Back to Journals » Journal of Asthma and Allergy » Volume 16
Real-World Study of Single-Inhaler Triple Therapy with Fluticasone Furoate/Umeclidinium/Vilanterol on Asthma Control in the US
Authors Bogart M, Germain G, Laliberté F, Mahendran M, Duh MS , DiRocco K , Noorduyn SG, Paczkowski R, Balkissoon R
Received 27 June 2023
Accepted for publication 7 November 2023
Published 1 December 2023 Volume 2023:16 Pages 1309—1322
DOI https://doi.org/10.2147/JAA.S424055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Michael Bogart,1 Guillaume Germain,2 François Laliberté,2 Malena Mahendran,2 Mei Sheng Duh,3 Kristi DiRocco,4 Stephen G Noorduyn,5,6 Rosirene Paczkowski,7 Ronald Balkissoon8
1U.S. Value Evidence and Outcomes, R&D U.S., GSK, Research Triangle Park, Durham, NC, USA; 2Groupe d’Analyse, Ltée, Montréal, QC, Canada; 3Analysis Group, Inc., Boston, MA, USA; 4U.S. Medical Affairs, GSK, Collegeville, PA, USA; 5Global Value Evidence and Outcomes, GSK, Mississauga, ON, Canada; 6Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada; 7Value Evidence and Outcomes, GSK, Collegeville, PA, USA; 8National Jewish Health, Denver, CO, USA
Correspondence: Kristi DiRocco, U.S. Medical Affairs, GSK, Collegeville, PA, USA, Tel +1 610-412-7175, Email [email protected]
Purpose: Real-world asthma control data among patients initiating fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) are limited. This study assessed rescue medication use and asthma-related exacerbations in patients with asthma before and after initiating single-inhaler FF/UMEC/VI using administrative claims data.
Patients and Methods: This retrospective, pre-post cohort study analyzed data from the IQVIA PharMetrics Plus database (September 18, 2016‒March 31, 2020). Patients aged ≥ 18 years that had ≥ 1 dispensing of single-inhaler FF/UMEC/VI 100/62.5/25 mcg (first dispensing = index date), ≥ 12 months of continuous health insurance enrollment prior to (pre-treatment) and following (post-treatment) FF/UMEC/VI initiation and ≥ 1 diagnosis of asthma during the pre-treatment period or on the index date were included. The primary endpoint was the number of oral corticosteroid (OCS) dispensings per patient per year during pre- and post-treatment periods. Secondary endpoints included asthma-related exacerbation rates and short-acting β2-agonist (SABA) use. Comparisons between pre- and post-treatment periods were made using risk and rate ratios.
Results: Overall, 890 patients with asthma initiating treatment with FF/UMEC/VI were included. The most recently dispensed controller medications prior to FF/UMEC/VI initiation were inhaled corticosteroids/long-acting β2-agonists (33.5%) and leukotriene modifiers (33.0%). Patients had a 29% reduction in the number of OCS dispensings (rate ratio [95% confidence interval (CI)]: 0.71 [0.65, 0.77], P < 0.001) during post-treatment versus pre-treatment, with a 23% reduction in the proportion of patients with ≥ 1 OCS dispensing post-treatment (risk ratio [95% CI]: 0.77 [0.73, 0.82], P < 0.001). Significant reductions in rates (rate ratio [95% CI]) of asthma-related exacerbations (0.59 [0.52, 0.67], P < 0.001) and SABA use (0.80 [0.74, 0.86], P < 0.001) were also observed.
Conclusion: In this real-world study, patients with asthma had significantly lower OCS use, asthma-related exacerbations, and SABA use following treatment initiation with FF/UMEC/VI compared with their pre-treatment period. These results suggest better asthma control following initiation of FF/UMEC/VI in a routine clinical practice setting.
Keywords: oral corticosteroids, rescue medication use, asthma control, FF/UMEC/VI, asthma exacerbations
Introduction
Between 30% and 50% of patients with asthma in the United States (US) are reported to have uncontrolled disease despite adherence to inhaled corticosteroid/long-acting β2-agonist (ICS/LABA) therapy.1,2 Patients whose asthma is uncontrolled experience worse quality of life, increased exacerbation frequency, healthcare costs, and risk of mortality compared with patients with well-controlled asthma.2–6
In the US, it has been found that 65% of patients with persistent asthma aged ≥12 years are prescribed oral corticosteroids (OCS), with 19% of patients classified as “high users” (cumulative dose of ≥450 mg within 90 days).7 Excessive need of OCS and/or short-acting β2-agonists (SABA) are often key indicators of poor asthma control7–9 and due to the serious side effects associated with OCS use,10 there is a risk of long-term complications and development of comorbid disease.11,12 Common adverse effects of OCS use include cataracts, hypertension, sleep apnea, osteoporosis, pneumonia, depression and anxiety.10 As such, the 2023 Global Initiative for Asthma (GINA) strategic report discourages the use of OCS as maintenance therapy except as a last resort.13 In addition, withdrawal from OCS therapy following long-term use can result in prolonged adrenal insufficiency that requires appropriate substitution and preventive measures.7
For patients with asthma who remain uncontrolled on a combination of ICS and LABA, the GINA report recommends a long-acting muscarinic antagonist (LAMA) as an add-on maintenance therapy option (ie, triple therapy).13 The addition of a LAMA to ICS/LABA for maintenance therapy was previously only available through multiple-inhaler triple therapy (MITT), usually requiring different devices or differing dosing regimens.13 Fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) in a fixed-dose combination inhaler (ELLIPTA dry-powder inhaler) was the first single-inhaler triple therapy (SITT) to receive approval from the US Food and Drug Administration (FDA) and the only SITT available in the US that is administered once daily.14
FF/UMEC/VI provides an alternative treatment paradigm for the management of patients with asthma who remain symptomatic on dual therapy;15 however, real-world data on asthma control among patients initiating FF/UMEC/VI are currently limited.
This retrospective cohort study assessed the impact of initiation of once-daily single-inhaler FF/UMEC/VI therapy on asthma control, as measured by the reduction in OCS use, asthma-related exacerbations, and rescue inhaler use, among patients with asthma in the US.
Materials and Methods
Data Source
De-identified data from the IQVIA PharMetrics Plus (IQVIA) database were obtained for the period of September 18, 2016, to March 31, 2020. The IQVIA database offers a diverse representation of employers, payers and providers, with geographic zones covering all 50 states of the US. It contains historical information on patient demographics, plan enrollment, and inpatient, outpatient, and pharmacy claims for approximately 40 million patients with both medical and pharmacy benefits in any given recent year.
Study Design
This was a retrospective, pre-post cohort study. The study design is shown in Figure 1. The index date was defined as the first pharmacy claim for off-label use of single-inhaler FF/UMEC/VI 100/62.5/25 mcg during the patient identification period from September 18, 2017 (approval date of FF/UMEC/VI for chronic obstructive pulmonary disease [COPD]) to March 31, 2019. The pre-treatment period was defined as the 12 months prior to, and including, the index date. The 12-month period beginning the day after the index date was defined as the post-treatment period. The study period was truncated on March 31, 2020 to minimize any confounding effects resulting from the COVID-19 pandemic. Each patient served as their own control by comparing study outcomes in the time periods prior to and following initiation of FF/UMEC/VI.
 |
Figure 1 Study design. Abbreviations: FF, fluticasone furoate; UMEC, umeclidinium; VI, vilanterol. |
Ethics Approval and Informed Consent
This study complied with all applicable laws regarding patient privacy. The IQVIA database contains anonymized patient records; therefore, these analyses did not require Institutional Review Board approval or consent, as the Office for Human Research Protections under the US Department of Health and Human Services does not consider research of fully deidentified information to involve human subjects {Electronic Code of Federal Regulations, #86}. The study was conducted in compliance with the Health Insurance Portability and Accountability Act.
Study Population
Patients were included in the study if they were aged ≥18 years at the index date, had ≥1 dispensing of single-inhaler FF/UMEC/VI 100/62.5/25 mcg, ≥1 medical claim with a primary or secondary diagnosis of asthma (International Classification of Diseases 10th Revision Clinical Modification [ICD-10-CM]: J45.xxx) during the pre-treatment period or on the index date, and ≥12 months of continuous health insurance enrollment both prior to and after the index date. Patients with ≥1 medical claim with a diagnosis of COPD (ICD-10-CM: J41.x–J44.x) in any position during the pre-treatment period or on the index date were excluded.
Study Endpoints
The primary endpoint was to describe and compare the mean (standard deviation [SD]) number of OCS dispensings per patient per year (PPPY), including the mean difference during the pre- and post-treatment periods.
OCS-related secondary endpoints assessed during the pre- and post-treatment periods were the proportion of patients with ≥1 OCS dispensing, the proportion of patients with no OCS exposure (0 mg/day), as well as exposure stratified by dose (>0–6, >6–12 and >12 mg/day), mean number of OCS bursts PPPY and chronic OCS use (maintenance use with a mean daily dose ≥5 mg and ≥10 mg). OCS exposure was calculated as the total OCS dosage dispensed during the period divided by the length of the period. Maintenance OCS use was defined as one or multiple OCS claims that had a gap of ≤14 days between the end date of a claim and the start date of the subsequent claim for at least 90 days. OCS bursts were defined as a pharmacy claim for an OCS medication with 2–28 days of supply and an average daily dose of ≥20 mg prednisone or equivalent.
Other secondary endpoints evaluated during the pre- and post-treatment periods included the rate of overall, inpatient/emergency department (IP/ED)-defined and systemic corticosteroid (SCS)-defined asthma-related exacerbations, and time-to-first overall, IP/ED-defined and SCS-defined asthma-related exacerbations. Overall asthma-related exacerbations included both IP/ED- and SCS-defined asthma-related exacerbations. IP/ED-defined asthma-related exacerbations included IP-defined exacerbations (ie, an asthma-related IP visit or an asthma-related ED visit resulting in an IP visit within +1 day) and ED-defined exacerbations (ie, an asthma-related ED visit). SCS-defined asthma-related exacerbations were defined as an asthma-related ED or outpatient visit with an SCS (OCS or injectable corticosteroid) medical or pharmacy claim within ±5 days. Asthma-related visits were identified as claims with a primary diagnosis of asthma. If ≥2 exacerbations occurred within 14 days of each other, this was classified as a single exacerbation according to the contributing event with the highest severity.
The mean number of SABA canisters per person year (PPY), and the proportion of patients with ≥1 SABA canister were other secondary endpoints evaluated during the pre- and post-treatment periods. Mean duration of FF/UMEC/VI treatment, mean number of FF/UMEC/VI dispensings and mean days of supply per dispensing were assessed during the post-treatment period and latest maintenance medication use prior to FF/UMEC/VI initiation was assessed during the pre-treatment period on the date closest to the index date. Duration of FF/UMEC/VI treatment was defined as the number of days from the first dispensing to the end of the days’ supply of the last dispensing.
Statistical Analysis
Descriptive statistics were summarized for patient characteristics during the pre-treatment period and treatment patterns of FF/UMEC/VI during the post-treatment period, as well as OCS treatment patterns and SABA canister use. Continuous variables were calculated as mean (SD), and relative frequencies and proportions were calculated for categorical variables.
During the pre- and post-treatment periods with FF/UMEC/VI, OCS outcomes, rates of asthma-related exacerbations, SABA canister use, and the proportion of patients using ≥1 SABA canisters were compared using rate ratios estimated from Poisson regressions and risk ratios estimated from log-binomial regressions, accounting for correlation between the pre- and post-treatment periods within the same patient using generalized estimating equations. 95% confidence intervals (CIs) and P-values were also reported using robust standard errors.
Rates of asthma-related exacerbations were reported PPY, calculated as the number of events, divided by person-years of observation and compared between the pre- and post-treatment periods. The time-to-first exacerbation (overall, IP/ED and SCS-defined) was estimated using a Kaplan–Meier survival analysis.
All analyses were conducted using SAS Enterprise Guide, Version 7.15 (SAS Institute Inc., Cary, NC, USA).
Results
Study Population, Demographics and Clinical Characteristics
A total of 5862 patients initiating treatment with FF/UMEC/VI were identified, of whom 890 patients (15.2%) were included in the study following application of the inclusion and exclusion criteria (Figure 2). Patients had a mean (SD) age of 52.0 (11.3) years, 56.9% of patients were female, and most patients were from the South US region (57.6%) (Table 1).
 |
Table 1 Patient demographics and clinical characteristics during the pre-treatment period |
Comorbidities
The most common asthma-related comorbidities were allergic rhinitis (50.4%), sinusitis (39.0%), upper respiratory tract infection (37.6%), and gastroesophageal reflux disease (31.7%) (Table 1).
Healthcare Resource Utilization (HRU) and Costs
Most patients had ≥1 asthma-related outpatient visit during the pre-treatment period, not including the index date (77.0%), and most patients were seen by a respiratory specialist (42.6%) or primary care physician (39.3%) closest to the index date. Mean (SD) total asthma-related healthcare costs were $5131 ($12,585), including outpatient visit costs of $2019 ($8625) and pharmacy costs of $2438 ($5754) (Table 1).
Asthma Medication During the Pre-Treatment Period
Patients most frequently used ICS/LABA (61.9%), leukotriene modifiers (51.7%), ICS (18.2%), MITT (13.9%), and LAMA (13.8%) as maintenance medications (Table 2). The most common rescue medication classes used by patients were antibiotics (81.0%), followed by SCS (77.1%), and SABA (72.2%) (Table 2).
 |
Table 2 Asthma medication use during the pre-treatment period |
Mean OCS Usage
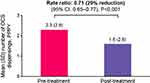
The rate of OCS dispensings was significantly lower during the post-treatment period (mean [SD] 1.6 [2.6] dispensings PPPY) compared with the pre-treatment period (mean [SD] 2.3 [2.9] dispensings PPPY) (Figure 3). The absolute difference in the mean number of OCS dispensings between the pre- and post-treatment period was 0.7, with a 29% reduction in the annual rate of OCS dispensing (rate ratio [95% CI]: 0.71 [0.65, 0.77], P < 0.001) in the post-treatment versus pre-treatment period (Figure 3).
Additional OCS Treatment Patterns
The proportion of patients with ≥1 OCS dispensing during the post-treatment period was significantly lower compared with the pre-treatment period (56.9% vs 73.4%; risk ratio [95% CI]: 0.77 [0.73, 0.82], P < 0.001) (Figure 4A). Similar findings were observed for the mean (SD) number of OCS bursts PPPY (0.8 [1.4] vs 1.2 [1.6] bursts PPPY; rate ratio [95% CI]: 0.70 [0.64, 0.77], P < 0.001) (Figure 4B). The proportion of patients with no OCS exposure showed a 62% improvement during the post-treatment period compared with the pre-treatment period (risk ratio [95% CI]: 1.62 [1.45, 1.82], P < 0.001) (Figure 4C).
The mean (SD) daily dose per dispensing was similar across the two time periods (post-treatment: 28.0 [12.9] mg; pre-treatment: 28.5 [11.6] mg) (Table 3). There was a significant reduction of 23% in the proportion of patients with OCS exposure >0–6 mg/day during the post-treatment period compared with the pre-treatment period (52.4% vs 68.0%; risk ratio [95% CI]: 0.77 [0.72, 0.83], P < 0.001) (Table 3). Chronic OCS use was similar across the two time periods (post-treatment: 6.9%; pre-treatment: 6.2%), with 3.9% receiving daily doses of ≥5 mg and 3.0% receiving ≥10 mg during the post-treatment period and 3.3% of patients receiving daily doses of ≥5 mg, and 2.9% of patients receiving ≥10 mg during the pre-treatment period (Table 3).
 |
Table 3 OCS exposure during the pre- and post-treatment periods |
Asthma-Related Exacerbations
During the pre-treatment period excluding the index date, 42.2% of patients experienced ≥1 asthma-related exacerbation (Table 1). IP/ED-defined exacerbations and SCS-defined exacerbations occurred in 8.7% and 38.1% of patients, respectively. The overall mean (SD) number of asthma-related exacerbations during the pre-treatment period excluding the index date was 0.79 (1.29) (Table 1).
The overall rate of asthma-related exacerbations was significantly lower during the post-treatment period, with a reduction of 41% compared with the pre-treatment period (0.50 vs 0.84 PPY; rate ratio [95% CI]: 0.59 [0.52, 0.67], P < 0.001) (Figure 5). Rates were also significantly lower for both IP/ED-defined (30% reduction) and SCS-defined (43% reduction) exacerbations (IP/ED-defined: 0.08 vs 0.12 PPY; rate ratio [95% CI]: 0.70 [0.51, 0.97], P = 0.032; SCS-defined: 0.41 vs 0.73 PPY; rate ratio [95% CI]: 0.57 [0.50, 0.65], P < 0.001) (Figure 5).
Kaplan–Meier plots show the time-to-first asthma-related exacerbation post-treatment, with overall events occurring in 11.9% of patients within 3 months, 19.2% within 6 months, 23.4% within 9 months, and 27.8% within 12 months (Supplementary Figure 1). The SCS-defined exacerbations followed a similar pattern with events occurring in 10.3% of patients within 3 months, 17.0% within 6 months, 20.9% within 9 months, and 25.3% within 12 months (Supplementary Figure 1). IP/ED-defined exacerbations occurred in 2.0% of patients within 3 months, 3.7% within 6 months, 4.6% within 9 months, and 5.6% within 12 months (Supplementary Figure 1).
SABA Use
There was a 20% reduction in SABA usage during the post-treatment period compared with the pre-treatment period (2.61 vs 3.27 canisters PPY; rate ratio [95% CI]: 0.80 [0.74, 0.86], P < 0.001). The proportion of patients using ≥1 SABA canister during the post-treatment period was also significantly lower compared with the pre-treatment period with an 18% reduction in risk of SABA usage (60.9% vs 74.6%; risk ratio [95% CI]: 0.82 [0.77, 0.86], P < 0.001) (Figure 6).
FF/UMEC/VI Treatment Patterns
Immediately prior to the initiation of FF/UMEC/VI, the most recently dispensed controller medications were ICS/LABA (33.5%), leukotriene modifiers (33.0%), ICS (5.7%), MITT (5.3%), and LAMA/LABA (2.5%). The mean (SD) duration of FF/UMEC/VI treatment was 246.8 (149.2) days, the mean number of dispensings was 5.7 (4.2), and the mean number of days of supply per dispensing was 36.1 (16.5).
Discussion
In this real-world observational study, patients with asthma who initiated treatment with FF/UMEC/VI demonstrated significant reductions in OCS and SABA use, as well as asthma-related exacerbations, relative to the period prior to initiation of FF/UMEC/VI. These findings are indicative of an improvement in asthma control upon initiation of FF/UMEC/VI.
This study found a 29% reduction in the mean number of OCS dispensings following initiation of FF/UMEC/VI. Post treatment, a 23% reduction in the proportion of patients with ≥1 OCS dispensing was observed compared with the pre-treatment period. Furthermore, of 890 patients included, 384 (43%) had no exposure to OCS during the post-treatment period; an improvement of 62% compared with the pre-treatment period. A 23% reduction was observed in the proportion of patients with OCS exposure between 0 and 6 mg/day following initiation of FF/UMEC/VI. The proportion of chronic users of OCS at ≥5 mg and ≥10 mg numerically increased following initiation of FF/UMEC/VI; however, the proportion of patients in these groups was small during both periods, and the difference was nonsignificant. Due to the association of OCS with both acute and long-term adverse effects10 and the risks posed by cumulative OCS use,11 reducing regular and rescue OCS use by 50% has been suggested as a realistic and important goal from a patient perspective.16 These findings indicate that a large proportion of patients could either reduce or eliminate their OCS usage by initiating FF/UMEC/VI.
This study also observed a 41% reduction in asthma-related exacerbations, including a 43% reduction in SCS-defined exacerbations and a 30% reduction in IP/ED-defined exacerbations. It has been shown that patients with asthma who experience exacerbations not only have higher HRU and healthcare costs but are also at greater risk of future exacerbations than those without a history of exacerbations.5,17–20 The TRIMARAN and TRIGGER studies (which compared single-inhaler extrafine combination beclometasone dipropionate/formoterol fumarate/glycopyrronium with beclometasone dipropionate/formoterol fumarate) also reported a 23% reduction in severe exacerbations, further highlighting the positive impact of SITT on reducing asthma-related exacerbations. A similar reduction was not seen in the CAPTAIN study, however the rate of exacerbations in the CAPTAIN population was low, likely due to the inclusion criteria not requiring a history of exacerbations.21 Furthermore, patients in the CAPTAIN study were first stabilized on FF/VI (100/25 mcg) before stepping up to FF/UMEC/VI (100/62.5/25 mcg); subsequently, the mean annualized severe exacerbation rate in the FF/VI group in CAPTAIN was much lower (0.38)21 than was observed in the pre-treatment period (0.84) of this study. It must be noted that the population of the present study was broader and more reflective of usual clinical practice than CAPTAIN. In CAPTAIN, patients were required to be uncontrolled (Asthma Control Questionnaire-6 [ACQ-6] scores of ≥1.5) despite ≥12 week treatment with ICS/LABA, with reduced lung function, with a best pre-bronchodilator morning forced expiratory volume in 1 second (FEV1) of between 30% and less than 85% of predicted normal value, and airway reversibility.21 These measures are not required to initiate FF/UMEC/VI in usual clinical practice. The findings of this study provide real-world evidence to suggest patients initiating FF/UMEC/VI in usual clinical practice experience a reduction in asthma-related exacerbations.
In addition, the proportion of patients using SABA canisters and the annual rate of SABA canister use reduced by 18% and 20%, respectively, following initiation of FF/UMEC/VI. Use of SABA canisters exceeding 2 per year can contribute to a decreased response to SABA as a reliever therapy and has been linked to increased OCS use, risk of severe exacerbations, ED visits, hospitalizations, and disease progression.22–25 Therefore the regular use of daily FF/UMEC/VI is associated with decreased reliance on SABA to manage asthma symptoms and a decreased exacerbation risk.
During the 12 months prior to initiating FF/UMEC/VI, almost two-thirds of patients were receiving ICS/LABA. The improvements in the outcomes examined in this study indicates improved asthma control, highlighting the benefit that initiating SITT with FF/UMEC/VI may provide. This supports the recommendations of the GINA 2023 strategy report, which highlights the benefits of adding a LAMA in patients whose asthma remains uncontrolled on medium- or high-dose ICS/LABA maintenance therapy.13
Strengths of this study include the use of a within-patient pre–post design allowing patients to act as their own controls, which enables comparisons with and without FF/UMEC/VI treatment within the same patient population and minimizes the risk of having unadjusted confounding factors. These data were collected prior to the COVID-19 pandemic and hence were not impacted by pandemic restrictions or changes in practice and patient behavior.
Patients with features of COPD were excluded from this study to ensure that OCS dispensings, SABA use and exacerbations were not COPD-related. This was both a strength and a limitation of the study. Here, we confirmed efficacy results from CAPTAIN within a real-world population and provided early data into exacerbation reduction within a population of patients with asthma. Nonetheless, FF/UMEC/VI is an established medicine for COPD26–28 and excluding patients with features of COPD permits a focused research question. However, there is a portion of patients with asthma who have features of COPD and this focus may mean that the study population included in this study is not representative of the more complex patient with asthma and features of COPD.29
This study observed the off-label use of FF/UMEC/VI 100/62.5/25 mcg in patients with asthma following FDA approval of its use in the treatment of COPD on September 18, 2017. Trends observed in this study represent the experiences of early adopters of FF/UMEC/VI in patients with asthma, prior to the regulatory approval for asthma (September 9, 2020) and any company commercialization. Therefore, results may not be representative of patients who initiate FF/UMEC/VI following marketing authorization. It should also be noted that the generalizability of the study results to the wider population (eg, other commercially insured populations, uninsured populations, or those with other types of public insurance) is limited as the IQVIA database is generally representative of commercially insured patients aged <65 years in the US.
There are some other limitations that should be highlighted regarding this study. The use of OCS and SABA as well as asthma-related exacerbation rates may not reflect asthma control as accurately as validated measures used in trials (eg, ACQ-6) or clinical parameters collected in practice (eg, peak expiratory flow or FEV1). However, these data are not available within large administrative claims datasets. These data are collected for administrative purposes and provide a broad overview of patient behavior and health utilization. As a result, elements captured in these datasets are vulnerable to error, for example, there may be diagnosis coding inaccuracies, and the presence of dispensed medication does not indicate that the medication was consumed or taken as prescribed by the patient. Furthermore, it cannot be ascertained from the administrative claims data whether a dispensing for a medication, such as antibiotics, was specific to the management of asthma. As such, asthma medication use may have been overestimated if patients had received the treatment for another condition.
Conclusion
To our knowledge, this is one of the first studies to provide real-world evidence on the impact of single-inhaler FF/UMEC/VI triple therapy for patients with asthma in usual practice in the US. Patients with asthma initiating FF/UMEC/VI had significant reductions in OCS use, SABA use, and asthma-related exacerbations compared with the period prior to treatment. These findings suggest substantial benefit for patients who initiate treatment with FF/UMEC/VI in routine clinical practice.
Data Sharing Statement
The data that support the findings of this study are available from IQVIA and are not publicly available. Restrictions apply to the availability of these data, which were used under license for the current study.
Acknowledgments
This study was funded by GSK (GSK study 214181). ELLIPTA is owned by or licensed to the GSK Group of Companies. IQVIA PharMetrics Plus is a trademark of IQVIA Inc. Editorial support (in the form of writing assistance including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing and referencing) was provided by Christopher Heath, PhD, and Suzanna Lim, PhD, of Fishawack Indicia Ltd, UK, part of Avalere Health, and was funded by GSK.
Abstracts based on this study were previously presented as poster presentations at the CHEST 2022 Annual Meeting, Nashville, Tennessee, October 18, 2022, poster No. 2068; and the CHEST 2023 Annual Meeting, Honolulu, Hawaiʻi, October 10, 2023, poster No. 4608.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GSK (214181).
Disclosure
KD, SGN, and RP are employees of GSK and hold stock and shares at GSK. MB is a former employee of GSK and holds stocks and shares at GSK. GG, FL, MM, and MSD are employees of Analysis Group, a consulting company that received research funds from GSK to conduct this study but did not receive payment for manuscript development. MSD also reports that Analysis Group has received grants from AbbVie, Apellis, AstraZeneca, Ayala Pharmaceuticals, Bayer, Blueprint Medicines, GSK, Humacyte, Janssen, Merck, Novartis, Pfizer, Sanofi, and Takeda, outside the submitted work. RB is a staff Pulmonary Physician at the National Jewish Health and is on speaker bureaus and advisory boards for GSK, AstraZeneca, Sanofi, Merck, and Regeneron. The authors report no other conflicts of interest in this work.
References
1. Davis J, Trudo F, Siddall J, Small M. Burden of asthma among patients adherent to ICS/LABA: a real-world study. J Asthma. 2019;56(3):332–340. doi:10.1080/02770903.2018.1455858
2. Lee LK, Obi E, Paknis B, Kavati A, Chipps B. Asthma control and disease burden in patients with asthma and allergic comorbidities. J Asthma. 2018;55(2):208–219. doi:10.1080/02770903.2017.1316394
3. Fernandes AGO, Souza-Machado C, Coelho RCP, et al. Risk factors for death in patients with severe asthma. J Bras Pneumol. 2014;40(4):364–372. doi:10.1590/S1806-37132014000400003
4. Katz PP, Yelin EH, Eisner MD, Blanc PD. Perceived control of asthma and quality of life among adults with asthma. Ann Allergy Asthma Immunol. 2002;89(3):251–258. doi:10.1016/S1081-1206(10)61951-5
5. Sullivan PW, Ghushchyan VH, Campbell JD, Globe G, Bender B, Magid DJ. Measuring the cost of poor asthma control and exacerbations. J Asthma. 2017;54(1):24–31. doi:10.1080/02770903.2016.1194430
6. Sullivan PW, Slejko JF, Ghushchyan VH, et al. The relationship between asthma, asthma control and economic outcomes in the United States. J Asthma. 2014;51(7):769–778. doi:10.3109/02770903.2014.906607
7. Tran TN, MacLachlan S, Hicks W, et al. Oral corticosteroid treatment patterns of patients in the United States with persistent asthma. J Allergy Clin Immunol Pract. 2021;9(1):338–346. e333. doi:10.1016/j.jaip.2020.06.019
8. Tavakoli H, Mark FitzGerald J, Lynd LD, Sadatsafavi M. Predictors of inappropriate and excessive use of reliever medications in asthma: a 16-year population-based study. BMC Pulm Med. 2018;18(1):1–8. doi:10.1186/s12890-018-0598-4
9. FitzGerald JM, Tavakoli H, Lynd LD, Al Efraij K, Sadatsafavi M. The impact of inappropriate use of short acting beta agonists in asthma. Respir Med. 2017;131:135–140. doi:10.1016/j.rmed.2017.08.014
10. Heatley H, Tran TN, Bourdin A, et al. Observational UK cohort study to describe intermittent oral corticosteroid prescribing patterns and their association with adverse outcomes in asthma. Thorax. 2022;78(9):860–867. doi:10.1136/thorax-2022-219642
11. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975–994. doi:10.1016/j.rmed.2009.01.003
12. Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi:10.1136/bmj.j1415
13. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2023. Available from: https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf.
14. GSK. FDA approves Trelegy Ellipta as the first once-daily single inhaler triple therapy for the treatment of both asthma and COPD in the US. Press release September 9, 2020. Available from: https://www.gsk.com/en-gb/media/press-releases/fda-approves-trelegy-ellipta-as-The-first-once-daily-single-inhaler-triple-therapy-for-The-treatment-of-both-asthma-and-copd-in-The-us.
15. FitzGerald JM, Sadatsafavi M. Triple therapy in a single inhaler: a new option for uncontrolled asthma. Lancet. 2019;394(10210):1690–1692. doi:10.1016/S0140-6736(19)32216-0
16. Pavord ID, Beasley R, Agusti A, et al. After asthma: redefining airways diseases. Lancet. 2018;391(10118):350–400. doi:10.1016/S0140-6736(17)30879-6
17. Schatz M, Meckley LM, Kim M, Stockwell BT, Castro M. Asthma exacerbation rates in adults are unchanged over a 5-year period despite high-intensity therapy. J Allergy Clin Immunol Pract. 2014;2(5):570–574.e571. doi:10.1016/j.jaip.2014.05.002
18. McDonald VM, Hiles SA, Godbout K, et al. Treatable traits can be identified in a severe asthma registry and predict future exacerbations. Respirology. 2019;24(1):37–47. doi:10.1111/resp.13389
19. Casey MF, Richardson LD, Weinstock M, Lin MP. Cost variation and revisit rate for adult patients with asthma presenting to the emergency department. Am J Emerg Med. 2022;61:179–183. doi:10.1016/j.ajem.2022.09.021
20. Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. doi:10.1186/s12890-017-0409-3
21. Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69–84. doi:10.1016/S2213-2600(20)30389-1
22. Stanford RH, Shah MB, D’Souza AO, Dhamane AD, Schatz M. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109(6):403–407. doi:10.1016/j.anai.2012.08.014
23. Nwaru BI, Ekström M, Hasvold P, Wiklund F, Telg G, Janson C. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi:10.1183/13993003.01872-2019
24. Quint JK, Arnetorp S, Kocks JWH, et al. Short-acting beta-2-agonist exposure and severe asthma exacerbations: SABINA findings from Europe and North America. J Allergy Clin Immunol Pract. 2022;10(9):2297–2309.e2210. doi:10.1016/j.jaip.2022.02.047
25. Noorduyn SG, Qian C, Johnston KM, et al. SABA use as an indicator for asthma exacerbation risk: an observational cohort study (SABINA Canada). ERJ Open Res. 2022;8(3):00140–02022. doi:10.1183/23120541.00140-2022
26. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
27. Ismaila AS, Haeussler K, Czira A, et al. Fluticasone Furoate/Umeclidinium/Vilanterol (FF/UMEC/VI) triple therapy compared with other therapies for the treatment of COPD: a network meta-analysis. Adv Ther. 2022;39(9):3957–3978. doi:10.1007/s12325-022-02231-0
28. Hanania NA, Bunner SH, Bengtson LGS, Ismaila AS, Bogart M. COPD exacerbations, costs, and health care resource utilization before and after initiation of Fluticasone Furoate/Umeclidinium/Vilanterol in routine care in the USA. Int J Chron Obstruct Pulmon Dis. 2023;18:407–418. doi:10.2147/COPD.S378867
29. Uchida A, Sakaue K, Inoue H. Epidemiology of asthma-chronic obstructive pulmonary disease overlap (ACO). Allergol Int. 2018;67(2):165–171. doi:10.1016/j.alit.2018.02.002
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.