin, processed in a paraffin tissue processing machine, and sliced at a thickness of 4 µm. Slides of gastric specimens were stained with H&E. The results were examined with a microscope at 20× magnification.
The histopathological results (Figure 3) showed that there were no pathological signs in the Normal control group (Figure 3A), indicating that no necrosis occurred in the Normal control group. The histopathological results of the Negative control group (Figure 3B) showed mild desquamation and severe necrosis. The group treated with sucralfate 100 mg/kg BW (Figure 3C) showed mild desquamation and no necrosis. Similar results were found in the treatment group treated with DLBS2411 at the dose of 25 mg/kg BW (Figure 3D). Histopathological results of the group treated with DLBS2411 at the dose of 50 mg/kg BW showed neither desquamation nor necrosis (Figure 3E). This finding was similar to that in the Negative control group.
Hematological Analysis
Blood tests performed in the hematological examination included the examination of leukocytes (WBCs), erythrocytes (RBCs), hemoglobin (HGB), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), and platelets (PLT). Hematological examinations were performed after the administration of treatments. No significant difference was found among the treatment groups and the Normal control group (Table 3).
 |
Table 3 Hematological Examination After Treatments |
Discussion
Gastric ulcer is caused not only by oversecretion of acid or pepsin, but also by reduced resistance of the stomach lining to gastric juices. Under normal conditions, the gastric mucosa is protected by bicarbonate-rich mucus that is secreted by the goblet cells or mucous cells which cover the entire luminal surface and extend down into the gland. Gastric mucosa damage can be induced by necrotizing agents, such as HCl and ethanol. These substances have been reported to be involved in the depression of gastric defensive mechanisms and stasis of gastric flow, which contribute to the development of hemorrhagic and necrotic lesions.21
Ethanol was reported to facilitate gastric secretion, which may contribute to some degree of ulceration.22,23 Histamine receptor-2 (H2)-mediated gastric secretion is not involved in alcoholic ulcers, since ethanol-induced lesions are not suppressed by cimetidine. Instead, it was proposed that static and radical reactions in the mucosa are the main cascade for the induction of alcoholic mucosa injury.3
Gastric mucus, produced in the pit cells of gastric pits, is considered essential for providing protection to the gastric mucosa. Changes in gastric mucus content have been shown to occur in association with the oral administration of ethanol or aspirin. Sucralfate is a complex formed from sucrose octasulfate and polyaluminum hydroxide that possesses anti-ulcer activity to protect the stomach. The effects of sucralfate on gastric mucus production appear to affect not only the amount of mucus secretion, but also the composition or quality of mucus.24 A previous study showed that DLBS2411 activated nuclear factor-ϰB (NF-ϰB) and induced phosphorylation of IKKα, which could stimulate mucus production and therefore induce gastric protection.25
Another common mechanism behind ethanol-induced gastric ulcer is oxidative stress.26 In our previous research, we conducted a free radical scavenging activity assay of DLBS2411 in vitro using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) method, and measured the reducing power of DLBS2411. The results from both assays showed that DLBS2411 yielded free radical scavenging (antioxidant) activity that was lower than but comparable to ascorbic acid.18 This antioxidant activity may also contribute to the gastroprotective effect yielded by DLBS2411 in this ethanol-induced gastric ulcer animal model.
The results of the current study confirmed our in vitro findings and demonstrated that both DLBS2411 at a dose of 50 mg/kg BW and sucralfate at a dose of 100 mg/kg BW acted as gastroprotective agents and reduced ethanol-induced ulceration in rats. These agents resulted in the protection of the stomach lining from damage caused by ethanol. It is also worth mentioning that the efficacy and safety of DLBS2411 have been investigated in clinical trials for gastric ulcer patients (trial numbers NCT01573403 and NCT02262169).
From the above results, it can be assumed that DLBS2411 could be used in clinical tests for patients with ulcers caused by long-term alcohol consumption. It can be concluded that DLBS2411 plays a gastroprotective role in this ethanol-induced animal model.
Conclusion
The gastroprotective effect of DLBS2411 at a dose of 50 mg/kg BW was more effective than DLBS2411 25 mg/kg BW and caused similar activity to sucralfate 100 mg/kg BW, according to the ULI, percentage of cure ratio, percentage of lesion area, number of ulcerations, and ulcer surface area shown from histopathological examination.
Acknowledgments
The authors would like to thank Irfan A Darfiansyah, James Sinambela, Arfken Andi, Marissa Winata, and Treesye AS Wijaya for their support with the test substance, and Neny Agustianingsih and Dewi Andriani for daily animal care. We also thank Imelda L Winoto and Maggy T Suhartono for reviewing and helpful discussion of the manuscript, and Isabela Anjani, Hery Kristiana, Theresia Monica Ginting, and Destrina Grace Simanjuntak for their help in editing the manuscript.
Disclosure
Dr Raymond Tjandrawinata reports a patent, “Cinnamomum burmanii Extract, Extraction Process and Its Use as Proton Pump Down-regulator, Enzyme Inhibitor, and Mucoprotector", issued. Both authors are employed by Dexa Medica. The authors declare that there was no competing interest in this study and report no other conflicts of interest in this work.
References
1. Zhang Y, Wang H, Mei N, et al. Protective effect of polysaccharide from Dendrobium nobile against ethanol-induced gastric damage in rats. Int J Biol Macromol. 2017;107:230–235. doi:10.1016/j.ijbiomac.2017.08.175
2. de Barros MP, Lemos M, Maistro EL, et al. Evaluation of antiulcer activity of the main phenolic acids found in Brazilian Green Propolis. J Ethnopharmacol. 2008;120:372–377. doi:10.1016/j.jep.2008.09.015
3. Bae DK, Park D, Lee SH, et al. Different antiulcer activities of pantoprazole in stress, alcohol and pylorus ligation induced ulcer models. Lab Anim Res. 2011;27:47–52. doi:10.5625/lar.2011.27.1.47
4. Liu ESL, Cho CH. Relationship between ethanol-induced gastritis and gastric ulcer formation in rats. Digestion. 2000;62:232–239. doi:10.1159/000007821
5. Manaf A, Tjandrawinata RR, Malinda D. Insulin sensitizer in prediabetes: a clinical study with DLBS3233, a combined bioactive fraction of Cinnamomum burmanii and Lagerstroemia speciosa. Drug Des Devel Ther. 2016;10:1279–1289. doi:10.2147/DDDT.S97568
6. Tandrasasmita OM, Wulan DD, Nailufar F, Sinambela J, Tjandrawinata RR. Glucose-lowering effect of DLBS3233 is mediated through phosphorylation of tyrosine and upregulation of PPARγ and GLUT4 expression. Int J Gen Med. 2011;4:345–357. doi:10.2147/IJGM.S16517
7. Nailufar F, Kristiana H, Tjandrawinata RR. Hepatoprotective activity evaluation of DLBS1433 bioactive fraction from Phaleria macrocarpa. Int J Pharma Bio Sci. 2017;8:313–319. doi:10.22376/ijpbs.2017.8.2.p313-319
8. Karsono AH, Tandrasasmita OM, Tjandrawinata RR. Molecular effect of bioactive fraction of Curcuma mangga (DLBS4847) as a downregulator of 5α-reductase activity pathways in prostatic epithelial cells. Cancer Manag Res. 2014;6:267–278. doi:10.2147/CMAR.S61111
9. Berlian G, Tandrasasmita OM, Tjandrawinata RR. Standardized bioactive fraction of Phaleria macrocarpa (Proliverenol) prevents ethanol-induced hepatotoxicity via down-regulation of NF-κB-TNFα-caspase-8 pathway. Asian Pac J Trop Biomed. 2016;6:686–691. doi:10.1016/j.apjtb.2016.06.007
10. Berlian G, Tandrasasmita OM, Suciptan DAS, Tjandrawinata RR. Forhidrol, a bioactive fraction of Phaleria macrocarpa (Scheff.) Boerl., increases reverse cholesterol transport pathway by down-regulation of cholesteryl ester transfer protein activity. J Biol Res. 2018;9(1).
11. Kartolo D, Suparman E, Tjandrawinata RR, Susanto L. Clinical experience with DLBS3233, a combination of Cinnamomum burmannii and Lagerstroemia speciosa, for polycystic ovary syndrome treatment. Asian J Pharm Clin Res. 2016;9:40–43.
12. Tjokroprawiro A, Murtiwi S, Tjandrawinata RR. DLBS3233, a combined bioactive fraction of Cinnamomum burmannii and Lagerstroemia speciosa, in type-2 diabetes mellitus patients inadequately controlled by metformin and other oral antidiabetic agents. J Complement Integr Med. 2016;13:413–420. doi:10.1515/jcim-2016-0031
13. Tan M, Gan D, Wei L, Pan Y, Tang S, Wang H. Isolation and characterization of pigment from Cinnamomum burmannii peel. Food Res Int. 2011;44:2289–2294. doi:10.1016/j.foodres.2010.05.022
14. Al-dhubiab BE. Pharmaceutical applications and phytochemical profile of Cinnamomum burmanii. Pharmacogn Rev. 2012;6:125–131. doi:10.4103/0973-7847.99946
15. Shan B, Cai Y, Brooks JD, Corke H. Antibacterial properties and major bioactive components of Cinnamomum stick (Cinnamomum burmanii): activity against foodborne pathogenic bacteria. J Agric Food Chem. 2007;55:5484–5490. doi:10.1021/jf070424d
16. Prasad KN, Yang B, Dong X, et al. Flavonoid contents and antioxidant activities from Cinnamomum species. Innov Food Sci Emerg Technol. 2009;10:627–632. doi:10.1016/j.ifset.2009.05.009
17. Nailufar F, Tandrasasmita OM, Tjandrawinata RR. DLBS3233 increases glucose uptake by mediating upregulation of PPARγ and PPARδ expression. Biomed Prev Nutr. 2011;1:71–78. doi:10.1016/j.bionut.2010.12.002
18. Tjandrawinata RR, Nailufar F, Arifin PA. Hydrogen potassium adenosine triphosphate activity inhibition and downregulation of its expression by bioactive fraction DLBS2411 from Cinnamomum burmannii in gastric parietal cells. Int J Gen Med. 2013;6:807–815. doi:10.2147/IJGM.S50134
19. Nailufar F, Tjandrawinata RR. The evaluation of proton pump inhibitor bioactive fraction DLBS2411 from Cinnamomum burmannii (Nees & T. Nees) in animal model of gastric ulceration healing. Am J Pharmacol Toxicol. 2017;12:79–88. doi:10.3844/ajptsp.2017.79.88
20. Morimoto Y, Shimohara K, Oshima S, Sukamoto T. Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimetidine. Jpn J Pharmacol. 1991;57(4):495–505. doi:10.1254/jjp.57.495
21. Ishikawa T, Donatini RS, Diaz IEC, Yoshida M, Bacchi EM, Kato ETM. Evaluation of gastroprotective activity of Plinia edulis (Vell.) Sobral (Myrtaceae) leaves in rats. J Ethnopharmacol. 2008;118:527–529. doi:10.1016/j.jep.2008.05.007
22. Chari S, Teyssen S, Singer MV. Alcohol and gastric acid secretion in human. Gut. 1993;34(6):843–847. doi:10.1136/gut.34.6.843
23. Gómez OG, Rodriguez RVG, Corona LQ, et al. Amelioration of ethanol-induced gastric ulcers in rats pretreated with phycobiliproteins of Arthrospira (Spirulina) maxima. Nutrients. 2018;10(6):763. doi:10.3390/nu10060763
24. Huaibao S, Shah PK, Audus KL. Sucralfate effects on mucus synthesis and secretion by human gastric epithelium in vitro. Int J Pharm. 1996;131:159–169. doi:10.1016/0378-5173(95)04270-9
25. Wulandari AS, Tandrasasmita OM, Tjandrawinata RR. Bioactive fraction DLBS2411 from Cinnamomum burmanii (Nees and T. Nees) blume as colon and gastroprotector by stimulating MUC5AC and Cyclooxygenase-2 gene expression. Int J Pharm Pharm Sci. 2016;8(8):202–207.
26. Mei X, Xu D, Xu S, Zheng Y, Xu S. Novel role of Zn(II)–curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem-Biol Interact. 2012;197:31–39. doi:10.1016/j.cbi.2012.03.006
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.