Back to Journals » Journal of Multidisciplinary Healthcare » Volume 17
Pulmonary Rehabilitation Associated with Noninvasive Ventilation on Physical Capacity and Quality of Life in Post-COVID-19: A Randomized Controlled Double-Blinded Clinical Trial Protocol
Authors Arêas GPT, Goulart CDL , Sant'Anna T, Fernandes TG, Alvim RDO, Borges FFDR, Amaral CMSSBD, Rodrigues SCF, Valente J, Ferreira JMBB, Rezende AG, Oliveira Júnior EFD, Lacerda MVGD, Almeida-Val FFD
Received 17 November 2023
Accepted for publication 29 February 2024
Published 5 April 2024 Volume 2024:17 Pages 1483—1490
DOI https://doi.org/10.2147/JMDH.S438120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Guilherme Peixoto Tinoco Arêas,1,2 Cássia da Luz Goulart,3 Thaís Sant’Anna,4 Tiótrefis Gomes Fernandes,4 Rafael de Oliveira Alvim,1 Fernanda Facioli dos Reis Borges,1 Camila Miriam Suemi Sato Barros do Amaral,3 Suzy Cristina França Rodrigues,1 Jefferson Valente,3 João Marcos Bemfica Barbosa Ferreira,5 Anna Gabriela Rezende,1,3 Edival Ferreira de Oliveira Júnior,6 Marcus Vinícius Guimarães de Lacerda,3,7,8 Fernando Fonseca de Almeida-Val1– 4
1Department of Physiological Sciences, Exercise Physiology Laboratory, Federal University of Amazonas, Manaus, AM, Brasil; 2Cardiopulmonary Laboratory, Getúlio Vargas University Hospital, UFAM, Manaus, AM, Brasil; 3Dr Heitor Vieira Dourado Tropical Medicine Foundation, Manaus, AM, Brasil; 4Faculty of Physical Education and Physiotherapy, Respiratory and Cardiovascular Physiotherapy Laboratory, Federal University of Amazonas, Manaus, AM, Brasil; 5Higher School of Health Sciences, Faculty of Medicine, State University of Amazonas, Manaus, AM, Brasil; 6Francisca Mendes University Hospital, Manaus, AM, Brasil; 7Leônidas and Maria Deane Institute, Fiocruz Amazonas, Manaus, AM, Brasil; 8University of Texas Medical Branch at Galveston, Galveston, TX, USA
Correspondence: Guilherme Peixoto Tinoco Arêas, Departamento de Ciências Fisiológicas, Universidade Federal Do Amazonas, Avenida Rodrigo Otávio, 6200, Manaus, AM, 69080-900, Brasil, Email [email protected]
Background: The coronavirus disease-2019 (COVID-19) pulmonary rehabilitation (PR) seems to be a better choice to improve physical and functional capacity after acute infection. However, there is a lack of evidence regarding the effects of different strategies to optimize post-acute phase rehabilitation and reduce long COVID-19 physical deteriorations.
Objective: To compare the use of a noninvasive ventilation (NIV) plus aerobic exercise strategy during PR program with to a standard PR (without NIV) on physical capacity and quality of life outcomes in post-COVID-19.
Methods: Double-blinded randomized controlled clinical trial. A total of 100 individuals discharged from hospital in a post-acute phase of severe COVID-19 will be randomized into two groups: PR + NIV (Group 1) and PR (Group 2). Inclusion criteria include participants who present symptomatic dyspnea II and III by the modified Medical Research Council, aged 18 years or older. Both groups will receive aerobic and resistance exercise, and inspiratory muscle training. However, group 1 will perform aerobic training with bilevel NIV. Cardiopulmonary exercise test will assess the O2 peak uptake, 6-minute walk test will assess the walking distance and short-form 36 will assess the quality of life before and after 8 weeks (after 24 PR sessions). Moreover, patients will be contacted by telephone every 3 months for one year to record possible adverse events, hospitalizations, and death. All data will be registered in RedCap, and analyses will be performed in the STATA v13 software.
Clinical Trial Registration: RBR-3t9pkzt.
Keywords: COVID-19, noninvasive ventilation, pulmonary rehabilitation
Introduction
Field experts have recommended pulmonary rehabilitation (PR), focusing mainly on physical exercise guided by physical therapists in post-COVID-19.1–3 Until now, no clinical trial has observed the effect of therapeutic exercise on the physical rehabilitation of post-COVID-19. However, the large volume of studies with positive effects of PR in patients diagnosed with chronic pulmonary disease has prompted the scientific community for an external validity of a post-acute phase of COVID-19 adapted PR.2,4
A strategy to enhance exercise tolerance in aerobic training within PR is the use of ventilatory pressurized modalities during aerobic exercise, as the bilevel noninvasive ventilation (NIV).5–7 Studies in patients with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF)7–9 have evidenced reduced intolerance to efforts during aerobic exercise training and improvement of the physical and functional capacity after physical training using a PR plus NIV strategy. However, positive effects are not widely observed in all pulmonary conditions. For instance, patients with different chronic respiratory failure diseases10 have been shown not to improve functional capacity after aerobic training associated NIV compared to aerobic training alone.5,11
Therefore, we hypothesized that the use of a bilevel NIV during aerobic training may significantly improve exercise-dependent physiological and functional capacities and promote positive change in quality of life (QoL) in comparison to those allocated to a PR program without the addition of NIV.
Methods
Trial Design
This study consists of a single-center, double-blinded, randomized (1:1 ratio), controlled, two-arm and parallel group. All phases of the study are described in Figure 1. This protocol was approved by the Human research Ethics Committee of the Universidade Federal do Amazonas (CAAE: 44971221.7.0000.5020). Any protocol amendments will be submitted to the Human research Ethics Committee and to the trial registry for approval before implementation.
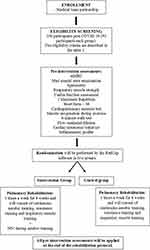 |
Figure 1 Description of the study phases. |
Eligibility Criteria, Recruitment Strategies, and Screening Procedures
Individuals living in Manaus, aged between 18 and 65 years old who developed severe or critical clinical COVID-19 requiring hospitalization and discharged afterwards, agree to all study procedures, and sign and Informed Consent Form (ICF) will be included in this study and randomized into one of the two study groups. Inclusion and exclusion criteria are described in Table 1.
 |
Table 1 The Full Eligibility Criteria |
Recruitment strategies will include social media advertisement, and in-person invitation. All training and assessments will be carried out in properly equipped exercise physiology laboratories at Universidade Federal do Amazonas (UFAM) under experienced, trained, and harmonized professionals. Only those individuals not under any other rehabilitation interventions will be included in this study in order to avoid biases. The severity of the acute illness is defined by the provisional clinical guidance of the World Health Organization (WHO).12
All participants will receive a participant identification number (PID), with all identifiable information restricted to intervention assessors. Moreover, to minimize the risk of bias for the outcome assessment, outcome assessors are not involved in the study data collection and are not aware of the treatment allocation. All outcome data will be entered into a separate database with no link to other study data.
Randomization
Participants will be randomly 1:1 assigned to either the NIV intervention group or the standard PR group. Randomization will be performed by R software 3.6.3 (University of Auckland, Auckland, New Zealand) through blockrand package and the random numbers will be organized in 4 and 6 blocks of participants, stratified by type of clinical presentation (severe or critical) and using permuted block of random sizes. The randomization process will be carried out by an external researcher not involved in the study, which will generate the randomization list on the computer. The same investigator will be responsible for maintaining and protecting the confidentiality of the allocation, in addition, will keep a copy of the list in a place inaccessible to participants and research staff.
Participants will be allocated to intervention groups through software-generated codes. To avoid possible errors and misdirection, random numbers will be documented with “I” for intervention and “C” for control. This process will be recorded to ensure that the envelopes are sealed with the respective names of the participants. All envelopes will be coated with aluminum foil to avoid possible transparency readings. The allocation sequence will be blinded to evaluators and study participants. The allocation of participants to the intervention groups will only be revealed after the individual has signed the consent form, thus ensuring that the status of the group is always unknown during the study.
Study Intervention
Assessments and Outcomes
Study Measurements, Outcomes, and Assessment Points
Primary outcomes consist of peak VO2 (mL*min−1), walking distance in meters, and SF-36 values (details in the Supplementary Materials). The secondary outcomes consist of physiological variables from pulmonary function test (details in the Supplementary Materials), CPET, muscle oxygenation by near-infrared spectroscopy (NIRS), arterial vascular endothelium function by flow-mediated dilation (FMD) (details in the Supplementary Materials), cardiac autonomic behavior by HR variability (HVR) (details in the Supplementary Materials) and inflammatory profile (details in the Supplementary Materials). All participants will undergo a full assessment before randomization, followed by the PR program and a one-year follow-up period.
6-Minute Walk Test
Functional capacity will be assessed through the 6MWT. Test will be performed in a 30-meter corridor without obstacles. The test will be performed twice, respecting the 30-minute interval between them as ATS recommendations.13 Briefly, participants will be encouraged to walk as far as possible in a stretch marked with colored ribbons on the ground, for a period of 6 minutes with standardized sentences. Participants may interrupt the test at any time, as needed, for resting, resuming it afterwards. A recovery time of six minutes after exercise will be considered. HR monitored by Polar United (Polar, Kemple, Finland), BP BIC sphygmomanometer (BIC, Itupeva, São Paulo) by the auscultatory method using Littmann Classic II stethoscope (3M, Minnesota, USA), SpO2 by digital oximeter Nonin 2500 (Nonin, Minnesota, USA) and the modified subjective effort perception scale Borg CR-10 will be used, monitored, and recorded in the moments of pre-test sitting rest, exercise peak, first, third, and sixth minutes of recovery. Assessments will be performed before and up to one week after training.
Muscle Strength
Determination of Muscle Strength Will Occur Through 1 Maximum Repetition (1-RM)
The test will be performed for each specific resistance exercise with the appropriate standards of execution.13 Up to six attempts will be allowed to identify the maximum weight, having a rest interval of five minutes between repetitions. The first attempt will be with submaximal loads, 5 kg for the grouping of the upper limbs and 10 kg for the lower limbs. 1 kg in the upper limbs and 2 kg in the lower limbs will be added, until approaching 1RM. The maximum load will be the last in which the individual performs a movement. The test will be performed every 2 weeks of resistance training.
Cardiopulmonary Exercise Test (CPET)
Physical capacity will be assessed by the CPET on a cicloergometer (GG – 04, Inbramed, Porto Alegre, Brazil) on a breath-by-breath metabolic analyzer PNOE (ENDO Medical, California, USA). The protocol will consist of the following phases: (I) rest period of 5 minutes standing on the treadmill; (II) warm-up at a speed of 3 km/h and without incline for 3 minutes; (III) incremental phase in the ramp protocol; (IV) Cool down for 1 minute at a speed of 3 km/h and no inclination; and (V) 5-minute passive recovery in the standing position on the treadmill (details in the Supplementary Materials).
The metabolic variables will be measured by the metabolic analyzer PNOE (ENDO Medical, California, USA) for gas analysis and absolute peak VO2 (mL*min−1), corrected peak VO2 (mL*kg−1*min−1), VO2 at ventilatory threshold (VT) (mL*min−1), carbon dioxide production (VCO2, mL*min−1), minute ventilation (VE, L*min−1), Fr (rpm), HR (bpm), chronotropic index, HR recovery (HRR), ventilatory equivalent for O2 and CO2 (VE/VO2 and VE/VCO2) and final partial pressure of O2 and CO2 (mmHg), O2 pulse (bpm*mL*min), test time and time to reach VT. The VE/VCO2 slope will be obtained by a linear regression analysis of the relationship between VE and VCO2.14 Additionally, the relationship between VO2 and VE, expressed by the efficiency of oxygen consumption (OUES) will be determined by the logarithmic curve using the equation: VO2 =a*log VE + b, where the constant “a” represents the rate of increase of VO2 in response to the increase in VE. Ventilatory power will be defined as peak systolic blood pressure (SBP) divided by the VE/VCO2 slope and circulatory power as peak VO2 * peak SBP.15
Muscle Oxygenation During Exercise
The availability and use of oxygen by peripheral tissues will be measured by NIRS using the Portamon (Artinis Medical Systems, Einsteinweg Netherlands) portable device for lower limbs and intercostal muscle. The method will allow the dynamic assessment of the relative concentrations of oxyhemoglobin (HbO2), deoxyhemoglobin (HHb), total hemoglobin (ie, the local blood volume) and the oxidative state of copper present in cytochrome c oxidase (HbT) during CPET before and after exercise training16 (details in the Supplementary Materials).
Hospitalization and Death Rate
After completion of PR, patients will be contacted by telephone every 3 months for one-year to record possible adverse events as cardiorespiratory decompensation, hospitalizations, and death. Participants will be advised to contact study staff if any event occurs within the 3-month period.
Interventions
Pulmonary Rehabilitation Program
PR will be performed 3 times a week for 8 weeks and will consist of continuous aerobic training, resistance training, and inspiratory muscle training. The difference in exercise protocol between groups is that NIV device will be used during continuous aerobic training in PR + NIV group. All procedures that compose the PR will be performed by trained, harmonized, and experienced physiotherapists in the trial procedures, always under the supervision of the unblinded investigator.
Continuous Aerobic Training
Continuous aerobic training will be carried out on a treadmill. In the first two weeks, individuals will start training with a speed that reaches 50% of the maximum heart rate (HR) achieved in cardiopulmonary exercise test (CPET) and/or perceived exertion of 3 on the Borg CR10 scale. In this phase, the volunteer will be submitted to 5 minutes of warm-up at minimum load on the treadmill, 15 minutes of constant load, and 10 minutes of cool down with minimum speed on the treadmill.
After 2 weeks, the individuals will perform the training with a speed that reaches 70% of the maximum HR reached in CPET and/or perceived exertion of 4–5 on the Borg CR 10 scale. In this phase, the volunteer will be submitted 5 minutes of warm-up at minimum load on the treadmill, 30 minutes of constant load and 10 minutes of cool down with minimum speed on the treadmill. If the volunteer has an arterial oxygenation (SpO2) ≤88%, additional O2 will be offered via catheter throughout the training. For patient monitoring, HR will be recorded by an HR monitor Polar United (Polar, Kempele, Finland) associated with an HR recording strap Polar H10 (Polar, Kempele, Finland), pressure values will be assessed every 3 minutes during the exercise and up to 5 minutes after stopping exercise, SpO2 by digital oximeter throughout the exercise and dyspnea perception by Borg CR10.
Resistance Training
Resistance training will be carried out with free load. Exercise will be performed in the upper limb flexion and extension of the elbow, anterior and lateral flexion of the shoulders. For the lower limb flexion and extension exercises of the hip, knee, and ankle will be performed, in addition to adduction and abduction exercises. In addition, sit-ups will be performed. In each session, 6 types of exercises will be performed. Initially, in the first two weeks, volunteers will perform 3 sets of 15 repetitions with 30% of 1 RM load. After the two weeks, volunteers will perform 5 sets of 8 repetitions with 50% of 1 RM load. If the volunteer has an arterial oxygenation (SpO2) ≤88%, additional O2 will be offered via catheter throughout the training. For patient monitoring, HR will be recorded by an HR monitor Polar United (Polar, Kempele, Finland) associated with an HR recording strap Polar H10 (Polar, Kempele, Finland), pressure values will be assessed every 3 minutes during the exercise and up to 5 minutes after stopping exercise, SpO2 by digital oximeter throughout the exercise and dyspnea perception by Borg CR10.17
Inspiratory Muscle Training
For inspiratory muscle training, it will be performed using the Power breathe clinic device (Powerbreathe, England, UK). Initially, in the first two weeks, the volunteers will perform the training of 5 sets of 6 inspirations with 1 minute of rest between sets with a load of 30% of the maximum inspiratory pressure (MIP). After 2 weeks, patients will undergo training with 5 sets of 6 breaths at 50% of MIP. If the volunteer has a drop in SpO2 ≤88%, additional O2 will be offered through a catheter between sets.
Noninvasive Ventilation
In the PR + NIV group, volunteers will perform continuous aerobic training associated with NIV Bilevel BIYH-730 Gaslive (Yuwell, Jiangsu, China) by face mask. The inspiratory pressure (IPAP) and expiratory pressure (EPAP) will have a difference of 5 cmH2O between them (ie, 4 cmH2O of EPAP and 9 cmH2O of IPAP). The choice of pressures will be made on the first day of the session, and the choice of pressure will be selected the most comfortable for each patient. If a volunteer, even with the use of NIV, has a drop in SaO2 ≤ 88%, additional O2 associated with NIV will be offered in all training sessions.
Statistical Analysis
Statistical analysis will consider intention-to-treat analysis. Initially, the nature of the distribution of variables will be evaluated using the Shapiro–Wilk test. The data will be described as mean, standard deviation and interval confidence 95%, or median and interquartile range depending on the distribution of variables. Frequencies will also be used. The investigations models will be centered on the analysis of the mean by the t Student’s test or median by Mann–Whitney test. In the parametric variance analysis, ANOVA two-way test (repeated measures, between factors) will be used, and in non-parametric variance analysis, Friedman and Kruskal Wallis test for intragroup and intergroup comparison will be used, and representative post hoc for each test will be done. For the primary outcome, p < 0.05 will be accepted as significant. For secondary outcomes, Bonferroni correction will be performed to adjust the p value. The analyzes will be performed using the statistical programs GraphPad Prism 7.0 (GraphPad, CA, USA) and R software (University of Auckland, Auckland, New Zealand). Effect size will be calculated using the GPower 3.1.2 (University of Dusseldorf, Dusseldorf, Germany)
Discussion
Although physical therapy plays an important role in all phases of recovery in COVID-19,10,18,19 the effort to optimize the impact of physical rehabilitation is lacking. To the best of our knowledge, this is the first protocol aiming to investigate the clinical effect of NIV associated with PR on physical capacity, functional capacity, and QoL of patients with sequelae of COVID-19. In addition, the study intends that, if NIV is more effective in improving the proposed primary outcomes, it should also analyze the various physiological cardiac, pulmonary, vascular, autonomic, and inflammatory mechanisms on the physical and functional improvement of these patients. Great debate may arise regarding the feasibility on investigating new approaches to rehabilitation in current times after vaccine roll-out. These taken together, and in consonance with real-life scenario of 2020’s epidemic peak and healthcare collapse, slow vaccination rates in 2021, especially in low- and middle-income countries, generalized poor access to post-acute care availability to individuals in these countries and lack of knowledge regarding optimized rehabilitation strategies in long covid, we believe that not only this study is feasible but also that it is necessary. The results of this study can provide high-quality clinical support for decision-making to a multidisciplinary team in the fight against the worst pandemic in the last 100 years and its consequences to public health worldwide.
Acknowledgments
The trial is supported by the Fundação de amparo à pesquisa do estado do Amazonas – (PCTI Grant 006/2020 process 01.02.016301.000063/2021-32-FAPEAM), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) – Brasil and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq): 408767/2022-7.
Disclosure
The authors have no conflicts to disclose.
References
1. Spruit MA, Holland AE, Singh SJ, Tonia T, Wilson KC, Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European respiratory society- and American thoracic society-coordinated international task force. Eur Respir J. 2020;56:2002197. doi:10.1183/13993003.02197-2020
2. Barker-Davies RM, O’Sullivan O, Senaratne KPP, et al. The Stanford Hall consensus statement for post-COVID-19 rehabilitation. Br J Sports Med. 2020;54:949–959. doi:10.1136/bjsports-2020-102596
3. Goulart CDL, Silva RN, Oliveira MR, et al. Lifestyle and rehabilitation during the COVID-19 pandemic: guidance for health professionals and support for exercise and rehabilitation programs. Expert Rev Anti Infect Ther. 2021;19:1385–1396. doi:10.1080/14787210.2021.1917994
4. Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation. Chest. 2007;131:4S–42S. doi:10.1378/chest.06-2418
5. Vitacca M, Kaymaz D, Lanini B, et al. Non-invasive ventilation during cycle exercise training in patients with chronic respiratory failure on long-term ventilatory support: a randomized controlled trial. Respirology. 2018;23:182–189. doi:10.1111/resp.13181
6. Marrara KT, Di Lorenzo VAP, Jaenisch RB, et al. Noninvasive ventilation as an important adjunct to an exercise training program in subjects with moderate to severe COPD. Respir Care. 2018;63:1388–1398. doi:10.4187/respcare.05763
7. da Goulart CL, Caruso FR, de Araújo ASG, et al. Can non-invasive ventilation modulate cerebral, respiratory, and peripheral muscle oxygenation during high-intensity exercise in patients with COPD-HF? Front Cardiovasc Med. 2022;8. doi:10.3389/fcvm.2021.772650
8. da Luz Goulart C, Caruso FR, Garcia de Araújo AS, et al. Non-invasive ventilation improves exercise tolerance and peripheral vascular function after high-intensity exercise in COPD-HF patients. Respir Med. 2020;173:106173. doi:10.1016/j.rmed.2020.106173
9. Mazzuco A, Souza AS, da Goulart CL, et al. Noninvasive ventilation accelerates oxygen uptake recovery kinetics in patients with combined heart failure and chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2020;40:414–420. doi:10.1097/HCR.0000000000000499
10. Raman B, Bluemke DA, Lüscher TF, Neubauer S. Long COVID: post-Acute sequelae of COVID-19 with a cardiovascular focus. Eur Heart J. 2022;43:1157–1172. doi:10.1093/eurheartj/ehac031
11. Chermont S, Quintão MMP, Mesquita ET, Rocha NN, Nóbrega ACL. Noninvasive ventilation with continuous positive airway pressure acutely improves 6-minute walk distance in chronic heart failure. J Cardiopulm Rehabil Prev. 2009;29:44–48. doi:10.1097/HCR.0b013e3181927858
12. Diaz J, Appiah J, Askie L, Baller A, Banerjee A, Barkley S. The Severity of the Acute Illness Was Defined by the Provisional Clinical Guidance of the World Health Organization (WHO). World Health Organization; 2021.
13. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi:10.1164/ajrccm.166.1.at1102
14. Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Technical considerations related to the minute ventilation/carbon dioxide output slope in patients with heart failure. Chest. 2003;124:720–727. doi:10.1378/chest.124.2.720
15. Hulkkonen J, Aatola H, Pälve K, et al. Determinants of exercise peak arterial blood pressure, circulatory power, and exercise cardiac power in a population based sample of Finnish male and female aged 30 to 47 years: the cardiovascular risk in young finns study. BMC Cardiovasc Disord. 2014;14:1–8. doi:10.1186/1471-2261-14-35.
16. Boushel R, Piantadosi CA. Near-infrared spectroscopy for monitoring muscle oxygenation. Acta Physiol Scand. 2000;168:615–622. doi:10.1046/j.1365-201x.2000.00713.x
17. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. doi:10.1249/00005768-198205000-00012
18. Borghi-Silva A, Back GD, Garcia de Araújo AS, et al. COVID-19 seen from a syndemic perspective: impact of unhealthy habits and future perspectives to combat these negative interactions in Latin America. Prog Cardiovasc Dis. 2022;71:72–78. doi:10.1016/j.pcad.2022.04.006
19. Silva RN, Goulart CDL, Oliveira MR, et al. Cardiorespiratory and skeletal muscle damage due to COVID-19: making the urgent case for rehabilitation. Expert Rev Respir Med. 2021;15:1107–1120. doi:10.1080/17476348.2021.1893169
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
