Back to Journals » Therapeutics and Clinical Risk Management » Volume 20
The Potential of Autologous Platelet-Rich Plasma Gel for Diabetic Foot Ulcer Care Among Older Adults: A Systematic Review and Meta-Analysis
Authors Platini H , Adammayanti KA, Maulana S , Putri PMK, Layuk WG, Lele JAJMN , Haroen H, Pratiwi SH, Musthofa F, Mago A
Received 28 October 2023
Accepted for publication 22 January 2024
Published 25 January 2024 Volume 2024:20 Pages 21—37
DOI https://doi.org/10.2147/TCRM.S433033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Hesti Platini,1 Keyzha Amartya Adammayanti,2 Sidik Maulana,3 Putu Moradha Kharisma Putri,2 Welly Grivin Layuk,2 Juan Alessandro Jeremis Maruli Nura Lele,2 Hartiah Haroen,4 Sri Hartati Pratiwi,1 Faizal Musthofa,5,6 Arpit Mago7
1Department of Medical-Surgical Nursing, Faculty of Nursing, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 2Clinical Clerkship Program, Faculty of Medicine, Universitas Kristen Indonesia (UKI), UKI Hospital East Jakarta, Special Capital Region, Indonesia; 3Master of Nursing Program, Faculty of Nursing, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 4Department of Community Health Nursing, Faculty of Nursing, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 5Nursing Internship Program, Faculty of Nursing, Universitas Padjadjaran, Sumedang, West Java, Indonesia; 6West Sapphire Medical-Surgical Ward, Santosa Bandung Central, Bandung, West Java, Indonesia; 7Department of Clinical Medicine, Jawaharlal Nehru Medical College, Belagavi, India
Correspondence: Hesti Platini, Department of Medical-Surgical Nursing, Faculty of Nursing, Universitas Padjadjaran, Bandung, West Java, 40132, Indonesia, Tel +62 822-1671-8363, Email [email protected]
Introduction: Poorly controlled diabetes mellitus can lead to the development of diabetic foot ulcers (DFU), which is a frequent complication in patients. However, several diabetes management guidelines for older adults do not mention the occurrence of DFUs. Nowadays, Autologous Platelet-Rich Gel (APG) is being used for treating diabetic ulcers. APG is an innovative platelet-derived product with many advantages, such as being low-cost, easy to produce, and readily available materials. Additionally, it does not lead to any rejection reaction.
Objective: This study aims to assess the safety and efficacy of APG as a novel treatment of DFU compared with standard treatment in older adult patients.
Methods: Randomized Controlled Trials (RCTs) were searched using PubMed, Cochrane, Google Scholar, Wiley, and PlosOne. The keywords have been arranged using the Boolean operator, including autologous platelet-rich gel, DFU, and elderly. The data was screened by inclusion and exclusion criteria. The final inclusion study was analyzed and synthesized by tabulation, clusterization, contextual and thematic approach, and assessed for risk of bias using ROB 2.0. Meta-analysis was conducted by using Review Manager 5.4 and the Mantel Haenszel method.
Results: Eight RCTs with 598 patients were eligible for the present analysis. Compared with standard care/conventional treatment, APG could significantly improve the healing wound in patients with diabetic foot ulcers (Relative risk (RR) 1.32, 95% confidence interval (CI) 1.22– 1.57, p < 0.0001), shortened the healing time (Mean difference [MD] − 16.97 days (95% CI: − 32.64 to − 1.29; p < 0.00001), shortened the length of hospital stay (MD= − 20.11, 95% CI: − 38.02, − 2.20; p = 0.03), and amputation rate (MD= 0.36, 95% CI: 0.16, 0.84; p = 0.02).
Conclusion: APG treatment can better treat DFU in terms of duration of healing, wound healing, length of hospital stay, and amputation prevention than the standard treatment.
Keywords: autologous platelet-rich plasma gel, diabetic foot ulcers, older adult
Introduction
The prevalence of Diabetes Mellitus increases significantly with age.1,2 Most diagnosed cases lie between the fourth and seventh decades of life.3 Recent statistics indicate that over 326 million working-age individuals are affected by DM, distinct from the 122.8 million individuals aged 65 years and above.3 These figures are expected to increase to 438.2 million and 253.4 million in the coming decades.3 One of the most frequent complications experienced by individuals with poorly managed DM is DFU. This condition often arises due to inadequate glycemic control, underlying neuropathy, peripheral vascular disease, or insufficient foot care.4 Additional factors contributing to the risk of DFU include vision impairment, irregular gait patterns, decreased mobility, and other medical conditions. Furthermore, the likelihood of significant amputations rises as individuals grow older in conjunction with the greater occurrence of these contributing factors. Diabetic feet are rarely mentioned in some guidelines for diabetes management in older adults.5,6 As people age, neuropathy, foot abnormalities, and peripheral arterial disease (PAD) increase, leading to an elevated risk of amputation, including those without diabetes. The management of foot ulcers necessitates a customized approach that considers the patient’s coexisting medical conditions and functional state and involves pressure reduction (off-loading), clearance of damaged tissue, treatment of infections and reduced blood flow (ischemia).7
In chronic wound healing, each stage presents distinct constraints. Hemostasis is often impeded by poor blood flow in diabetics; prolonged inflammation characterizes the inflammation stage, hindering healing; during proliferation, inadequate tissue formation and angiogenesis occur due to diminished cellular response; and in maturation, an imbalance in collagen synthesis affects scar strength.8 These constraints underscore the challenges faced in treating chronic wounds, particularly in patients with underlying conditions like diabetes.8 Conventional treatments, which typically include wound debridement, offloading, infection control, and the use of standard dressings, often fail to offer effective relief for diabetic refractory ulcers. Current research has revealed that refractory ulcer pathophysiology involves imbalances in the microenvironment, including low levels of growth factors and bioactive substances, which significantly impede healing. To address this deficiency, emerging cellular therapies and biological products have been developed and are highly valued by medical professionals. Platelet-derived products, in particular, have been in use since 1986. In the first clinical study conducted by Knighton et al, it was found that locally applying platelet-derived wound healing factors (PDWHFs) led to the healing of chronic refractory ulcers.9
In this century, APG has emerged as a more modern platelet-derived product used for the clinical management of diabetic ulcers. Saldalamacchia et al conducted the first controlled trial assessing APG’s safety and efficacy in treating DFU. Their findings provided crucial evidence for the importance of topical APG application in treating diabetic skin ulcers.10 APG is an economical and safe source of growth factors with no known immunological risks. Due to its alleged ability to expedite the healing process, APG has gained widespread usage in the wound repair field.11,12 Being the second generation of platelet-derived preparations, APG offers several added benefits, including readily available materials, ease of product, affordability, and no risk of rejection reaction. An increasing amount of research indicates that APG is superior to standard care or conventional treatment for chronic wound management.13,14 APG is gaining global attention as conventional treatments yield inadequate results, and DFU remain prevalent in the aging population. This study aims to assess the effectiveness of APG treatment in contrast to current conventional methods for treating DFU.
To our knowledge, no meta-analysis specifically addresses the treatment of APG in the older adult population with DFU. We analyzed the efficacy of APG in each of Wagner’s classifications as a tool for treating DFU to demonstrate the clinical effect of APG. We performed a recent meta-analysis over the last ten years to assess the safety and efficacy of APG as a novel treatment of DFU compared with standard treatment. Therefore, we systematically reviewed APG’s clinical efficacy and safety as a breakthrough therapy in older adult patients with DFU.
Methods
Study Design and Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement was used to conduct a systematic review.15 Randomized controlled trials (RCTs) were retrieved from PubMed, Cochrane, Google Scholar, Wiley, and PlosOne medical databases. We conducted a search from 2013–2021 using the following Boolean Operator-organized keywords: (autologous platelet-rich gel) AND (diabetic foot ulcer) [See Supplementary File 1]. The search results were downloaded to a personal database, filtered, extracted, analyzed, and synthesized for qualitative and quantitative data. Following the PRISMA flowchart, the data collection procedure for this study was carried out. This included identifying relevant studies in the databases, screening for duplicates, titles, and abstracts, evaluating the complete eligibility text, and extracting and analyzing the included studies.
Eligibility Criteria
The study was screened by inclusion and exclusion criteria. The inclusion criteria following the PICOS framework, include:
Population (P): Patients with DFU between the ages of roughly 50 years and under Wagner grade 1–4
Intervention (I): APG as a topical application
Comparison (C): Placebo or other biomaterials (These biomaterials include, but are not limited to, hydrocolloid dressings, alginate dressings, collagen-based dressings, and foam dressings)
Outcome (O): the complete healing of the wound
Study (S)= Randomized controlled trial.
The study also evaluated several secondary outcomes, including the length of hospital stay, healing time, reduction in wound size, and any adverse effects. These adverse effects were identified as advanced infections, prickling sensations, sensations of formication, and amputation In the context of this study, “formication” is specifically defined as an itchy sensation akin to ants crawling on the skin, a symptom occasionally reported in diabetic foot ulcers. The term “prickling sensation” was used to describe a sharp, piercing pain experienced at the ulcer sites. Regarding infections, they were characterized by the presence of microbial cultures within the wound following treatment with APG. Additionally, for the purposes of this study, “older adult” refers to patients aged around 65 years and older, aligning with the commonly used definition in the literature. The exclusion criteria were case series, case reports, retrospective studies, animal studies, technical studies, and reviews without a peer review process. Furthermore, the EndNote X9 software (Clarivate, Philadelphia, PA, USA) was utilized to remove duplicate studies. Subsequently, three independent reviewers screened the titles and abstracts of the studies based on accessibility criteria. Any discrepancies between the reviewers were resolved through discussion to reach a consensus.
Data Extraction
The information was manually gathered and evaluated from the studies that met the inclusion criteria and recorded on the extraction spreadsheet. The data recorded in the extraction included the author, research design, sample size, country, and the feasibility and effectiveness of Autologous Platelet-Rich Gel. Other collected and analyzed outcomes included the duration of healing, healing time, and any negative results. Both qualitative and quantitative methods were employed to analyze the study.
Statistical Analysis
Statistical analysis was conducted using Review Manager version 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). The clinical outcome was evaluated using a 95% confidence interval (CI) with continuous data (Mean Differences) and dichotomous data (Risk Ratio). The p-values < 0.05 were deemed significant. The primary results utilized in the statistical analysis were the mean difference between APG and standard care, which was demonstrated by a reduction in time, surface area, and hospital stay duration. Also shown is the risk ratio for wound healing and infection. The efficacy of APG on all outcomes was evaluated by displaying the mean difference, risk ratio, 95% confidence interval, and p-value in a forest plot. Riley et al suggested using an inverse variance and DerSimonian-Laird random-effects model to analyze potential heterogeneity outside the study. In addition, we assessed the heterogeneity using the estimated effect statistics (I2) based on the Cochrane threshold, with a threshold of 0% indicating insignificance, 25% indicating low heterogeneity, 50% indicating moderate heterogeneity, and 75% indicating high heterogeneity.16 According to Von Hippel, I2 can be significant when there are few investigations. As suggested by Duval and Tweedie, we also conducted a sensitivity analysis using trim and fill.17 When substantial heterogeneity existed, a sensitivity analysis was conducted using the Jamovi 2.2.5 software. In addition, we conducted a systematic review to analyze and synthesize the data using a qualitative approach that included tabulation, clustering, thematic analysis, and contextual descriptions, as shown in the illustrative study.
Risk of Bias Assessment
Final inclusion studies were evaluated for risk of bias using the Revised Tool for Risk of Bias in Randomized Trials (RoB 2.0), which consists of five domains for initiative studies. The author assessed the risk of bias according to the algorithm provided by Cochrane. The results were inputted into the domain file for bias (.xlsx). This file was then used on the ROBVIS website to visualize the resulting data properly.
Results
Study Selection
A comprehensive search across multiple databases resulted in the identification of a substantial number of studies. Specifically, the initial search yielded 1165 records from various sources, including PubMed (n = 33), Cochrane (n = 360), Google Scholar (n = 552), Wiley (n = 33), and PlosOne (n = 187). From these, 765 records were removed due to duplication, leaving 400 records for screening. Upon further evaluation, 30 reports were sought for retrieval. However, only 20 of these reports were assessed for eligibility after excluding 10 reports that could not be retrieved. The reasons for exclusion at this stage included irretrievable full-text (n = 2), observational study design (n = 2), clinical trial registry (n = 1), incomplete data (n = 1), and non-English language (n = 1). Ultimately, Eight studies met the inclusion criteria and were included in the analysis (see Figure 1).18–25
Characteristics of Included Study
The included investigations were carried out between 2013 and 2021. This study included 8 randomized controlled trials.18–25 With sample sizes ranging from 48 to 129 participants, enrolling 598 patients in the intervention (n=434) and control (n=434) groups. The distribution of ages was comparable between the APG and the control group. According to Wagner, the studies included in the analysis had DF lesions from grades 1 to 5. The characteristics of these investigations are summarized in Table 1.
 |
Table 1 Characteristics of Included Studies |
Risk of Bias
The Cochrane Risk of Bias Tool for Randomized Controlled Trials 2.0 (Cochrane RoB Tool 2.0) was utilized to evaluate the risk of bias based on seven distinct factors. According to Xie et al24 excluding two studies, attrition bias was minimal. Serra R. et al23 reported a high level of bias due to the fact that some patients in the control group withdrew from the study before its conclusion. In contrast, the study by Serra R et al.23 After collecting sufficient information on the nature of the treatment, all patients were administered care. All included data originated from low-risk studies, so reporting bias was low. No additional bias was mentioned in the included studies. Consequently, the included studies demonstrated that the dangers were uncertain. Figure 2 depicts individual assessment, while Figure 3 provides a summary.
 |
Figure 2 Traffic light plot’s risk of bias.18–25 |
 |
Figure 3 Summary risk of bias. |
The Effect of Autologous Platelet-Rich Gel in Healing Wounds Among Older Adult with Diabetic Foot Ulcer Patients
DFU, a common complication among diabetic patients, particularly in the older adult, were the focus of our subgroup analysis regarding wound repair. The studies we included exclusively involved DFU patients. Notably, based on the Wagner classification of diabetic foot, one study provided specific data on DFU repair. This study employed a fix effects model due to the significant heterogeneity among studies (Chi2 = 7.78, p = 0.25, I2 = 23%), revealed that APG significantly enhances 1.38 time higher the healing rate in patients with DFU compared to conventional treatments (Relative risk (RR) 1.32, 95% confidence interval (CI) 1.22–1.57, p < 0.0001). When focusing on specific grades of lesions, APG is recommended for grade 2 (RR=2.12, 95% CI: 1.01–0.44; p = 0.05) and grade 3 (RR=1.36, 95% CI: 0.70–2.64; p = 0.37) lesions. However, it’s important to note that the results for these specific grades were not statistically significant, as illustrated in Figure 4 of this study. Additionally, the funnel plot analysis, which assesses the risk of bias and heterogeneity, showed a generally uniform distribution of studies. However, one study emerged as an outlier, presenting either a significantly larger effect size or a smaller standard error compared to the other studies included in the meta-analysis (see Figure 5). This outlier warrants careful consideration as it may impact the overall interpretation of this findings.
 |
Figure 4 Forest plot of the effect of APG on wound healing.18,20–25 |
 |
Figure 5 Funnel plot of the effect of APG on wound healing. |
The Effect of Autologous Platelet-Rich Gel in Healing Duration Among Older Adult with Diabetic Foot Ulcer Patients
The included studies on healing duration were divided into two sub-group, including healing duration per days and weeks. APG treatment statistically significant reduces −16.97 days healing duration (95% CI: −32.64 to −1.29; p < 0.00001). However, no statistically significant reduce the healing duration (MD= −5.60 weeks, 95% CI: −18.92, 7.72, p = 0.41). This significant reduction per day indicates that APG treatment is more effective in speeding up the healing process of DFU compared to standard treatment, as illustrated in Figure 6. Despite these promising results, the study revealed considerable heterogeneity (tau=112.69, I2 = 93%, p = < 0.00001), as shown in Figure 7. The heterogeneity suggests variability in the outcome across different studies.
 |
Figure 6 Forest plot of the effect of APG on healing duration.18,19,25 |
 |
Figure 7 Funnel plot of the effect of APG on healing duration. |
The Effect of Autologous Platelet-Rich Gel in Infection Among Older Adult with Diabetic Foot Ulcer Patients
This study comparing the incidence of infection in DFU patients treated with standard care versus APG, significant variations were observed over different time intervals. In the early stages, after one week (RR=0.56, 95% CI 0.34 to 0.91; p = 0.02) and two weeks (RR=0.13, 95% CI 0.02 to 0.04; p = 0.01), standard care was associated with a higher incidence of infection compared to APG. This suggests that APG may be more effective in preventing infection during the initial weeks of treatment. However, as the treatment progressed, the difference in infection rates between standard care and APG became less pronounced. By the fourth week (RR=0.20, 95% CI 0.02 to 1.63; p = 0.13), eighth week (RR=1.00, 95% CI 0.07 to 15.21; p = 1.00), and twelfth week (RR=0.52, 95% CI 0.19 to 1.38; p = 0.19), the incidence of infection in patients receiving standard care was not significantly higher than in those treated with APG. Overall, the total results indicate that standard care does not significantly cause more infections compared to APG (RR=0.41, 95% CI 0.26 to 0.64; p = 0.41), suggesting that APG may be superior to conventional care in preventing infection in DFU patients, as shown in Figure 8.
 |
Figure 8 Forest plot of the effect APG on infection.18,24,25 |
 |
Figure 9 Funnel plot of the effect APG on infection. |
Furthermore, the distribution of studies within the funnel plot (Figure 9) was uniform, indicating comparability in precision and effect magnitude across studies. This uniformity suggests minimal or non-existent publication bias, thereby enhancing the reliability of the meta-analysis results. Additionally, a homogeneous funnel plot implies that the aggregate estimate of the treatment effect is likely reliable, further affirming the effectiveness of APG treatments in the context of DFU.
The Effect of Autologous Platelet-Rich Gel in Surface Area Reduction Among Older Adult with Diabetic Foot Ulcer Patients
There is lack of evidence that APG was recommended for surface area reduction. Study conducted by Rainys et al22 showed that APG was not statistically significant in reducing the surface area of DFU.
The Effect of Autologous Platelet-Rich Gel in Length Hospital Stay Among Older Adult with Diabetic Foot Ulcer Patients
Figure 10 demonstrates that APG treatment may abbreviate hospital stays and reduce hospitalization costs compared to conventional treatment (MD= −20.11, 95% CI: −38.02, −2.20; p = 0.03). The distribution of studies in the funnel plot may appear no statistically heterogeneous (see Figure 11).
 |
Figure 10 Forest plot of the effect APG on length hospital stay.24,25 |
 |
Figure 11 Funnel plot of the effect APG on length hospital stay. |
The Effect of Autologous Platelet-Rich Gel in Amputation Rate Among Older Adult with Diabetic Foot Ulcer Patients
Figure 12 demonstrates that Conventional treatment may potential increase risk of amputation rate compared to APG treatment (MD= 0.36, 95% CI: 0.16, 0.84; p = 0.02). There is no statistically significant heterogeneity in this outcome (I2= 0%, p= 0.63) and the distribution of studies in the funnel plot may appear no statistically heterogeneous, see Figure 13.
 |
Figure 12 Forest plot of the effect APG on amputation rate.19,21,25 |
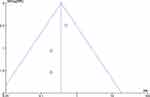 |
Figure 13 Funnel plot of the effect APF on amputation rate. |
Discussion
It is also probable that the incidence of DFUs in patients aged 45 to 64 will increase. Moreover, DFUs can result in unemployment, disability, and even mortality among middle-aged working adults, contributing to increased family, social, and healthcare burdens.26,27 Lower Extremity Amputation (LEA) is the most severe and feared complication of DFU, with diabetes continuing to be the leading cause of LEA worldwide. The prevalence of foot ulcers accounts for 85% of all cases of diabetic lower extremity amputation (LEA).28,29 Approximately two-thirds of all amputations are estimated to occur in patients aged 60 and older.30
The DFU treatment protocol, as outlined in this study, primarily includes proper wound dressing, early treatment of infections, and decompression techniques. “Decompression” in this context refers to off-loading or pressure relief methods, which are crucial for healing. The protocol, encompassing these key components, has demonstrated approximately a 60% success rate in healing DFUs within one year.18–20,23–25 Many chronic ulcers do not heal despite ongoing treatment and may persist for extended periods. In contrast, others may reappear after successful healing and require advanced wound care treatments to heal correctly. Approximately 40% of DFU cases returned after 31 months of follow-up, and 12.3% of cases remained unhealed after the follow-up period. In addition, there is evidence that at 3-year recurrence and amputation rate is between 10 and 20%.13,31–33 Currently, both APG and DFU are being considered for use in cancer treatment.
The APG is a plasma-based solution that contains a high concentration of platelets and numerous growth factors secreted by these platelets. APG, readily available and prepared as a secondary agent derived from platelets, has been established as an effective adjunctive treatment for chronic and acute lesions. This therapy, composed predominantly of platelets, leukocytes, fibrin, growth factors, and cytokines, can combat infection34 and modulate an immune response.35 There is no increased risk of adverse events associated with the use of APG treatment, which has the potential to reduce hospital stay duration and some hospitalization expenses.
Through this study, the efficacy of APG on the impact of DFU in older adult patients has been evaluated for the first time. We conducted a meta-analysis of healing time, wound healing, and infection. Regarding healing duration, wound healing, and infection, this study indicates that APG treatment is preferable for treating DFU. The risk of bias was low, except for two studies. Some studies reported substantial bias because, in the final analyses, some patients in the control group withdrew from the study, and, in other studies, all patients were treated after obtaining adequate information on the type of treatment. All data included in the trials posed a low risk of bias, resulting in minimal reporting bias. In addition, no other forms of bias were addressed in any of the included studies. Consequently, the included studies had hazy risks or raised some concerns.
According to the results of this study, healing time with APG is faster than with conventional treatment with MD −11.32. This finding aligns with a previous meta-analysis conducted by Li Y et al that APG substantially accelerated the healing of chronic DFU compared to standard care with MD −9.18.36 In addition, a meta-analysis by Ding H. et al determined that wounds recover within four weeks (p < 0.001).37 Patients with DFU who received APG had faster wound healing than those who received standard care. In a similar study, Knighton et al found that patients treated with APG experienced complete wound healing in an average of 10.6 weeks.9 According to the data, the results of the wound recovery rate study by Ding et al show a similar trend. 85.8% of the cohort received APG treatment on average, ranging from 68.4% to 100%. In contrast, the mean percentage for the control group ranged from 18.2% to 75.0%.37
Regarding the incidence of infection in DFU patients, the results of this study indicate that AGP is more optimal for infection prevention than conventional treatment. This is comparable to the results obtained by Sun SY et al. Antibiotics are ineffective at curing wounds, but APG, combined with negative pressure therapy, eradicates bacterial infections and heals wounds.38 Based on this study's findings, APG treatment may reduce hospital costs and abbreviate hospital stays for DFU. Li Y and colleagues conducted an analogous investigation. The duration of hospitalization was significantly shorter for patients in the APG group compared to those in the control group.36
Diabetic ulcers result from an imbalance in the metalloproteinases (MMPs) and MMP inhibitors, exacerbated by oxygen and nutrient deprivation of lesion tissue due to diabetic neuropathy and vascular disease. The inability of epithelial cells to produce healing agents such as platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) is hindered in the absence of oxygen and essential nutrients. These factors diminish normal wound healing response.18,39,40 APG is a biotechnology offered as a supplement for treating diabetic ulceration. APGs are commonly used to promote wound healing in surgical procedures. By containing cytokines, chemokines, growth factors, and fibrin scaffolds, advanced wound dressings such as APGs are believed to stimulate natural healing responses. APGs contain platelet alpha granules, which contain growth factors such as PDGF, VEGF, and transforming growth factor (TGF beta 3), thereby promoting cell differentiation and proliferation and facilitating the formation of new cells. These growth factors can also stimulate angiogenesis and nourish ischemic cells.18,39,40 APGs are believed to serve as a wound-site defense mechanism by releasing signals that attract macrophages. In addition, a limited number of white blood cells are present in APG.41
In exploring the future of APG for DFU care, particularly among older adults, emerging combinations with other regenerative technologies present significant promise. The integration of APG with biological scaffolds offers a novel approach, providing a structural matrix to enhance cellular adhesion and proliferation, thereby potentially improving wound healing outcomes.42–44 Furthermore, the synergy between APG and extracellular vesicles opens up avenues for enhanced therapeutic efficacy; these vesicles can act as carriers of bioactive molecules, amplifying the regenerative properties of APG.43 Additionally, The integration of APG with stem cell therapy, particularly using Mesenchymal Stem Cells (MSCs), represents a promising avenue in DFU treatment. MSCs offer a versatile approach due to their ability to differentiate into a variety of cell types such as osteoblasts, cartilage cells, and nerve cells, making them ideal for tissue repair and regeneration.45 The therapeutic potential of MSCs in treating diabetic lower limb ischemia, ulcers, and neuropathy has been demonstrated in both animal models and clinical research.45 These approach could revolutionize the treatment of DFU by providing a robust cellular foundation for regeneration, particularly in cases where traditional therapies are less effective. These advanced applications of APG not only promise enhanced efficacy in treatment but also pave the way for innovative strategies in managing DFU in older adults.
The study conducted by Meamar et al46 examined the effectiveness of human placenta-derived mesenchymal stem cells (hPDMSCs) incorporated into gelatin nanofiber, with and without added APG, for the healing of diabetic foot ulcers (DFUs). The study showed the expression of mesenchymal markers via flow cytometry and a significant increase in the proliferation of hPDMSCs on electrospun gelatin nanofibers (GNS) scaffolds. There was a significant difference in wound size reduction of 71% in the Group with hPDMSCs after ulcer resurfacing with APG gel, and 36% in the usual care Group. In addition, tissue biopsy showed the formation of new capillaries in the Group with hPDMSCs coated with APG.
Implication for Practice
This study demonstrates that APG, as an additional therapy for DFU, can provide significant clinical benefits, especially in the area of wound repair, thus corroborating previous research findings. This study has made significant progress by evaluating the safety and efficacy of APG as a new approach to treating DFU compared to the standard treatment. Therefore, we comprehensively evaluated APG’s clinical safety and efficacy as an innovative treatment for DFU in older adult patients. Based on this study finding, APG substantially decreased the time required for DFUs to recover. This study evaluates the efficacy of APG in accelerating wound healing, an aspect previously neglected by other studies.20,22 No matter the patient’s age, the results of this study indicate that the administration of APG can substantially reduce the time required for DFU healing.
The findings of this study not only have significant implications for practice in the multidisciplinary management of DFU but also carry important considerations for policy and clinical decision-making. Nurses and other health professionals are crucial in the effective treatment and management of DFU, providing direct patient care, administering treatments like APG, and monitoring wound progress. Their role in patient education about wound care, blood glucose management, and lifestyle modifications is vital in preventing complications and promoting healing.
From a policy perspective, this study highlight the need for healthcare systems to prioritize comprehensive DFU management strategies. This includes funding and support for ongoing training of healthcare professionals in the latest DFU treatment modalities, as well as patient education programs. Policies that facilitate interdisciplinary collaboration among podiatrists, diabetes educators, dietitians, and primary care providers can significantly enhance patient outcomes. For clinical decision-makers, incorporating the findings into practice guidelines can guide healthcare professionals in optimizing treatment strategies. Decision-makers should consider evidence-based approaches like APG in DFU treatment protocols, acknowledging its efficacy in improving healing rates. Additionally, emphasizing the importance of patient education and adherence to treatment regimens in clinical guidelines can lead to more effective management and reduced recurrence of DFU.
However, this study possesses several limitations. First, the included trials had a high or uncertain risk of bias, which may have contributed to the underpowered studies. The results of this study may also have been affected by confounding variables such as the severity of DFU and the patient’s comorbidities. The APG dose administered to each patient was not standardized. Future research should include high-quality clinical trials with exhaustive data and standardized APG dosages. Moreover, we also acknowledge a notable limitation in our data: the absence of certain critical clinical outcomes such as minor amputation rate, major amputation rate, overall mortality rate, and cardiovascular mortality rate. While we were able to incorporate the total amputation rate into our analysis, the unavailability of detailed data on these other outcomes in the included studies prevented a more comprehensive evaluation of the clinical impact of treatments for DFU. This gap highlights a significant area for future research. We recommend that subsequent studies in this field should aim to systematically collect and analyze data on both minor and major amputation rates, as well as mortality rates associated with DFU. Such data are essential for understanding the broader implications of DFU treatments and can significantly contribute to the development of more effective treatment strategies. Moreover, understanding the impact of DFU treatments on mortality, particularly cardiovascular mortality, is crucial given the high risk of cardiovascular complications in diabetic patients.
Conclusions
This study provides compelling evidence that APG is a highly effective treatment for DFU, offering multiple benefits. Notably, APG significantly reduces the duration of healing, length of hospital stays, amputation rate, and improves wound healing demonstrating its effectiveness in managing DFUs. Furthermore, APG exhibits distinct antimicrobial properties, effectively converting positive bacterial cultures to negative, highlighting its unique role in infection control. Importantly, its use is safe, showing no substantial alterations in patients’ blood chemistry or hematology, including albumin levels.
APG’s efficacy is particularly evident in treating Wagner Grade 1 DF lesions and, while not statistically significant, its benefits extend to Wagner Grades 2 and 3 lesions. Given its proven advantages in wound healing and safety profile, the adoption of APG in treating DFU in older adult patients is highly recommendable. This approach not only enhances treatment outcomes but also aligns with the growing need for more effective, patient-friendly interventions in managing chronic diabetic complications.
Data Sharing Statement
More data is available from the author. Please get in touch with the corresponding author for more data.
Acknowledgment
The Research and Community Engagement Director of Universitas Padjadjaran, Bandung, Indonesia, funded the Article Processing Charge (APC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. NCD Risk Factor Collaboration (NCD-RisC). Worldwide Trends in Diabetes since 1980: a Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet. 2016;387(10027):1513–1530. doi:10.1016/S0140-6736(16)00618-8
2. Moradi-Lakeh M, Forouzanfar MH, El Bcheraoui C, et al. High Fasting Plasma Glucose, Diabetes, and Its Risk Factors in the Eastern Mediterranean Region, 1990-2013: findings From the Global Burden of Disease Study 2013. Diabetes Care. 2017;40(1):22–29. doi:10.2337/dc16-1075
3. Ogurtsova K, da Rocha Fernandes JD, Huang Y, et al. IDF Diabetes Atlas: global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabet Res Clin Pract. 2017;128:40–50. doi:10.1016/j.diabres.2017.03.024
4. Oliver TI, Mutluoglu M. Diabetic Foot Ulcer. StatPearls Publishing; 2023.
5. American Diabetes Association. 11. Older Adults: standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S119–S125. doi:10.2337/dc18-S011
6. Sinclair A, Morley JE, Rodriguez-Mañas L, et al. Diabetes Mellitus in Older People: position Statement on Behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012;13(6):497–502. doi:10.1016/j.jamda.2012.04.012
7. Pataky Z, Vischer U. Diabetic Foot Disease in the Elderly. Diabetes Metab. 2007;33(Suppl 1):S56–65. doi:10.1016/s1262-3636(07)80057-7
8. Han G, Ceilley R. Chronic Wound Healing: a Review of Current Management and Treatments. Adv Ther. 2017;34(3):599–610. doi:10.1007/s12325-017-0478-y
9. Knighton DR, Ciresi KF, Fiegel VD, Austin LL, Butler EL. Classification and Treatment of Chronic Nonhealing Wounds. Successful Treatment with Autologous Platelet-Derived Wound Healing Factors (PDWHF). Ann Surg. 1986;204(3):322–330. doi:10.1097/00000658-198609000-00011
10. Saldalamacchia G, Lapice E, Cuomo V, et al. A Controlled Study of the Use of Autologous Platelet Gel for the Treatment of Diabetic Foot Ulcers. NMCD. 2004;395–396. doi:10.1016/s0939-4753(04)80029-2
11. Ding S-L, Ji L-F, Zhang M-Z, et al. Safety and Efficacy of Intra-Articular Injection of Platelet-Rich Plasma for the Treatment of Ankle Osteoarthritis: a Systematic Review and Meta-Analysis. Int Orthop. 2023;47(8):1963–1974. doi:10.1007/s00264-023-05773-2
12. Suthar M, Gupta S, Bukhari S, Ponemone V. Treatment of Chronic Non-Healing Ulcers Using Autologous Platelet Rich Plasma: a Case Series. J Biomed Sci. 2017;24(1):16. doi:10.1186/s12929-017-0324-1
13. Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, et al. Autologous Platelet-Rich Plasma for Treating Chronic Wounds. Cochrane Database Syst Rev. 2016;2016(5):CD006899. doi:10.1002/14651858.CD006899.pub3
14. Fletcher J. Differences between Acute and Chronic Wounds and the Role of Wound Bed Preparation. Nurs Stand. 2008;22(24):62,64–68. doi:10.7748/ns2008.02.22.24.62.c6412
15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372. doi:10.1136/bmj.n71
16. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ. 2003;327(7414):557–560. doi:10.1136/BMJ.327.7414.557
17. Duval S, Tweedie R. Trim and Fill: a Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics. 2000;56(2):455–463. doi:10.1111/j.0006-341x.2000.00455.x
18. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet-Rich Plasma for the Treatment of Clean Diabetic Foot Ulcers. Ann Vasc Surg. 2016;38:206–211. doi:10.1016/j.avsg.2016.04.023
19. Alamdari NM, Shafiee A, Mirmohseni A, Besharat S. Diabetes & Metabolic Syndrome: clinical Research & Reviews Evaluation of the Ef Fi Cacy of Platelet-Rich Plasma on Healing of Clean Diabetic Foot Ulcers: a Randomized Clinical Trial in Tehran, Iran. Diabetes Metab Syndrome. 2021. doi:10.1016/j.dsx.2021.03.005
20. Goda AA, Metwally M, Ewada A, Ewees H. Platelet-Rich Plasma for the Treatment of Diabetic Foot Ulcer: a Randomized, Double-Blind Study. Egypt J Surg. 2018;37(2):56.
21. Gude W, Hagan D, Abood F, Clausen P. Aurix Gel Is an Effective Intervention for Chronic Diabetic Foot Ulcers: a Pragmatic Randomized Controlled Trial. Adv Skin Wound Care. 2019;32(9):416–426. doi:10.1097/01.ASW.0000577140.19174.9e
22. Rainys D, Surgeon P, Cepas A, et al. Effectiveness of Autologous Platelet-Rich Plasma Gel in the Treatment of Hard-to- Heal Leg Ulcers: a Randomised Control Trial. Diabetes Metab Syndrome. 2019;28(10).
23. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of Platelet-Rich Gel to Enhance Healing of Transmetatarsal Amputations in Diabetic Dysvascular Patients. Int Wound J. 2013;10(5):612–615. doi:10.1111/iwj.12052
24. Xie J, Fang Y, Zhao Y. ScienceDirect Autologous Platelet-Rich Gel for the Treatment of Diabetic Sinus Tract Wounds: a Clinical Study. J Surg Res. 2019;9:1–9. doi:10.1016/j.jss.2019.09.069
25. Li L, Chen D, Wang C, et al. Autologous Platelet-Rich Gel for Treatment of Diabetic Chronic Refractory Cutaneous Ulcers: a Prospective, Randomized Clinical Trial. Wound Repair Regen off Publ Wound Heal Soc. 2015;23(4):495–505. doi:10.1111/wrr.12294
26. Pan J, Jia W. Early-Onset Diabetes: an Epidemic in China. Front Med. 2018;12(6):624–633. doi:10.1007/s11684-018-0669-1
27. Kasiya MM, Mang’anda GD, Heyes S, et al. The Challenge of Diabetic Foot Care: review of the Literature and Experience at Queen Elizabeth Central Hospital in Blantyre, Malawi. Malawi Med J. 2017;29(2):218–223. doi:10.4314/mmj.v29i2.26
28. Pecoraro RE, Reiber GE, Burgess EM. Pathways to Diabetic Limb Amputation. Basis for Prevention. Diabetes Care. 1990;13(5):513–521. doi:10.2337/diacare.13.5.513
29. Boulton AJM. The Diabetic Foot: from Art to Science. The 18th Camillo Golgi Lecture. Diabetologia. 2004;47(8):1343–1353. doi:10.1007/s00125-004-1463-y
30. Unwin N. Epidemiology of Lower Extremity Amputation in Centres in Europe, North America and East Asia. Br J Surg. 2000;87(3):328–337. doi:10.1046/j.1365-2168.2000.01344.x
31. Winkley K, Stahl D, Chalder T, Edmonds ME, Ismail K. Risk Factors Associated with Adverse Outcomes in a Population-Based Prospective Cohort Study of People with Their First Diabetic Foot Ulcer. J Diabetes Complications. 2007;21(6):341–349. doi:10.1016/j.jdiacomp.2007.09.004
32. Dubský M, Jirkovská A, Bem R, et al. Risk Factors for Recurrence of Diabetic Foot Ulcers: prospective Follow-up Analysis in the Eurodiale Subgroup. Int Wound J. 2013;10(5):555–561. doi:10.1111/j.1742-481X.2012.01022.x
33. Moulik PK, Mtonga R, Gill GV. Amputation and Mortality in New-Onset Diabetic Foot Ulcers Stratified by Etiology. Diabetes Care. 2003;26(2):491–494. doi:10.2337/diacare.26.2.491
34. Zundert V, Moojen DJF, Everts PAM, Schure R, Overdevest EP, Knape JTA. Antimicrobial Activity of Platelet-Leukocyte Gel against Staphylococcus Aureus. Diabetes Metab Syndrome. 2008:404–410. doi:10.1002/jor.20519
35. Diss A, Dohan DM, Mouhyi J, Mahler P. Osteotome Sinus Floor Elevation Using Choukroun’s Platelet-Rich Fibrin as Grafting Material: a 1-Year Prospective Pilot Study with Microthreaded Implants. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod. 2008;105(5):572–579. doi:10.1016/j.tripleo.2007.08.021
36. Li Y, Gao Y, Gao Y, et al. Autologous Platelet-Rich Gel Treatment for Diabetic Chronic Cutaneous Ulcers: a Meta-Analysis of Randomized Controlled Trials. J Diabetes. 2019;11(5):359–369. doi:10.1111/1753-0407.12850
37. Ding H, Fu X-L, Miao -W-W, Mao X-C, Zhan M-Q, Chen H-L. Efficacy of Autologous Platelet-Rich Gel for Diabetic Foot Wound Healing: a Meta-Analysis of 15 Randomized Controlled Trials. Adv Wound Care. 2019;8(5):195–207. doi:10.1089/wound.2018.0861
38. Sun S, Wang C, Chen D, et al. Combating Superbug Without Antibiotic on a Postamputation Wound in a Patient with Diabetic Foot. Int J Low Extrem Wounds. 2016;15(1):74–77. doi:10.1177/1534734615595736
39. OuYang H, Tang Y, Yang F, et al. Platelet-Rich Plasma for the Treatment of Diabetic Foot Ulcer: a Systematic Review. Front Endocrinol. 2023;14:1256081. doi:10.3389/fendo.2023.1256081
40. Babaei V, Afradi H, Gohardani HZ, Nasseri F, Azarafza M, Teimourian S. Management of Chronic Diabetic Foot Ulcers Using Platelet-Rich Plasma. J Wound Care. 2017;26(12):784–787. doi:10.12968/jowc.2017.26.12.784
41. Lacci KM, Dardik A. Platelet-Rich Plasma: support for Its Use in Wound Healing. Yale J Biol Med. 2010;83(1):1–9.
42. Rohman G, Langueh C, Ramtani S, et al. The Use of Platelet-Rich Plasma to Promote Cell Recruitment into Low-Molecular-Weight Fucoidan-Functionalized Poly(Ester-Urea-Urethane) Scaffolds for Soft-Tissue Engineering. Polymers. 2019;11(6). doi:10.3390/polym11061016
43. Ding Z-Y, Tan Y, Peng Q, Zuo J, Li N. Novel Applications of Platelet Concentrates in Tissue Regeneration (Review). Exp Ther Med. 2021;21(3):226. doi:10.3892/etm.2021.9657
44. Yang L, Rong G-C, Wu Q-N. Diabetic Foot Ulcer: challenges and Future. World J Diabetes. 2022;13(12):1014–1034. doi:10.4239/wjd.v13.i12.1014
45. Wu Q, Chen B, Liang Z. Mesenchymal Stem Cells as a Prospective Therapy for the Diabetic Foot. Stem Cells Int. 2016;2016:4612167. doi:10.1155/2016/4612167
46. Meamar R, Ghasemi-Mobarakeh L, Norouzi M-R, Siavash M, Hamblin MR, Fesharaki M. Improved Wound Healing of Diabetic Foot Ulcers Using Human Placenta-Derived Mesenchymal Stem Cells in Gelatin Electrospun Nanofibrous Scaffolds plus a Platelet-Rich Plasma Gel: a Randomized Clinical Trial. Int Immunopharmacol. 2021;101:108282. doi:10.1016/j.intimp.2021.108282
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

