Back to Journals » Journal of Inflammation Research » Volume 17
Prognostic Value of Fibrinogen to Prealbumin Ratio (FPR) in Resectable Gastric Cancer
Authors Li H , Sun Y, Wang C , Xue Y
Received 27 October 2023
Accepted for publication 13 February 2024
Published 27 February 2024 Volume 2024:17 Pages 1325—1335
DOI https://doi.org/10.2147/JIR.S440832
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Hongwei Li,1,* Yufei Sun,2,* Cong Wang,1 Yingwei Xue1
1Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 2Department of Anesthesia, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yingwei Xue, Tel +86-13304646901, Email [email protected]
Background: The ratio of fibrinogen to prealbumin (FPR) is associated with the prognosis of many cancers. However, the prognostic significance of FPR in resectable gastric cancer has not been clarified.
Methods: A total of 760 patients with resectable gastric cancer participated in this study. The receiver operating characteristic curve (ROC) was used to calculate the optimal cutoff value of each immunonutrition marker. Univariate and multivariate Cox regression analyses were used to confirm the prognostic value of FPR in patients with gastric cancer and to select appropriate variables for the construction of nomogram.
Results: Utilizing ROC analysis, we calculated the optimal cutoff value for FPR and stratified 760 patients into high and low FPR groups. Subsequent examination revealed notable distinctions in baseline characteristics between these groups. For instance, Patients with higher FPR tend to be older and have more lymph node metastasis. Statistical analysis through the chi-square test confirmed the significance of these differences (P < 0.05). In addition, the results of the multivariate Cox proportional hazards regression analysis indicate that the factors related to OS were age (P = 0.001), T stage (P < 0.001), N stage (P < 0.001), radical resection (P < 0.001), and FPR (P < 0.024). The nomogram is composed of the above five variables. ROC analysis showed that the area under the curve (AUC) of the nomogram was 0.859 (95% CI: 0.831– 0.887), and the sensitivity and specificity were 77.4% and 82.1%, respectively.
Conclusion: FPR is a potential marker in patients with resectable gastric cancer. The nomogram based on FPR shows good predictive ability, which is helpful for clinicians to judge the prognosis of patients and choose targeted treatment strategies.
Keywords: gastric cancer, fibrinogen to prealbumin ratio, nomogram, prognosis
Introduction
Gastric cancer is one of the types of cancer with the highest morbidity and mortality in the world. Although with the improvement of medical standards and the improvement of people’s living conditions, the incidence and mortality of gastric cancer have decreased, the prognosis of patients with gastric cancer is still not optimistic, and there is a tendency to be younger.1,2 The treatment of patients with gastric cancer is still comprehensive, based on surgery and supplemented by chemotherapy and immunotherapy.3 However, due to the different physical conditions and severity of the disease, different treatment strategies may be needed. Therefore, it is a meaningful work to find an easily available preoperative prognostic factor so as to provide a reference for doctors when making treatment plans for patients with gastric cancer.
Inflammatory cells participate in the formation of the tumor microenvironment and play a significant role in the occurrence and development of tumor.4 Some studies have found that inflammation scores composed of more than two types of inflammatory cells are independent prognostic factors for a variety of cancers.5–7 In addition, as a soluble plasma glycoprotein synthesized by the liver, fibrinogen plays an important role in blood coagulation and inflammation and is considered to be closely related to the biological behavior of tumors. High levels of fibrinogen may lead to a poor prognosis for patients.8,9 For malignant tumors of the digestive system, the nutritional status of patients is very important to their prognosis. To some extent, the levels of albumin(ALB) and prealbumin(PALB) reflect the nutritional status of the patient. Some studies have shown that patients with lower ALB or PALB may have a shorter survival time.10,11 According to previous studies, FPR is significantly associated with the prognosis of patients with a variety of cancers, and a high FPR suggests a shorter survival time for patients.12,13 For gastric cancer, researchers have come to different conclusions. Tang et al believe that FPR is an independent prognostic risk factor for patients with gastric cancer.14 However, Zhang et al found that the correlation between FPR and OS in patients with gastric cancer was not significant.15 Therefore, whether FPR can accurately predict the prognosis of patients with gastric cancer is still a controversial issue.
Therefore, the purpose of this study is to explore whether there is a significant correlation between preoperative FPR levels and prognosis in patients with gastric cancer through overall and subgroup analysis and to analyze the relationship between FPR and different clinicopathological features. In addition, we established a FPR-based nomogram to predict the five-year overall survival time (OS) of patients with resectable gastric cancer.
Method
Patients
This study included 760 patients with gastric cancer who were treated in the cancer hospital affiliated with Harbin Medical University from January 2016 to December 2016. The diagnosis of the patient is based on the tissue samples obtained during gastroscopy, and the postoperative pathological tissue is examined by the pathologist to confirm the diagnosis.
The exclusion criteria were as follows: (1) patients who received neoadjuvant chemotherapy or conversion therapy before the operation. (2) patients who have not received a radical operation. (3) Distant metastases occur in cancer. (4) Intravascular coagulation. (5) hematological malignant tumor. (6) The patient suffers from a chronic disease. (7) Patients with incomplete follow-up information.
Data Extraction
A total of 13 indicators were collected for analysis. It includes sex, age, pT stage, pN stage, pTNM stage, Borrmann classification, lymph node metastasis (LNM), radical resection (R0, R1, R2), and five immune nutrition markers. Specifically, it includes the FPR, the ratio of fibrinogen to albumin (FAR), the ratio of neutrophils to lymphocytes (NLR), the prognostic nutritional index (PNI = albumin + 5 * lymphocytes), and the ratio of lactate dehydrogenase to lymphocytes (LLR).The hematological samples of the patients were collected within one week before the operation. R0 resection is defined as complete resection of the tumor, and the microsurgical margin is negative. R1 resection is defined as the presence of tumor residue at the incisal margin under the microscope. R2 resection is defined as tumor residue visible to the naked eye. The pTNM staging was in accordance with the eighth edition of the American Joint Commission on Cancer (AJCC).
Follow-Up
Patients were followed up every 3–6 months by telephone or email until they had been followed up for five years. The last follow-up date is January 1, 2022.
Statistical Analysis
OS is defined as the period from the date of the operation to the patient’s death for any reason. The classification variables are expressed as frequency and percentage, and the differences are obtained by the chi-square test. Continuous variables are described as medians and quartiles. The subject’s working characteristic curve is used to evaluate the predictive performance of the feature. The most approximate index was used to determine the cutoff value of the inflammation index. The logarithmic rank test and Kaplan-Meier method were used to analyze the survival curve. The independent prognostic factors of patients with gastric cancer were determined by univariate and multivariate analysis of the Cox proportional hazard model, and then the hazard ratio (HR) and 95% confidence interval (CI) of each variable were evaluated. Use Rstudio’s “SvyNom” and “rms” packages to draw a nomogram. In addition, the ROC curves of this study were drawn using MedCalc. All the statistical analysis of this study was carried out through R software version 4.2.3 and MedCalc software version 20.010. P < 0.05 was considered to be statistically significant.
Result
Patient Characteristics
The clinicopathological features are shown in Table 1. Most of the patients were male (71.8%), and 369 patients were less than 60 years old (48.6%). According to the eighth edition of the AJCC staging system, the number of patients in stages I, II, and III were 235 (30.9%), 198 (26.1%), and 227 (43.0%), respectively. 675 patients underwent R0 resection (88.8%). The optimal cutoff values for FPR, FAR, NLR, PNI, and LLR are 0.0106, 0.0731, 2.1476, 47.25, and 93.7209, respectively. The corresponding AUC are 0.688 (95% CI: 0.654–0.721), 0.636 (95% CI: 0.601–0.671), 0.578 (95% CI: 0.542–0.613), 0.590 (95% CI: 0.554–0.626), and 0.563 (95% CI: 0.527–0.598) (Figure 1). According to the cutoff level of FPR, patients were divided into two groups (FPR > 0.0106 and FPR ≤ 0.0106). As shown in Table 1, there are notable distinctions in baseline characteristics between high FPR group and low FPR group. The five-year survival rate of the low FPR group was 76.1%, and the average survival time was 52.1994 months (95% CI: 50.741–53.647). The five-year survival rate of the high FPR group was 47.7%, and the average survival time was 39.270 months (95% CI: 36.942–41.597, Figure 2).
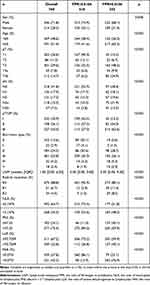 |
Table 1 Clinicopathological Features of All GC Patients |
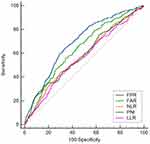 |
Figure 1 ROC curve analysis of different inflammatory indexes with OS as the outcome. |
 |
Figure 2 Kaplan meier survival curve of FPR. |
Univariate and Multivariate Cox Regression Analysis for OS
This study utilized univariate and multivariate Cox proportional hazards regression analyses to evaluate the prognosis of resectable gastric cancer patients. Death was defined as the endpoint for both univariate and multivariate Cox proportional hazards regression analyses. The calculation commenced from the date of surgical treatment, and if a patient experienced mortality within the subsequent five years, it was recorded as an endpoint event (death). Univariate Cox regression analysis showed that the characteristics included in this study, except gender, were significantly correlated with the prognosis of patients (P < 0.001). However, multivariate Cox regression analysis showed that the factors associated with OS were age (P < 0.01), T stage (P < 0.001), N stage (P < 0.001), radical resection (P < 0.001), and FPR (0.024, Table 2).
 |
Table 2 Univariate and Multivariate Analysis for OS |
Subgroup Analysis of the Predictive Value of FPR for OS
In order to further evaluate the prognostic significance of FPR in patients with gastric cancer, patients were divided into 10 subgroups according to age, sex, radical resection, pTNM stage, and other pathological features. Univariate Cox regression analysis was performed on 13 indexes collected in each subgroup. If a feature is proved to be related to the prognosis of patients by univariate Cox regression analysis. They will be involved in multivariate Cox regression analysis. The detailed results of univariate and multivariate Cox proportional hazard regression analysis in each subgroup are shown in Tables S1-S9. Through multivariate COX proportional hazard regression analysis, the performance of FPR in each subgroup is shown in Table 3. We found that the prognostic significance of FPR was significant in 5 subgroups: patients under 60 years old (P=0.031, HR=1.59, 95% CI: 1.04–2.44), female patients (P=0.0387, HR=2.02, 95% CI: 1.04–3.92), R0 resection group (P=0.0308, HR=1.5, 95% CI: 1.04–2.18), stage II patients group (P=0.0328, HR=1.99, 95% CI: 1.06–3.73), and stage III patient group (p=0.0128, HR=1.5, 95% CI: 1.09–2.06). In other subgroups, such as the male patients and the patients over 60 years old, FPR is not an independent risk factor for the patients. Although univariate Cox proportional hazards regression analysis has demonstrated a significant correlation between FPR and OS in these subgroups. The possible reason for this result could be that compared to other more meaningful characteristics, FPR has a weaker predictive power for OS in these subgroups. However, when considering all subgroups together, FPR holds significant importance for the prognosis of patients with resectable gastric cancer and is a potential prognostic biomarker. In addition, because none of the variables in the R1 resection group passed the univariate Cox regression analysis (P < 0.05), we only obtained the Cox regression analysis results for nine subgroups.
 |
Table 3 Subgroup Analysis of the Prognostic of FPR for OS |
Predictive Nomogram for OS
Based on the results of multivariate Cox regression analysis, we used age, T stage, N stage, radical resection, FPR, and other five variables to develop a nomogram to predict the five-year survival rate of patients (Figure 3A). The total points of each patient was calculated according to the results of the nomogram and analyzed by ROC. The results showed that the AUC of the nomogram was 0.859 (95% CI: 0.831–0.887), and the sensitivity and specificity were 77.4% and 82.1%, respectively (Figure 3B). According to the best cutoff value obtained by ROC curve analysis, the patients were divided into a high-risk group (total points > 141) and a low-risk group (total points ≤141). The results of the Kaplan-Meier survival curve showed that the survival time of patients in the high-risk group was relatively short (P < 0.0001, Figure 3C). According to Table 2, our analysis reveals that, in comparison to age and FPR, T stage, N stage, and radical resection exhibit smaller P values and larger HR in the results of the multivariate Cox proportional hazards regression analysis. Consequently, we designate these three variables as the most significantly associated with OS. Subsequently, we used these three variables and the total points from the nomogram to construct a forest map with a 5-year mortality rate as the outcome. The forest map results showed that after adjusting the three most significant factors in multivariate Cox regression analysis, the total points based on the nomogram was still an independent prognostic factor for patients (Figure 3D).
Discussion
In recent years, the link between cancer and inflammation has attracted more and more researchers’ attention. When cancer occurs, cancer cells tend to migrate from the primary tumor to the blood circulation, and natural killer (NK) cells kill them in the process of tumor cell circulation. Therefore, the level of NK cells in patients can reflect the immune status and prognosis of the human body to a certain extent.16,17 Fibrinogen is an acute-phase protein. When there is a malignant tumor or systemic inflammatory reaction in the human body, the IL-6 synthesis pathway is activated, which increases the release of fibrinogen, and the level of fibrinogen in the blood will show an increasing trend.18 Previous studies have shown that tumor cells can induce platelet aggregation and form thrombin, which promotes fibrinogen to gather around tumor cells to form a dense fibrin layer, which makes tumor cells escape the killing effect of NK cells.19 Some researchers have also found that when the level of fibrinogen in cancer patients increases, the expression of vimentin (a mesenchymal marker) increases while the expression of E-cadherin (an epithelial cell marker) decreases, thus promoting tumor progression.20 The nutritional status of malignant tumors is also significantly related to the prognosis of patients.21,22 Although ALB is more often used to evaluate the nutritional status of patients, in fact, the half-life of PALB is shorter and is a more sensitive indicator of nutritional status.23 In addition, it has been reported that PALB is an acute-phase negative protein, which is often reduced when inflammation occurs in the human body.24 Therefore, FPR is a simple and potential prognostic marker, and it is necessary to explore its correlation with the prognosis of patients with gastric cancer.
In this study, we demonstrated that FPR is an independent prognostic factor for patients with resectable gastric cancer. Through ROC analysis, when predicting the prognosis of patients with gastric cancer, the AUC of FPR was 0.688, which was higher than the other four inflammation scores. The AUC of the FPR-based nomogram is 0.859. Furthermore, through subgroup analysis, we identified that FPR holds significant prognostic relevance for resectable gastric cancer patients in the majority of subgroups. These subgroups include: patients under 60 years old, female patients group, R0 resection group, stage II patients group, and stage III patient group.
Previous Mate analysis pointed out that FPR has a high prognostic value for malignant tumors of the digestive system.25 Xie et al found that FPR is an independent prognostic factor for resectable colorectal cancer.26 In addition, similar conclusions have been drawn in digestive system tumors such as gastric cancer, pancreatic cancer, esophageal cancer, and so on: the prognosis of patients tends to become worse with the increase of FPR.14,27,28 Some researchers believe that the combined score of FPR and PNI can predict the prognosis of elderly patients with gastric cancer, but FPR is not an independent prognostic factor for elderly patients with gastric cancer, which is different from our results.15 Additionally, in the subgroup analysis, we employed univariate and multivariate Cox analyses to mitigate the influence of confounding factors, delving deeper into the significance of FPR in predicting prognosis across different subsets of gastric cancer patients. We also found that FPR seems to have a high prognostic value for female patients. Then we conducted a ROC analysis of female patients, and the results showed that the AUC of FPR reached 0.731 (Figure 4). This may be a novel and meaningful discovery, but large-scale prospective experimental studies are needed to verify our results and explore the possible mechanism behind this phenomenon. In addition, we use five features, including FPR, to establish a nomogram, and the results show good accuracy, which can provide a reference for clinicians to judge the prognosis of patients and formulate treatment strategies.
 |
Figure 4 ROC curve analysis of the FPR in female patients with gastric cancer. |
It must be admitted that this study still has some limitations. First of all, this is a retrospective study, and the subjects are from the same center, which will inevitably increase the risk of selection bias. Secondly, our sample size is small. In addition, different patients receive different postoperative treatment options, which may affect our research results. Therefore, large prospective randomized controlled trials are needed to address these limitations and test the accuracy of our findings.
Conclusion
FPR is a potential marker for resectable gastric cancer and has high prognostic value, especially in gastric cancer patients under 60 years old and female patients. The nomogram based on FPR shows good predictive ability, which is helpful for clinicians to judge the prognosis of patients and choose targeted treatment strategies.
Data Sharing Statement
The data involved in this study can be obtained from the correspondents.
Ethical Approval
This study was approved by the Ethics Committee of Harbin Medical University Cancer Hospital, and the research process was in accordance with the 1964 Helsinki Declaration. All patients included in this study have signed written informed consent.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Nn10 program of Harbin Medical University Cancer Hospital, China (No. Nn10 PY 2017-03).
Disclosure
The authors have declared that there is no conflict of interest for this work.
References
1. Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396(10251):635–648. doi:10.1016/s0140-6736(20)31288-5
2. Wong MCS, Huang J, Chan PSF, et al. Global Incidence and Mortality of Gastric Cancer, 1980-2018. JAMA Network Open. 2021;4(7):e2118457. doi:10.1001/jamanetworkopen.2021.18457
3. Wang Y, Zhang L, Yang Y, Lu S, Chen H. Progress of Gastric Cancer Surgery in the era of Precision Medicine. Int J Biol Sci. 2021;17(4):1041–1049. doi:10.7150/ijbs.56735
4. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi:10.1038/nature01322
5. Cummings M, Merone L, Keeble C, et al. Preoperative neutrophil:lymphocyte and platelet:lymphocyte ratios predict endometrial cancer survival. Br J Cancer. 2015;113(2):311–320. doi:10.1038/bjc.2015.200
6. Li J, Cao D, Huang Y, et al. The Prognostic and Clinicopathological Significance of Systemic Immune-Inflammation Index in Bladder Cancer. Front Immunol. 2022;13:865643. doi:10.3389/fimmu.2022.865643
7. Jomrich G, Paireder M, Kristo I, et al. High Systemic Immune-Inflammation Index is an Adverse Prognostic Factor for Patients With Gastroesophageal Adenocarcinoma. Ann Surg. 2021;273(3):532–541. doi:10.1097/sla.0000000000003370
8. Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):960–970. doi:10.1016/j.ctrv.2015.10.002
9. Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3(8):1894–1904. doi:10.1111/j.1538-7836.2005.01365.x
10. Yamashita K, Ushiku H, Katada N, et al. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41(10):1324–1332. doi:10.1016/j.ejso.2015.05.021
11. Zu H, Wang H, Li C, Xue Y. Preoperative prealbumin levels on admission as an independent predictive factor in patients with gastric cancer. Medicine. 2020;99(11):e19196. doi:10.1097/md.0000000000019196
12. Huang L, Mo Z, Hu Z, et al. Diagnostic value of fibrinogen to prealbumin ratio and gamma-glutamyl transpeptidase to platelet ratio in the progression of AFP-negative hepatocellular carcinoma. Cancer Cell Int. 2020;20:77. doi:10.1186/s12935-020-1161-y
13. Liao YC, Fu M, Wang XF, Cheng XX. Combined fibrinogen-to-pre-albumin ratio and carbohydrate antigen 19-9 score is a promising metric to predict progression of metastatic colorectal mucinous adenocarcinoma. J Clin Lab Anal. 2021;35(5):e23757. doi:10.1002/jcla.23757
14. Tang S, Lin L, Cheng J, et al. The prognostic value of preoperative fibrinogen-to-prealbumin ratio and a novel FFC score in patients with resectable gastric cancer. BMC Cancer. 2020;20(1):382. doi:10.1186/s12885-020-06866-6
15. Zhang X, Zhao W, Chen X, et al. Combining the Fibrinogen-to-Pre-Albumin Ratio and Prognostic Nutritional Index (FPR-PNI) Predicts the Survival in Elderly Gastric Cancer Patients After Gastrectomy. Onco Targets Ther. 2020;13:8845–8859. doi:10.2147/ott.S264199
16. Liotta LA. Cancer cell invasion and metastasis. Sci Am. 1992;266(2):54–9, 62–3. doi:10.1038/scientificamerican0292-54
17. Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2’-deoxyuridine. J Natl Cancer Inst. 1970;45(4):773–782.
18. Yamaguchi T, Yamamoto Y, Yokota S, Nakagawa M, Ito M, Ogura T. Involvement of interleukin-6 in the elevation of plasma fibrinogen levels in lung cancer patients. Jpn J Clin Oncol. 1998;28(12):740–744. doi:10.1093/jjco/28.12.740
19. Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100(5):859–865. doi:10.1111/j.1349-7006.2009.01115.x
20. Shu YJ, Weng H, Bao RF, et al. Clinical and prognostic significance of preoperative plasma hyperfibrinogenemia in gallbladder cancer patients following surgical resection: a retrospective and in vitro study. BMC Cancer. 2014;14:566. doi:10.1186/1471-2407-14-566
21. Argilés JM. Cancer-associated malnutrition. Eur J Oncol Nurs. 2005;9(Suppl 2):S39–50. doi:10.1016/j.ejon.2005.09.006
22. Kheirouri S, Alizadeh M. Prognostic Potential of the Preoperative Controlling Nutritional Status (CONUT) Score in Predicting Survival of Patients with Cancer: a Systematic Review. Adv Nutr. 2021;12(1):234–250. doi:10.1093/advances/nmaa102
23. Devoto G, Gallo F, Marchello C, et al. Prealbumin serum concentrations as a useful tool in the assessment of malnutrition in hospitalized patients. Clin Chem. 2006;52(12):2281–2285. doi:10.1373/clinchem.2006.080366
24. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. doi:10.1056/nejm199902113400607
25. Li B, Deng H, Zhou Z, Tang B. The Prognostic value of the Fibrinogen to pre-albumin ratio in malignant tumors of the digestive system: a systematic review and meta-analysis. Cancer Cell Int. 2022;22(1):22. doi:10.1186/s12935-022-02445-w
26. Hailun X, Huang S, Yuan G, Tang S, Gan J. Prognostic Significance of Preoperative Fibrinogen-to-Prealbumin Ratio in Patients with Stage I-III Colorectal Cancer Undergoing Surgical Resection: a Retrospective Cohort Study. Biomed Res Int. 2021;2021:3905353. doi:10.1155/2021/3905353
27. Li C, Fan Z, Guo W, et al. Fibrinogen-to-prealbumin ratio: a new prognostic marker of resectable pancreatic cancer. Front Oncol. 2023;13:1149942. doi:10.3389/fonc.2023.1149942
28. Feng JF, Wang L, Jiang YH, Yang X. A novel prognostic index in patients with resectable esophageal squamous cell carcinoma: fibrinogen/prealbumin ratio. Rev Invest Clin. 2020;72(1):46–54. doi:10.24875/ric.19003184
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

