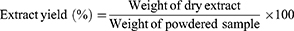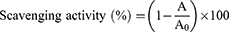Back to Journals » Journal of Experimental Pharmacology » Volume 16
Phytochemical Investigation and in vitro Antimicrobial and Antioxidant Activities Evaluation of Erianthemum aethiopicum Wiens and Polhill
Authors Gonfa T , Temesgen A, Kiros T , Muthusaravanan S , Erba Urgessa O , Teklu T
Received 28 November 2023
Accepted for publication 1 February 2024
Published 13 February 2024 Volume 2024:16 Pages 71—80
DOI https://doi.org/10.2147/JEP.S452098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Teshome Gonfa,1 Ayalew Temesgen,1 Tsegu Kiros,1 Sivasubramanian Muthusaravanan,1 Olyad Erba Urgessa,2 Tadele Teklu2
1Department of Chemistry, College of Natural and Computational Sciences, Haramaya University, Dire Dawa, Ethiopia; 2School of Biological Sciences and Biotechnology, College of Natural and Computational Sciences, Haramaya University, Dire Dawa, Ethiopia
Correspondence: Teshome Gonfa; Tsegu Kiros, Department of Chemistry, Haramaya University, P. O. Box: 138, Dire Dawa, Oromia, Ethiopia, Tel +251936566688, Email [email protected]; [email protected]
Background: Erianthemum aethiopicum Wiens and Polhill (Loranthaceae) is a parasitic plant native to north eastern Africa and Ethiopia. In Ethiopia, it is traditionally used to treat breast swelling, mastitis, morning illnesses and vomiting.
Objective: This study aimed to screen the main phytochemical constituents; determine the total amounts of phenolics, flavonoids, and tannins; and evaluate the antimicrobial (against Escherichia coli, Staphylococcus sciuri, Candida glaebosa and Cryptococcus albidus) and antioxidant (against DPPH radical and ferric ion) activities of E. aethiopicum leaves extracts.
Methods: Powdered E. aethiopicum leaves were macerated using n-hexane, chloroform, ethyl acetate, ethanol, and methanol. All crude extracts were qualitatively screened for phytochemical identification. The total phenolic, flavonoid, and condensed tannin contents of the chloroform, ethanol, and methanol extracts were determined by UV-Vis spectrophotometry. The n-hexane, chloroform, and methanol extracts were evaluated for their antimicrobial activity against the aforementioned microbes using agar disc diffusion and broth micro-dilution techniques. Chloroform, ethanol, and methanol extracts were also evaluated for antioxidant activity by DPPH and ferric ion reduction antioxidant power (FRAP) assays.
Results: Methanol (17.56 ± 16%) and ethanol (16.45 ± 19%) showed better extraction efficiency. Flavonoids, polyphenols, tannins, terpenoids, saponins, and sterols were detected in all extracts. The highest total content of phenolics (22.63 ± 0.69 mgGAE/gDCE), flavonoids (5.38 ± 0.52 mgCE/gDCE) and tannins (39.18 ± 38 mg CE/g DCE), as milligram of gallic acid and catechin per gram of dried crude extract, were recorded in the methanolic extract. The methanolic extract also presented best anti -DPPH strength (IC50, 4.31 μg/mL) and ferric ion reduction power (absorbance of 0.71) though found weak compared to the ascorbic acid (IC50 of 0.49 μg/mL and absorbance of 0.93, respectively).
Conclusion: All evaluated extracts displayed antifungal activity against both Cryptococcus albidus and Candida glaebosa strains (minimum inhibitory concentration values of 12.5– 25 mg/mL), whereas they were found to have negligible activity against all tested bacterial strains. This report provides preliminary information for further phytochemical investigation of Erianthemum aethiopicum to isolate potential antioxidant and antifungal compounds.
Keywords: Erianthemum aethiopicum, antimicrobial assay, DPPH assay, FRAP assay, phytochemical analysis, minimum inhibitory concentration
Introduction
Since time immemorial, natural sources, mainly medicinal plants, have been the guardians of millions of lives. The use of medicinal plants in local medicine is still in its place, especially in the developing world where modern medicine is inaccessible and unaffordable.1 The wide array of bioactivities of medicinal plants including anticancer, antimicrobial, antioxidant and anti-inflammatory are attributed to the major phytochemical constituents such as polyphenols and flavonoids. Antioxidant agents originating from plants can block the production of oxidants, thereby preventing oxidative stress-causing illnesses including cardiovascular syndromes, diabetes, cancer tumors, hypertension, skin irritation, and neural disorders.2
In Ethiopia, close to 80% of the people rely on local medicine, with 95% plus of the formulation being based on medicinal plants.3,4 It is believed that approximately 6000–7000 plant sources are estimated to be found in Ethiopian flora of which 1000 of them are used traditionally to alleviate various human and animal diseases.5 Even though the reality is this, most of the previous studies conducted on these medicinal plants remained mainly as a survey of ethno-botanical aspects of the plants. Even studies that focused on the bioactivity and phytochemical analysis of plants were few in number and limited to some plant species. The societies of the Eastern and Western parts of Hararghe, particularly those around the Harla and Dengego valleys, have used plenty of plant species directly for human and animal healthcare.6
Erianthemum aethiopicum Wiens and Polhill (genus: Erianthemum, family: Loranthaceae), one of the members of African mistletoes, is a parasitic climber plant native to North-Eastern Africa and indigenous to Ethiopia. In Ethiopia, it is known by the vernacular name “Degalo serkema” (Afan Oromo), and has tomentose twigs with white branches and short hairs and grows on Acacia or Commiphora woodland. It is extensively distributed in tropical areas of East African countries, mainly Ethiopia, Kenya, and Somalia. In the prehistoric town of Harla in Eastern Ethiopia, the leaves of E. aethiopicum are used as therapeutic agents to cure breast swelling, mastitis, morning sickness, and vomiting.6,7 However, to our knowledge, no previous scientific studies have been conducted on the screening and determination of the main classes of phytochemicals and assessment of the antimicrobial and antioxidant action of E. aethiopicum leaves. This is the first study to investigate the major phytochemicals and examine in vitro antimicrobial and antioxidant effects of different E. aethiopicum leaves extracts.
Materials and Methods
Plant Material
Erianthemum aethiopicum leaves (Figure 1) were collected from prehistoric Harla town, Dire Dewa, Eastern Ethiopia (9° 27′, 9° 39′N latitude, 41° 38′, 42° 20′E longitude), in February 2022. The taxonomic identity of the plant was confirmed at the herbarium of Haramaya University, Ethiopia, and a representative sample with voucher number AHU196 was retained. Freshly collected leaves were air-dried at an ambient temperature under illuminated conditions. The dried samples were then coarsely milled using a grinding apparatus, packed in capped glass bottles, and stored at 4°C °C for subsequent experiments.
 |
Figure 1 Photo of Erianthemum aethiopicum plant (By Teshome Gonfa, February, 2022). |
Consumable Materials
Methanol (MeOH, 99.8%), ethanol (EtOH, 99.5%), chloroform (99.8% AR), n-hexane (AR), ethyl acetate (EtOAc), hydrochloric acid (HCl, 37%), Folin-Ciocalteu’s phenol reagent (2 N), DPPH (2, 2-Diphenyl picrylhydrazyl), gallic acid, catechin, ascorbic acid, potassium ferric cyanide, sodium nitrite, aluminum chloride (AlCl3), trichloroacetic acid (Cl3COOH), sodium hydroxide (NaOH), ferric chloride (FeCl3), sodium carbonate (Na2CO3), vanillin, Mueller-Hinton agar (MHA), Potato Dextrose agar (PDA), and dimethyl sulfoxide (DMSO) were used as chemicals and reagents. Whatman No.1 filter paper (Whatman International Ltd, England), a Rota evaporator (rotary vacuum, Jainsons, India), and a UV-Vis spectrophotometer (Cecil CE4001 UV/Vis, Cambridge, England) were used for extraction filtration, solvent evaporation, and absorbance measurements, respectively.
Microorganism Culture Collection
The Ethiopian Public Health Institute (EPHI) provided us with two bacterial pathogens (Escherichia coli ATCC 12435 and Staphylococcus sciuris sciuri ATCC 25923) and two fungal pathogens (Candida glaebosa ATCC 76484 and Cryptococcus albidus ATCC 34140).
Extraction Process
Powdered leaves samples of E. aethiopicum (40.00 g) were macerated in n-hexane, chloroform, ethyl acetate, ethanol, and methanol (300 mL each) and extracted for two days on an orbital shaker to avoid any temperature under shedding. Each extract solution was filtered followed by solvent evaporation. The extraction efficiency of each solvent was determined in terms of the crude extract yield percentage, calculated using the following formula (1):
Qualitative Phytochemical Profiling
All leaves extracts were qualitatively screened for the presence or absence of the major classes of phytochemical constituents. The screening test was performed using the commonly applied procedures described previously.8–10
Quantitative Determination of Phytochemicals
Determination of Total Phenolic Content (TPC)
The TPC of the chloroform, ethanol, and methanol extracts was determined at a wavelength of 765 nm, following the technique described by Siddiqui et al11 and the detailed procedure described by Gonfa et el.12 The results were calculated from the graph obtained and presented as milligrams of gallic acid equivalent per gram of dried crude extract (mg GAE/g DCE) in triplicates.
Measurement of Total Flavonoid Content (TFC)
The TFC of the three extracts mentioned above were estimated using the aluminum chloride colorimetric method13 and the detailed procedure was similar to that described by Gonfa et el.12 The absorbance value of each tested analyte was recorded at a wavelength of 415 nm. The TFC value, recorded in triplicate, was calculated as milligrams of catechin equivalent per gram of dried crude extract (mg CE/g DCE).
Total Condensed Tannin Content (TCTC) Determination
The TCTC content of each extract was analyzed using the modified Broadhurst method with reference to the standard catechin.12,14 The content was determined using absorbance values recorded at 515 nm using a UV-Vis spectrophotometer and expressed in terms of milligram equivalents of catechin (mg CE/g DCE) per dried crude extract. The experiments were conducted in triplicates.
In vitro Antimicrobial Activity Evaluation
Prior to evaluation of the antimicrobial activity assay, each tested organism was sub-cultured and the respective inoculum was standardized based on the standard procedure.15 A paper disc diffusion assay was followed to study the antimicrobial effectiveness of the extracts on selected pathogens as per the clinical and laboratory standards institute (CLSI).16 Then, an original solution of each analyte was prepared by reconstituting dry extract (g) in DMSO (mL), followed by the preparation of three corresponding dilutions (50, 75, 125 mg/mL) using the same solvent. MHA and PDA media were prepared according to the instructions described for the respective containers. Then, 100 μL of each plant extract solution was infused onto individual filter paper disc (6 mm width) and allowed to dry at room temperature. The impregnated discs were aseptically placed on the surfaces of MHA and PDA Petri plates. The plates were then sealed with Parafilm and incubated at 37°C for 18 h for bacterial pathogens and at 35°C for 24 h for yeast pathogens. Commercial ciprofloxacin (5 μg) and fluconazole (5 μg) discs were used as standard antibacterial and antifungal agents, respectively. DMSO was used as the negative control. After incubation, the diameters of the inhibited areas were recorded using calipers (in mm). The experiment was repeated thrice.
Minimum Inhibitory Concentration (MIC) Test
The MIC against fungal pathogens was determined using the broth microdilution technique.17 Two-fold serial dilutions of the extracts showing antifungal activity up to the lowest concentration (50 mg/mL) in the disc diffusion method were prepared in a plastic test tube containing the appropriate broth medium to obtain various concentrations (50, 25, 12.5, 6.25, and 3.125 mg/mL). Fungal yeast inoculums were added to give 0.5 x103–2.5 × 103 CFU/mL. Each tube was covered with sterile cotton and incubated at 35 °C for 48 h. The test tube, which showed a clear appearance, indicated no fungal growth, and the concentration attributed to fungal growth inhibition was considered to be the MIC (mg/mL).
In vitro Antioxidant Activity Examination
Chloroform, methanol, and ethanol leaves extracts enriched with major secondary metabolites were assessed for their antioxidative potential using DPPH free radical and potassium ferric ion reduction antioxidant power (PFRAP) assays.
Anti-DPPH Activity
The antioxidative ability against DPPH oxidant of the aforementioned three extracts (chloroform, ethanol, and methanol), and the positive control (ascorbic acid) was examined following detailed procedures described elsewhere.12,18 Six serially diluted solutions (5, 12.5, 25, 50, 75, and 100 μg/mL) of each extract were derived from the corresponding original solution (0.5 mg/mL). Next, 2 mL of fresh DPPH solution (0.004% g/mL in MeOH) was added to 2 mL of each dilution and incubated at 25 °C for half an hour. The absorbance of each tested analyte, including the DPPH solution, was measured three times at a wavelength of 517 nm. The antioxidative action of each solution against DPPH was determined by the percentage of scavenging activity, calculated using Equation (2):
where A0 and A represent the absorbance of the DPPH solution and target analyte, respectively.
Potassium Ferric Ion Reducing Power (PFRAP) Analysis
The PFRAP of each extract was investigated using the K3Fe(CN)6-FeCl3 procedure with six dilutions (5, 12.5, 25, 50, 75, and 100 μg/mL), and the absorbance values were measured at a wavelength of 700 nm.19 Ascorbic acid was employed as an antioxidant drug, and the overall antioxidative activity against ferric ions of each analyte was presented in terms of absorbance values.
Data Analysis
Multivariate analysis using SPSS software version 20 was used to calculate the mean ± standard deviation (SD) of individual values recorded from triplicate experimental analyses. One-way analysis of variance (ANOVA) was used to compare significant differences in the obtained results at a confidence level of 95%.
Results and Discussion
Percentage of Crude Extract Yield
In this study, the alcoholic solvents provided the highest percentage of extract yield with 17.56 ± 16% for methanol and 16.45 ± 19% for ethanol. The next lowest extract yield was recorded for chloroform (4.65 ± 11%), ethyl acetate (3.03 ± 0.6%), and n-hexane (1.73 ± 0.7%). This indicates that the plant may be enriched in polar phytochemical constituents. This was supported by qualitative phytochemical profiling, although the number of phytochemicals screened was limited. Therefore, methanol and ethanol are better solvents for the extraction of phytochemicals from the leaves powder of E. aethiopicum. In line with this, the same solvents were suggested to exploit polar compounds, such as flavonoids and phenolics (Amalraj et al, 2021).20
Qualitative Phytochemical Profiles
The preliminary phytochemical profiles of various leaves extracts are presented in Table 1. Flavonoids, phenolics, and saponins were detected in all, except ethyl acetate, extracts. Phytosterols and terpenoids were positively screened for all extracts. However, alkaloids, free anthraquinones, and coumarins were absent in all extracts. The methanol and ethanol extracts demonstrated a strong presence of the tested class of compounds, both in number and quantity. The present phytochemical screening results indicate that the ethnomedicinal uses of E. aethiopicum leaves can be attributed to the presence of flavonoids, phenolics, terpenoids, saponins, and tannins (Table 1).
 |
Table 1 Qualitative Chemical Profiles of Five Leaves Extracts |
Total Contents of Phenolics, Flavonoids and Tannins
The concentrations of flavonoids, phenolics, and tannins in each extract are presented in Table 2. The total flavonoids content (TFC) of each extract was determined as milligrams of catechin equivalent per gram of dried crude extract (mg CE/g DCE) using the obtained equation (y = 0.003x + 0.2426; R2 = 0.9938). Accordingly, the highest TFC content (5.38 ± 0.52 mgCE/1gDCE) was observed in the methanol extract followed by the ethanol (4.12 ± 0.10 mgCE/1gDCE) and chloroform (2.16 ± 0.13 mgCE/1gDCE) extracts (Table 2). The TFC was found to be significantly different (P ≤ 0.05). Based on the generated linear regression equation (y = 0.0018x + 0.0893, R2 = 0.9972) of the gallic acid standard curve, the extracts showed a statistically significant difference (P< 0.05) in the total polyphenolics content (TPC) ranged from 13.93 ± 0.86–22.63 ± 0.69mgGAE/gDCE with the highest content recorded in the methanol extract (22.63 ± 0.69), followed by ethanol (21. 52 ± 0.56) (Table 2).
 |
Table 2 Total Phenolic, Flavonoid and Tannin Contents (Mean ± SD, n = 3) of Three Different Leaves Extracts |
Similarly, the total condensed tannin content (TCTC) in each extract was estimated using the equation: y = 0.0008x+ 0.0826 (R2= 0.995). The highest TCTC value was recorded for the methanolic extract (39.18 ± 38 mgCE/gDCE), followed by the ethanol extract (12.77 ± 0.14). In general, the obtained results (Table 2) were found to be in line with the intensity of the qualitative phytochemical profiles obtained (Table 1), which revealed that flavonoids, polyphenolics, and tannins were strongly detected in both methanol and ethanol extracts.
Antioxidant Activity Determination
Anti-DPPH Activity
The percentage scavenging activity and IC50 values against DPPH free radicals of the chloroform, ethanol, and methanol extracts are presented in Figure 2 and Table 3. The results demonstrated that the methanolic extract displayed the best anti-DPPH activity (IC50 value of 4.31μg/mL), which was slightly comparable to that of ascorbic acid (IC50 value of 0.49 µg/mL). Whereas the ethanolic and chloroform extracts showed a weaker DPPH inhibitory effect with higher IC50 values (6.73 µg/mL and 12.75µg/mL, respectively) compared to methanolic extract and ascorbic acid.
 |
Table 3 DPPH Oxidant Scavenging Activity Percentage (Mean ±SD, n=3) and IC50 Values of Three Different Leaves Extracts |
 |
Figure 2 DPPH scavenging percentage (%) against six concentrations (µg/mL) of three leaves extracts (chloroform, ethanol and methanol) and ascorbic acid. |
The DPPH solution (0.004%, (m/v), used for the estimation of antioxidant activity, contains a purple free radical that changes into a yellow color that is stable when reacting with plant extracts enriched with antioxidants.21 Our finding suggested that the alcoholic extracts demonstrated the greatest scavenging percentage against DPPH oxidant, which was in agreement with the highest total phenolic and flavonoid contents. This indicates that the plant is a potential source of natural antioxidants.
FRAP Assay
The ferric ion reduction antioxidative potentials recorded at various concentrations of the tested extracts are shown in Figure 3. The methanolic and ethanolic extracts presented the best reductive capacity with higher absorbance values (0.71 and 0.42, respectively), although it was found to be weaker than that of ascorbic acid (0.93) at a similar dose (200 μg/mL). Generally, a significant difference (P ≤ 0.05) in the FRAP mean value was observed between the tested extracts according to the obtained statistical data.
 |
Figure 3 Absorbance of FRAP versus various dilutions (μg/mL) of three leaves extracts (chloroform, ethanol and methanol) and ascorbic acid. |
The bright yellow color of ferric ions (Fe3+) in the ferricyanide complex ([K3Fe(CN)6]) was changed to dark blue ferrous ions (Fe2+) depending on the concentration of the plant extract.
Several studies have demonstrated that plants enriched with polyphenols, including flavonoids, are interesting classes of bioactive phytochemicals that exhibit antimastitis/breast related infections,22,23 preventing morning sickness24,25 and vomiting during pregnancy.26,27 Hence, E. aethiopicum is a source of numerous phytochemicals and polyphenols that possess antioxidant activities and traditionally possess the aforementioned activities, supporting the present study.
In vitro Antimicrobial Activity Evaluation
The antimicrobial activities of the n-hexane, chloroform, and methanol extracts against the tested bacteria (E. coli and S. sciuris) and fungal pathogens (C. albidus and C. glaebosa) were presented in Table 4 and Figures S1–S4 (see Supplementary Materials for inhibition zone images). The result showed that all the extracts showed a positive inhibitory action on both C. albidus and C. glaebosa fungal species up to 50 mg/mL dose with respective diameter of cleared zone values of 7.25 ± 0.25–8.83 ± 0.38 mm and 7.25 ± 0.25–10.17 ± 0.38 mm. The n-hexane and methanol extracts revealed improved action on C. albidus (8.83 ± 0.38 to 12.17 ± 0.58 mm) and C. glaebosa (10.17 ± 0.38 to 14.83 ± 0.80 mm), respectively, at concentrations of 50–125 mg/mL. However, their activity was still weak compared to that of the fluconazole standard (22.92 ± 0.72 mm). Whereas not all the tested extracts (n-hexane, chloroform and methanol) showed any activity against the bacterial species at all concentrations, except the n-hexane extract which tried to block spreading of E. coli bacterium (7.00 ± 0.38 to 8.08 ± 0.24 mm) up to 50 mg/mL.
 |
Table 4 In vitro Antimicrobial Activity Diameter of Cleared Zone Values (Mean ± SD, Millimeter) of Three Different Leaves Extracts Against Human Pathogenic Fungal and Bacterial Strains |
MIC Test
All the tested extracts were subjected to antifungal activity evaluation to determine their MIC values. This was because they showed good activity against the two fungal strains at a minimum concentration (50 mg/mL) using the agar disc diffusion method discussed above. Accordingly, both the methanol and n-hexane extracts showed MIC of 12.5 mg/mL, while the chloroform extract exhibited 25 mg/mL against both C. albidus and C. glaebosa fungal species.
Overall, The current promising antifungal activity of n-hexane extract could be due to the presence of various important phytochemicals including terpenoids, phytosterols, flavonoids, and phenolic acids.28–30 The activity observed in methanolic and chloroform extracts may also be attributed to the flavonoids and polyphenolic constituents.31 Congruently, previous studies also reported that fungal pathogens cause infection in or around the human breast,32–34 and nausea and vomiting.35–37 Therefore, this is the first report on the antifungal activities of the E. aethiopicum leaves extracts, which support the traditional claims or the folkloric usage of this plant and could be a source of novel antifungal drugs.
Conclusion
In the present study, methanol and ethanol extracts showed better extraction efficiency by providing a comparatively higher extract yield. Flavonoids, phenolics, phytosterols, tannins, and saponins were observed in almost all extracts, with a strong presence observed in the alcoholic extracts. The highest total flavonoid, phenolic, and tannin contents were recorded in the methanol and ethanol extracts. The methanol extract showed the highest antioxidant activity against DPPH, although it was not comparable to that of ascorbic acid. All tested extracts presented good antifungal action against both C. albidus and C. glaebosa species. However, against the bacterial species, no extracts showed any activity at all concentrations, except the n-hexane extract, which inhibited the growth of E. coli bacterium up to 50 mg/mL. In general, this study concluded that methanol and ethanol could be used as extraction solvents for target compounds, such as flavonoids and phenolics, from E. aethiopicum leaves. This plant could also be considered as a potential source of antifungal agents. Therefore, as this study is reported here for the first time, it can be used as preliminary information to isolate bioactive compounds and evaluate additional biological activities of E. aethiopicum leaves.
Acknowledgments
The authors acknowledge the Research and Extension Office of Haramaya University for financial support. They would also like to thank the Department of Chemistry and School of Biology and Biotechnology, Haramaya University, for the laboratory facilities and the Ethiopia Institute of Biodiversity for providing pathogen isolates.
Funding
This research was supported by the Research and Extension Office of the Vice President of Haramaya University, Ethiopia [grant number HURG-2020-06-02-11].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Iordache AM, Nechita C, Podea P, et al. Comparative amino acid profile and antioxidant activity in sixteen plant extracts from Transylvania, Romania. Plants. 2023;12(11):2183. doi:10.3390/plants12112183
2. Koval D, Plocková M, Kyselka J, Skřivan P, Sluková M. Buckwheat secondary metabolites: potential antifungal agents. J Agricul Food Chem. 2020;68(42):11631–11643. doi:10.1021/acs.jafc.0c04538
3. Assefa B, Glatzel G, Buchmann C. Ethnomedicinal uses of Hagenia abyssinica (Bruce) JF Gmel. Among rural communities of Ethiopia. J Ethnobiol Ethnomed. 2010;6(1):1–10. doi:10.1186/1746-4269-6-20
4. Demie G, Negash M, Awas T. Ethnobotanical study of medicinal plants used by indigenous people in and around Dirre Sheikh Hussein heritage site of South-eastern Ethiopia. J Ethnopharmacol. 2018;220:87–93. doi:10.1016/j.jep.2018.03.033
5. Yineger H, Yewhalaw D. Traditional medicinal plant knowledge and use by local healers in Sekoru District, Jimma Zone, Southwestern Ethiopia. J Ethnobiol Ethnomed. 2007;3(1):1–7. doi:10.1186/1746-4269-3-24
6. Belayneh A, Bussa NF. Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):1–17. doi:10.1186/1746-4269-10-18
7. Gairola S, Bhatt A, Govender Y, Baijnath H, Procheş Ş, Ramdhani S. Incidence and intensity of tree infestation by the mistletoe Erianthemum dregei (Eckl. & Zeyh.) V. Tieghem in Durban, South Africa. Urban Forest Urban Green. 2013;12(3):315–322. doi:10.1016/j.ufug.2013.03.012
8. Harborne A. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. Springer science & business media; 1998.
9. María R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505. doi:10.1016/j.jksus.2017.03.009
10. Kumar J, Kaur A, Narang P. Phytochemical screening and metal binding studies on floral extract of Solanum nigrum. Mater Today. 2020;26:3332–3336.
11. Siddiqui N, Rauf A, Latif A, Mahmood Z. Spectrophotometric determination of the total phenolic content, spectral and fluorescence study of the herbal Unani drug Gul-e-Zoofa (Nepeta bracteata Benth). J Taibah Univ Sci. 2017;12(4):360–363. doi:10.1016/j.jtumed.2016.11.006
12. Gonfa T, Teketle S, Kiros T, Yildiz F. Effect of extraction solvent on qualitative and quantitative analysis of major phyto-constituents and in-vitro antioxidant activity evaluation of Cadaba rotundifolia Forssk leaf extracts. Cogent Food Agric. 2020;6(1):1853867. doi:10.1080/23311932.2020.1853867
13. Truong D-H, Nguyen DH, Ta NTA, Bui AV, Do TH, Nguyen HC. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Qual. 2019;3:2019.
14. Medini F, Fellah H, Ksouri R, Abdelly C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J Taibah Univ Sci. 2014;8(3):216–224. doi:10.1016/j.jtusci.2014.01.003
15. Rangkadilok N, Tongchusak S, Boonhok R, et al. In vitro antifungal activities of longan (Dimocarpus longan Lour). Seed Extract Fitoterapia. 2012;83(3):545–553. doi:10.1016/j.fitote.2011.12.023
16. Fothergill AW. Antifungal susceptibility testing: clinical laboratory and standards institute (CLSI) methods. In: Interactions of Yeasts, Moulds, and Antifungal Agents: How to Detect Resistance. Springer; 2011:65–74.
17. Gullo FP, Sardi JC, Santos VA, et al. Antifungal activity of maytenin and pristimerin. Evid Based Compl Alter Med. 2012;2012:1. doi:10.1155/2012/340787
18. Khorasani Esmaeili A, Mat Taha R, Mohajer S, Banisalam B. Antioxidant activity and total phenolic and flavonoid content of various solvent extracts from in vivo and in vitro grown Trifolium pratense L. Biomed Res Int. 2015;2015:2. doi:10.1155/2015/643285
19. Hou W-C, Lin R-D, Cheng K-T, et al. Free radical-scavenging activity of Taiwanese native plants. Phytomedicine. 2003;10(2–3):170–175. doi:10.1078/094471103321659898
20. Amalraj S, Mariyammal V, Murugan R, Gurav SS, Krupa J, Ayyanar M. Comparative evaluation on chemical composition, in vitro antioxidant, antidiabetic and antibacterial activities of various solvent extracts of Dregea volubilis leaves. S Afr J Bot. 2021;138:115–123. doi:10.1016/j.sajb.2020.12.013
21. Akhtar MS, Hossain MA, Said SA. Isolation and characterization of antimicrobial compound from the stem-bark of the traditionally used medicinal plant Adenium obesum. J Traditional Complementary Med. 2017;7(3):296–300. doi:10.1016/j.jtcme.2016.08.003
22. Li W, Guo Y, Zhang C, et al. Dietary phytochemicals and cancer chemoprevention: a perspective on oxidative stress, inflammation, and epigenetics. Chem Res Toxicol. 2016;29(12):2071–2095. doi:10.1021/acs.chemrestox.6b00413
23. Moga MA, Dimienescu OG, Bălan A, et al. Pharmacological and therapeutic properties of Punica granatum phytochemicals: possible roles in breast cancer. Molecules. 2021;26(4):1054. doi:10.3390/molecules26041054
24. Bisht D, Kumar D, Kumar D, Dua K, Chellappan DK. Phytochemistry and pharmacological activity of the genus Artemisia. Arch Pharmacal Res. 2021;44(5):439–474. doi:10.1007/s12272-021-01328-4
25. Malode GP, Parbat AY, Shaikh AR, Panchale WA, Manwar JV, Bakal RL. Phytochemistry, pharmacology and botanical aspects of Murraya Koenigii in the search for molecules with bioactive potential-A review. GSC Adv Res Rev. 2021;6(3):143–155. doi:10.30574/gscarr.2021.6.3.0055
26. Sanwal SK, Rai N, Singh J, Buragohain J. Antioxidant phytochemicals and gingerol content in diploid and tetraploid clones of ginger (Zingiber officinale Roscoe). Sci Hortic. 2010;124(2):280–285. doi:10.1016/j.scienta.2010.01.003
27. Suo S, Lai Y, Li M, et al. Phytochemicals, pharmacology, clinical application, patents, and products of Amomi fructus. Food and Chemical Toxicology. 2018;119:31–36. doi:10.1016/j.fct.2018.05.051
28. Garcıa VN, Gonzalez A, Fuentes M, et al. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003;87(1):85–88. doi:10.1016/S0378-8741(03)00114-4
29. Liu Q, Luyten W, Pellens K, et al. Antifungal activity in plants from Chinese traditional and folk medicine. J Ethnopharmacol. 2012;143(3):772–778. doi:10.1016/j.jep.2012.06.019
30. Javaid A, Amin M. Antifungal activity of methanol and n-hexane extracts of three Chenopodium species against Macrophomina phaseolina. Nat Product Res. 2009;23(12):1120–1127. doi:10.1080/14786410802617433
31. Singh M, Govindarajan R, Nath V, Rawat AKS, Mehrotra S. Antimicrobial, wound healing and antioxidant activity of Plagiochasma appendiculatum Lehm. Et Lind. J Ethnopharmacol. 2006;107(1):67–72. doi:10.1016/j.jep.2006.02.007
32. Reddy BT, Torres HA, Kontoyiannis DP. Breast implant infection caused by Trichosporon beigelii. Scand J Infect Dis. 2002;34(2):143–144. doi:10.1080/00365540110026895
33. Guarro J, Chander J, Alvarez E, et al. Apophysomyces variabilis infections in humans. Emerg Infect Dis. 2011;17(1):134. doi:10.3201/eid1701.101139
34. Kuhn N, Homsy C. Rare presentation of breast implant infection and breast implant illness caused by Penicillium species. Eplasty. 2022;2022:22.
35. Samaranayake LP, Cheung K, Samaranayake YH. Candidiasis and other fungal diseases of the mouth. Dermatol Ther. 2002;15(3):251–269. doi:10.1046/j.1529-8019.2002.01533.x
36. Karlson-Stiber C, Persson H. Cytotoxic fungi—an overview. Toxicon. 2003;42(4):339–349. doi:10.1016/S0041-0101(03)00238-1
37. El-Shabrawi MH, Kamal NM, Jouini R, Al-Harbi A, Voigt K, Al-Malki T. Gastrointestinal basidiobolomycosis: an emerging fungal infection causing bowel perforation in a child. J Med Microbiol. 2011;60(9):1395–1402. doi:10.1099/jmm.0.028613-0
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.