Back to Journals » Journal of Inflammation Research » Volume 17
Prognostic Nutritional Index Predicts Efficacy and Immune-Related Adverse Events of First-Line Chemoimmunotherapy in Patients with Extensive-Stage Small-Cell Lung Cancer
Authors Zhang B , Chen J, Yu H, Li M, Cai M, Chen L
Received 21 November 2023
Accepted for publication 12 March 2024
Published 18 March 2024 Volume 2024:17 Pages 1777—1788
DOI https://doi.org/10.2147/JIR.S450804
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Baishen Zhang,1 Jing Chen,2 Hui Yu,2 Meichen Li,2 Muyan Cai,3 Likun Chen2
1Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Guangdong Provincial Clinical Research Center for Cancer, Guangzhou, People’s Republic of China; 2Department of Medical Oncology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Guangdong Provincial Clinical Research Center for Cancer, Guangzhou, People’s Republic of China; 3Department of Pathology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Guangdong Provincial Clinical Research Center for Cancer, Guangzhou, People’s Republic of China
Correspondence: Muyan Cai, Department of Pathology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Guangdong Provincial Clinical Research Center for Cancer, 651 Dongfeng E Road, Yuexiu District, Guangzhou, 510060, People’s Republic of China, Email [email protected] Likun Chen, Department of Medical Oncology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Guangdong Provincial Clinical Research Center for Cancer, 651 Dongfeng E Road, Yuexiu District, Guangzhou, 510060, People’s Republic of China, Email [email protected]
Background: Currently, there is a lack of well-established markers to predict the efficacy of chemoimmunotherapy in small-cell lung cancer (SCLC). Neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), advanced lung cancer inflammation index (ALI) and prognostic nutritional index (PNI) are associated with prognosis in several tumors, whereas their predictive role in SCLC remains unclear.
Methods: A retrospective study was conducted at Sun Yat-sen University Cancer Center, involving extensive-stage SCLC (ES-SCLC) patients who received first-line chemoimmunotherapy between January 2020 and December 2021. Peripheral blood biomarkers were extracted from medical records and their correlation with prognosis and immune-related adverse events (IRAEs) was analyzed.
Results: A total of 114 patients were included. Patients with a low PLR, high ALI and high PNI had prolonged progression-free survival (PFS) compared to those with a high PLR, low ALI and low PNI. Patients with a low NLR, low PLR, high ALI and high PNI had prolonged overall survival (OS) compared to those with a high NLR, high PLR, low ALI and low PNI. Cox regression model showed that PNI was an independent risk factor for both PFS and OS. ROC curve showed that PNI outperforms NLR, PLR and ALI in predicting both PFS and OS. The PNI-based nomogram demonstrated strong predictive capability for both PFS and OS. In addition, there was a significant correlation between PNI and IRAEs.
Conclusion: A high baseline PNI might be associated with improved prognosis and the occurrence of IRAEs in ES-SCLC patients treated with first-line chemoimmunotherapy.
Keywords: peripheral blood markers, predictive ability, chemoimmunotherapy, small-cell lung cancer, immune-related adverse events
Introduction
Lung cancer is a highly prevalent and lethal malignancy worldwide. Among its histological subtypes, small-cell lung cancer (SCLC) accounts for approximately 14% of all lung cancer cases.1 SCLC is characterized by its remarkable aggressiveness, rapid growth and propensity for metastasis, and a majority of patients already have local or distant metastases at diagnosis.2
For extensive-stage small-cell lung cancer (ES-SCLC) patients, systemic therapy plays a pivotal role in treatment, and platinum-based combination chemotherapy has been regarded as the standard approach over the past three decades.3,4 However, its overall efficacy is modest, with a median overall survival (mOS) ranging from 8 to 13 months.5,6
In recent years, there has been a growing trend in using immunotherapy in the treatment of ES-SCLC.7 A clinical trial conducted on SCLC patients, IMpower133,8 found that combining chemotherapy with immunotherapy (atezolizumab) significantly improved mOS compared with chemotherapy alone (12.3 months vs 10.3 months, HR = 0.70, 95% CI 0.54 to 0.91; P=0.007). Another clinical trial on SCLC, CASPIAN,9 also showed that adding immunotherapy (durvalumab) to chemotherapy significantly improved mOS (13 months vs 10.3 months, HR = 0.73, 95% CI 0.59 to 0.91; p=0.0047). Therefore, chemotherapy plus immunotherapy has become a better first-line treatment option for ES-SCLC.10
However, the efficacy benefit of adding immune checkpoint inhibitors to chemotherapy is still not satisfactory in ES-SCLC, and it may lead to various immune-related adverse events (IRAEs) in some patients.11,12 Therefore, it is an urgent clinical challenge to identify potential beneficiaries in order to enhance clinical efficacy and manage IRAEs.
Exploring the predictive biomarkers for the efficacy of immunotherapy has been a prominent research focus. Several studies have investigated the efficacy biomarkers of chemoimmunotherapy in ES-SCLC, but the results have been less than satisfactory.13 And there remains a lack of well-established marker to predict the efficacy of chemoimmunotherapy in ES-SCLC patients currently.14,15
Studies have demonstrated a close association between inflammation and cancer, as it modulates the immune microenvironment and triggers the activation of carcinogenic signaling pathways that facilitate the proliferation and metastasis of cancer cells.16,17 The prognostic value of some peripheral blood biomarkers associated with inflammation has been studied. The neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), advanced lung cancer inflammation index (ALI), and prognostic nutritional index (PNI) are some of the blood-based markers that have been extensively studied in this regard. NLR is calculated by dividing the absolute neutrophil count by the absolute lymphocyte count,18 while PLR is calculated by dividing the platelet count by the lymphocyte count.19 ALI is derived from NLR and albumin levels,20 and PNI is calculated based on albumin and lymphocyte counts.21 These markers reflect the balance between inflammation and immune response, as well as the nutritional and overall health status of the patient. Studies have shown that high NLR and PLR, as well as low ALI and PNI are associated with poor clinical outcomes and reduced response to immunotherapy in various cancers.18–21 However, no studies have explored the association between these markers with the efficacy of first-line chemotherapy plus immunotherapy for ES-SCLC.
Therefore, this study retrospectively collected data on NLR, PLR, ALI, and PNI at baseline in ES-SCLC patients treated with first-line chemoimmunotherapy, and analyze the correlation between these markers and patients’ outcomes. The objective was to identify a promising marker for predicting efficacy in ES- SCLC patients treated with first-line chemoimmunotherapy.
Materials and Methods
Study Design and Patient Population
All patients with histologically confirmed ES-SCLC at Sun Yat-sen University Cancer Center from January 1, 2020 to December 30, 2020 were retrospectively enrolled. The patients exhibited measurable lesions according to the Response Evaluation Criteria in Solid Tumors, version1.1 (RECIST v1.1)22 and their major organ functions were within normal range. Patients were treated with etoposide plus platinum plus PD-L1 inhibitors, specifically atezolizumab (N=48) and durvalumab (N=66). (Figure S1)
Evaluation of Baseline NLR, PLR, ALI and PNI Score
NLR was calculated as the ratio of absolute neutrophil count to absolute lymphocyte count. PLR was calculated as the ratio of absolute platelet count to absolute lymphocyte count. ALI was calculated as Body Mass Index × serum albumin (g/L) × 0.1 /NLR. And PNI was calculated as serum albumin(g/L) +5 × absolute lymphocyte count. The cut-off values utilized in this study were adopted from previous literature, which were 5 for NLR,23,24 185 for PLR,25 18 for ALI,20 and 45 for PNI.26,27
Efficacy Assessments and Evaluation of Adverse Events
Treatment efficacy was evaluated by two oncologists using the RECIST v1.1. Physical examinations, hematologic and biochemical tests were conducted at baseline and after every 3–4 weeks (within a week before each chemoimmunotherapy session), and tumors were assessed using contrast-enhanced computed tomography scans of the chest and upper abdomen, as well as contrast-enhanced magnetic resonance imaging scans of the brain at baseline and after every second cycle of treatment (6–8 weeks). Treatment toxicity was evaluated by two independent medical professionals according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.28
Statistical Analysis
The descriptive statistics were used to summarize all variables. Categorical variables were analyzed using appropriate statistical tests such as Chi-square or Fisher’s exact test. Survival data were estimated using Kaplan-Meier curves and compared using a Log rank test. The risk of disease progression and death was evaluated using Cox proportional hazards regression model, and the hazard ratio (HR) and 95% confidence interval (CI) were calculated. Univariate and multivariate Cox proportional hazards regression analyses were conducted to assess the prognostic value for progression-free survival (PFS) and overall survival (OS). The analysis of ROC and AUC curves was performed using the timeROC package, while the ggplot2 package was used for visualizing the results. The development of nomogram and the calibration curve analysis was performed using the survival package, while the rms package was used for visualizing the results.
Statistical analysis was performed with SPSS (version 25), GraphPad Prism (version 7) and R software (version 4.0). A two-sided p-value of < 0.05 was considered statistically significant. The last follow-up date was July 30, 2023.
Results
Baseline Characteristics
A total of 114 patients with histologically confirmed ES-SCLC were treated with first-line chemoimmunotherapy from January 2020 to December 2021. The median age of enrolled patients was 62 years, and most patients were male (102/114, 89.5%). 82 patients had a history of smoking (71.9%), and 88 patients had extrathoracic metastases at baseline (77.2%). In addition, patients were stratified according to the cut-off values of NLR, PLR, ALI, and PNI reported in the previous literature respectively. The baseline characteristics of the patients are summarized in Table 1.
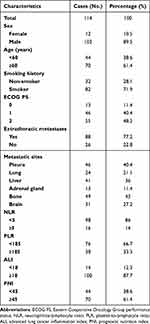 |
Table 1 Baseline Characteristics for Small-Cell Lung Cancer Patients |
Association Between Peripheral Blood Markers and Progression-Free Survival
The association between peripheral blood markers and PFS in SCLC patients was analyzed. As shown in Figure 1, Patients with low PLR had prolonged PFS compared to those with high PLR (7.5 months vs 6.5 months, HR=0.66, 95% CI 0.36–1.18, p=0.031). Patients with high ALI had prolonged PFS compared to those with low ALI (7 months vs 5.4 months, HR=0.46, 95% CI 0.25–0.85, p=0.014). And patients with high PNI had prolonged PFS compared to those with low PNI (8.2 months vs 5.4 months, HR=0.36, 95% CI 0.23–0.55, p<0.001). However, there was no statistically significant difference in PFS between high NLR group and low NLR group (p=0.162).
The Cox regression analysis incorporated various factors including sex, age, smoking status, ECOG PS, extrathoracic metastases, pleura metastases, lung metastases, liver metastases, adrenal gland metastases, bone metastases, brain metastases, NLR, PLR, ALI and PNI. Both univariate and multivariate analysis showed that extrathoracic metastases and PNI were independent prognostic factors for PFS (Table S1).
Association Between Peripheral Blood Markers and Overall Survival
In terms of the association between peripheral blood markers and OS, it was found that compared with a high NLR, high PLR, low ALI and low PNI, a low NLR (17.7 months vs 14.1 months, HR=0.51, 95% CI 0.27–0.95, p=0.035), low PLR (19.5 months vs 14.1 months, HR=0.59, 95% CI 0.35–0.98, p=0.041), high ALI (18.5 months vs 14 months, HR=0.28, 95% CI 0.14–0.57, p<0.001) and high PNI (25 months vs 11.1 months, HR=0.29, 95% CI 0.18–0.47, p<0.001) indicated improved OS outcomes in ES-SCLC patients (Figure 2).
Cox regression analysis included factors including sex, age, smoking status, ECOG PS, extrathoracic metastases, pleura metastases, lung metastases, liver metastases, adrenal gland metastases, bone metastases, brain metastases, NLR, PLR, ALI and PNI. Both univariate and multivariate analysis showed that ECOG PS, liver metastases and PNI were independent prognostic factors for OS (Table S2).
Subgroup Analysis
In subgroup analysis based on baseline characteristics, PFS favored high PNI over low PNI in most subgroups (Figure S2A), whereas the benefit was not statistically significant in female patients, patients without extrathoracic metastases or patients with adrenal gland metastases. Similarly, OS favored high PNI over low PNI in most subgroups (Figure S2B), whereas the benefit was not statistically significant in patients without extrathoracic metastases or patients with adrenal gland metastases.
Receiver Operating Characteristic Curve Analysis
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive values of peripheral blood markers for prognosis. The results indicated that PNI outperformed NLR, PLR and ALI in predicting 6-month PFS with an area under the curve (AUC) of 0.658 and 12-month PFS with an AUC of 0.778 (Figure 3A and B). Furthermore, PNI was superior to NLR, PLR, and ALI in predicting 1-year OS with an AUC of 0.771 and 2-year OS with an AUC of 0.756. (Figure 3C and D)
Time-dependent AUC curves were generated to further compare the predictive accuracy of multiple peripheral blood markers for PFS and OS. As a result, PNI outperformed NLR, PLR and ALI in predicting PFS (Figure 3E) and OS (Figure 3F) throughout the entire observation period.
Nomogram
To better predict the clinical outcomes of ES-SCLC patients, nomograms based on the results of Cox regression analysis were developed. The nomogram in Figure 4A, incorporating PNI and extrathoracic metastasis, could be used to predict PFS. And the calibration curve of the nomogram showed there was good agreement between the predicted and observed PFS probabilitied at 6 and 12 months (Figure 4B). Furthermore, the nomogram in Figure 4C, incorporating ECOG, PNI, and liver metastases, could be used to predict OS. And the calibration curve of the nomogram showed good agreement between the predicted and observed OS probabilitied at 1 and 2 years (Figure 4D).
Immune-Related Adverse Events
Of all patients, 53 patients (46.5%) experienced 5 different IRAEs of any grade, and none experienced high-grade IRAEs. The most common IRAE (any grade) was hypothyroidism (31/114, 27.2%). Other observed IRAEs included pneumonitis (10/114, 8.8%), increased aminotransferase (10/114, 8.8%), rash (8/114, 7.0%) and pruritus (6/114, 5.3%). The median PFS of 53 patients with IRAEs was significantly longer than that of 61 patients without irAEs (8.2 months vs 5.6 months; HR=0.36, 95% CI 0.24–0.56; P<0.001) (Figure S3A). Similarly, patients with irAEs had a better median OS than those without irAEs. (19.8 months vs 14.1 months; HR=0.52, 95% CI 0.32–0.86; P=0.010) (Figure S3B).
IREAs were present in 41 (53.9%) patients in the low PLR group and 39 (55.7%) in the high PNI group, respectively. Univariate analysis revealed a significant association between low PLR (p=0.026) or high PNI (p=0.014) and the occurrence of IRAEs of any grade. Furthermore, multivariate logistic regression analysis indicated that high PNI (p= 0.042) independently predicted the onset of IRAEs (Table 2). Besides, the median PNI of patients with IRAEs was significantly higher compared to those without IRAEs (48.85 vs 45.15, p<0.001) (Figure S4 and Table S3).
 |
Table 2 Peripheral Blood Markers and Immune-Related Adverse Events |
Discussion
To the best of our knowledge, this is the first study to investigate the role of peripheral markers in the prognosis of ES-SCLC patients receiving chemotherapy plus immunotherapy. By comparing NLR, PLR, ALI and PNI, we found for the first time that baseline PNI showed a prominent predictive ability for prognosis in ES-SCLC patients undergoing chemotherapy plus immunotherapy. Furthermore, we developed a PNI-based nomogram that can effectively predict the prognosis of these patients. In addition, it was observed that SCLC patients with a high PNI were more likely to experience IRAEs, and the high incidence of IRAEs was associated with improved PFS and OS in these patients.
The expression level of PD-L1 in tumor tissue has been utilized as a biomarker to assist clinicians in deciding whether to choose immunotherapy in non-small-cell lung cancer.29 However, current clinical trial findings do not support the use of PD-L1 as a predictor of immunotherapy efficacy in SCLC. In IMpower133, there was no significant correlation observed between PD-L1 expression level and long-term survival benefits from immunotherapy.30 And similar conclusions were drawn from KEYNOTE-604 and CASPIAN.9,31
SCLC is typically characterized by high tumor mutational burden (TMB),32 however the predictive role of TMB in immunotherapy efficacy for SCLC patients remains controversial. The CheckMate 032 trial evaluated the efficacy of nivolumab in SCLC patients and observed that those with a high TMB had significantly prolonged PFS and OS.33 However, it was found that TMB level were not associated with the efficacy of atezolizumab in SCLC patients in the IMpower133 trial.30 Therefore, whether TMB could be used as a biomarker to predict the efficacy of immunotherapy in SCLC needs to be verified by further clinical trials.
In addition to tumor tissue biomarkers, there has been growing interest in identifying blood-based biomarkers that can predict the efficacy of immunotherapy in solid tumors. In numerous studies, it has been suggested that NLR,18 PLR,19 ALI34,35 and PNI26 are closely associated with the prognosis of patients with cancer undergoing immunotherapy. However, our study showed that NLR did not serve as a reliable predictor of PFS in patients with SCLC receiving immunotherapy (Figure 1A), and its ability to predict OS was only moderate (Figure 2A). As for PLR, ALI and PNI, these three variables showed statistically significant predictive capacity for PFS and OS in Kaplan-Maier analysis. However, when multivariate analysis was performed, only PNI remained an independent prognostic factor for SCLC patients (Table S1 and Table S2). In addition, PNI, rather than NLR, PLR and ALI, could significantly predict the prognosis (both PFS and OS) of SCLC patients even when the cutoff value was set at the median (Figure S5). Therefore, PNI is superior to NLR, PLR, and ALI in predicting prognosis of SCLC patients receiving immunotherapy.
While immunotherapy brings survival benefits to ES-SCLC patients, it is inevitably accompanied by various IRAEs, which may result in treatment interruption and even more serious harm.36,37 Therefore, it is important to search for potential indicators that can predict IRAEs. However, no reliable predictor of IRAEs in SCLC has been identified so far.36,37 Previous studies have reported an association between PNI and the occurrence of IRAEs in non-small cell lung cancer,38 and our findings suggested a similar association between a high PNI and the occurrence of IRAEs in SCLC. Interestingly, it was also observed that patients who experienced concurrent IRAEs had a favorable prognosis. This phenomenon has been documented in other cancer types undergoing immunotherapy.39,40 The mechanism might be associated with the heightened PNI, indicating an active immune system, which could potentially trigger an exaggerated response upon immunotherapy-induced immune system activation. As a result, an active immune system leaded to a higher incidence of adverse events on the one hand, and a better prognosis on the other hand.
Based on the findings of this study, we cautiously make the following recommendations to clinicians: For ES-SCLC patients with PNI≥45, chemotherapy plus immunotherapy is preferred as the first-line treatment in order to achieve optimal survival outcomes. However, special attention should be paid to the monitoring of potential IRAEs throughout the treatment course, as these patients have a relatively high incidence of IRAEs. For patients with PNI<45, although the benefit of adding a PD-L1 inhibitor to chemotherapy may not be as pronounced as in patients with PNI≥45, and even the median OS (in patients with PNI<45 receiving chemotherapy plus immunotherapy) is similar to what has been reported in previous studies of extensive-stage SCLC patients who received chemotherapy alone. However, there is no evidence to directly suggest that chemotherapy plus immunotherapy is not superior to chemotherapy in these patients. Therefore, the preferred first-line treatment option is still chemotherapy plus immunotherapy, while chemotherapy alone can be considered as an alternative regimen for patients who have economic constraints. Meanwhile, it is important to closely monitor the response to treatment in order to detect resistance early and make adjustments to regimen, as these patients tend to experience disease progression relatively early.
The study also had several limitations that need to be acknowledged. Firstly, it was a retrospective study conducted at a single center, which may have introduced some biases in data analysis. Secondly, PD-L1 inhibitors were approved for the treatment of ES-SCLC in approximately 2020, considering sufficient survival data to be collected, our study only included patients diagnosed in 2020–2021. As a result, the sample size was relatively small. Therefore, it is still necessary to validate our findings in prospective studies with larger sample sizes. Thirdly, comorbidities commonly associated with ES-SCLC, such as cachexia, malnutrition, and infection, could induce alterations in peripheral blood markers. Consequently, the treatment outcome of these patients could be significantly influenced by changes in peripheral blood markers. Given the intricate interplay among these factors, we failed to have a clear elucidation of the specific mechanism. Finally, due to the limited number of cases associated with each specific type of IRAEs, we did not extensively differentiate the correlation between the five IRAEs and peripheral blood markers, nor did we analyze the temporal relationship between the timing of IRAE occurrence and peripheral blood markers, which could be the direction of future research.
Despite the aforementioned limitations, PNI could serve as a cost-effective, non-invasive, user-friendly, and easily interpretable test method. Furthermore, it holds potential in predicting patient prognosis and IRAEs, aiding clinicians in making better clinical treatment decisions.
Conclusion
A high baseline PNI might be associated with improved prognosis and the occurrence of IRAEs in ES-SCLC patients treated with first-line chemoimmunotherapy. The findings of this study could serve as a valuable reference for identifying ES-SCLC patients who could benefit from immunotherapy. Moreover, it is anticipated that these results would provide valuable insights for future prospective studies in this area.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Approval of the study protocol was obtained from the ethics and research committee of Sun Yat-sen University Cancer Center (approval number: B2023-489-01). All patients’ individual consent for this retrospective analysis was waived. All samples were anonymized. We covered patient data confidentially and conducted this study in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank all the patients and their families for their cooperation and participation. Additionally, the authors are thankful to all research staff and coinvestigators involved in this investigation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Wang Q, Gümüş ZH, Colarossi C, et al. SCLC: epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J Thoracic Oncol. 2023;18(1):31–46. doi:10.1016/j.jtho.2022.10.002
2. Megyesfalvi Z, Gay CM, Popper H, et al. Clinical insights into small cell lung cancer: tumor heterogeneity, diagnosis, therapy, and future directions. Ca a Cancer J Clinicians. 2023;73(6):620–652. doi:10.3322/caac.21785
3. Noda K, Nishiwaki Y, Kawahara M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. New Engl J Med. 2002;346(2):85–91. doi:10.1056/NEJMoa003034
4. Okamoto H, Watanabe K, Nishiwaki Y, et al. Phase II study of area under the plasma-concentration-versus-time curve-based carboplatin plus standard-dose intravenous etoposide in elderly patients with small-cell lung cancer. J Clin Oncol. 1999;17(11):3540–3545. doi:10.1200/JCO.1999.17.11.3540
5. Pelayo Alvarez M, Gallego Rubio O, Bonfill Cosp X, Agra Varela Y. Chemotherapy versus best supportive care for extensive small cell lung cancer. Cochrane Database Syst Rev. 2009;1(4):Cd001990.
6. Wolf M, Tebbe S, Fink T. First-line chemotherapy in metastatic small-cell lung cancer (SCLC). Lung Cancer. 2004;45 Suppl 2:S223–234.
7. Zhou T, Zhang Z, Luo F, et al. Comparison of First-Line Treatments for Patients With Extensive-Stage Small Cell Lung Cancer: a Systematic Review and Network Meta-analysis. JAMA network open. 2020;3(10):e2015748. doi:10.1001/jamanetworkopen.2020.15748
8. Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. New Engl J Med. 2018;379(23):2220–2229. doi:10.1056/NEJMoa1809064
9. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, Phase 3 trial. Lancet. 2019;394(10212):1929–1939. doi:10.1016/S0140-6736(19)32222-6
10. Mathieu L, Shah S, Pai-Scherf L, et al. FDA Approval Summary: atezolizumab and Durvalumab in Combination with Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung Cancer. oncologist. 2021;26(5):433–438. doi:10.1002/onco.13752
11. Barrows ED, Blackburn MJ, Liu SV. Evolving role of immunotherapy in small cell lung cancer. Semi Cancer Biol. 2022;86(Pt 3):868–874. doi:10.1016/j.semcancer.2022.02.021
12. Zhang C, Wang H. Accurate treatment of small cell lung cancer: current progress, new challenges and expectations. Biochim. Biophys. Acta, Rev. Cancer. 2022;1877(5):188798. doi:10.1016/j.bbcan.2022.188798
13. Memmott RM, Wolfe AR, Carbone DP, Williams TM. Predictors of Response, Progression-Free Survival, and Overall Survival in Patients With Lung Cancer Treated With Immune Checkpoint Inhibitors. J Thoracic Oncol. 2021;16(7):1086–1098. doi:10.1016/j.jtho.2021.03.017
14. Longo V, Catino A, Montrone M, et al. What Are the Biomarkers for Immunotherapy in SCLC? Int J Mol Sci. 2021;22(20):11123. doi:10.3390/ijms222011123
15. Gelsomino F, Lamberti G, Parisi C, et al. The evolving landscape of immunotherapy in small-cell lung cancer: a focus on predictive biomarkers. Cancer Treat Rev. 2019;79:101887. doi:10.1016/j.ctrv.2019.08.003
16. Hou J, Karin M, Sun B. Targeting cancer-promoting inflammation - have anti-inflammatory therapies come of age? Nat Rev Clin Oncol. 2021;18(5):261–279. doi:10.1038/s41571-020-00459-9
17. Ritter B, Greten FR. Modulating inflammation for cancer therapy. J Exp Med. 2019;216(6):1234–1243. doi:10.1084/jem.20181739
18. Guo Y, Xiang D, Wan J, Yang L, Zheng C. Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: a Meta-Analysis and Systematic Review. Cancers. 2022;14(21):5297. doi:10.3390/cancers14215297
19. Xu H, He A, Liu A, Tong W, Cao D. Evaluation of the prognostic role of platelet-lymphocyte ratio in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Int Immunopharmacol. 2019;77:105957. doi:10.1016/j.intimp.2019.105957
20. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13(1):158. doi:10.1186/1471-2407-13-158
21. Wang Z, Wang Y, Zhang X, Zhang T. Pretreatment prognostic nutritional index as a prognostic factor in lung cancer: review and meta-analysis. Int j Clin Chem. 2018;486:303–310. doi:10.1016/j.cca.2018.08.030
22. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
23. Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi:10.1186/s40425-018-0383-1
24. Gu XB, Tian T, Tian XJ, Zhang XJ. Prognostic significance of neutrophil-to-lymphocyte ratio in non-small cell lung cancer: a meta-analysis. Sci Rep. 2015;5(1):12493. doi:10.1038/srep12493
25. Gu X, Sun S, Gao XS, et al. Prognostic value of platelet to lymphocyte ratio in non-small cell lung cancer: evidence from 3430 patients. Sci Rep. 2016;6(1):23893. doi:10.1038/srep23893
26. Johannet P, Sawyers A, Qian Y, et al. Baseline prognostic nutritional index and changes in pretreatment body mass index associate with immunotherapy response in patients with advanced cancer. J Immunother Cancer. 2020;8(2):e001674. doi:10.1136/jitc-2020-001674
27. Watanabe I, Kanauchi N, Watanabe H. Preoperative prognostic nutritional index as a predictor of outcomes in elderly patients after surgery for lung cancer. Jpn J Clin Oncol. 2018;48(4):382–387. doi:10.1093/jjco/hyy014
28. National Institutes of Health NCI. Common Terminology Criteria for Adverse Events (CTCAE), version 4.03; 2009. Available at https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf.
29. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer. 2020;126(2):260–270. doi:10.1002/cncr.32468
30. Liu SV, Reck M, Mansfield AS, et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol. 2021;39(6):619–630. doi:10.1200/JCO.20.01055
31. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol. 2020;38(21):2369–2379. doi:10.1200/JCO.20.00793
32. Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nature Reviews Disease Primers. 2021;7(1):3. doi:10.1038/s41572-020-00235-0
33. Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell. 2018;33(5):853–861.e854. doi:10.1016/j.ccell.2018.04.001
34. Li Q, Ma F, Wang JF. Advanced lung cancer inflammation index predicts survival outcomes of hepatocellular carcinoma patients receiving immunotherapy. Front Oncol. 2023;13:997314. doi:10.3389/fonc.2023.997314
35. Mountzios G, Samantas E, Senghas K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open. 2021;6(5):100254. doi:10.1016/j.esmoop.2021.100254
36. Hou W, Zhou X, Yi C, Zhu H. Immune Check Point Inhibitors and Immune-Related Adverse Events in Small Cell Lung Cancer. Front Oncol. 2021;11:604227. doi:10.3389/fonc.2021.604227
37. Fu Y, Zheng Y, Wang PP, Ding ZY. Toxicities of Immunotherapy for Small Cell Lung Cancer. Front Oncol. 2021;11:603658. doi:10.3389/fonc.2021.603658
38. Peng L, Wang Y, Liu F, et al. Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–1822. doi:10.1007/s00262-020-02585-w
39. Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi:10.1186/s40425-019-0805-8
40. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–148. doi:10.1016/j.ejca.2015.11.016
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




