Back to Journals » Drug Design, Development and Therapy » Volume 13
Neonatal outcomes and congenital malformations in children born after dydrogesterone application in progestin-primed ovarian stimulation protocol for IVF: a retrospective cohort study
Authors Huang J, Xie Q , Lin J, Lu X, Wang N, Gao H, Cai R, Kuang Y
Received 27 March 2019
Accepted for publication 11 July 2019
Published 26 July 2019 Volume 2019:13 Pages 2553—2563
DOI https://doi.org/10.2147/DDDT.S210228
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Jialyu Huang,* Qin Xie,* Jiaying Lin, Xuefeng Lu, Ningling Wang, Hongyuan Gao, Renfei Cai, Yanping Kuang
Department of Assisted Reproduction, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, People’s Republic of China
*These authors contributed equally to this work
Purpose: Dydrogesterone (DYG) has been demonstrated to be an alternative progestin in the progestin-primed ovarian stimulation (PPOS) protocol with comparable oocyte retrieval and pregnancy outcomes. However, its safety regarding neonatal outcomes and congenital malformations is still unclear.
Patients and methods: This retrospective cohort study included 3556 live-born infants after in vitro fertilization and vitrified embryo transfer cycles using the DYG + human menopausal gonadotropin (hMG) protocol (n=1429) or gonadotropin-releasing hormone (GnRH)-agonist short protocol (n=2127) from January 2014 to December 2017. Newborn information was gathered from standardized follow-up questionnaires and/or access to medical records within 7 days after birth. Associations between ovarian stimulation protocols and outcome measures were analyzed by binary logistic regression after adjusting for confounding factors.
Results: In both singletons and twins, birth characteristics regarding mode of delivery, newborn gender, gestational age, birthweight, length at birth and Z-scores were comparable between the two protocols. For adverse neonatal outcomes, the two protocols showed no significant differences on the rates of low birthweight, very low birthweight, preterm birth, very preterm birth, small-for-gestational age, large-for-gestational age and early neonatal death after adjustment. Furthermore, the incidence of major congenital malformations in the DYG + hMG protocol (1.12%) was similar to that in the GnRH-agonist short protocol (1.08%), with the adjusted odds ratio of 0.98 (95% confidence interval [CI]: 0.40–2.39) and 0.90 (95% CI: 0.33–2.41) in singletons and twins, respectively.
Conclusion: Our data suggested that compared with the conventional GnRH-agonist short protocol, application of DYG in the PPOS protocol was a safe option for the newborn population without compromising neonatal outcomes or increasing congenital malformation risks.
Keywords: neonatal outcomes, congenital malformations, dydrogesterone, progestin-primed ovarian stimulation, in vitro fertilization
Introduction
Since the first birth of Louise Brown in the United Kingdom in 1978,1 in vitro fertilization (IVF) has flourished for more than 40 years and brought hope to many infertile couples as a major treatment alternative. Currently, more than seven million children have been conceived via IVF/intracytoplasmic sperm injection (ICSI), accounting for 1.6% of births in the United States and 1.0% in mainland China.2,3 However, compared with spontaneously achieved pregnancies, infants born after IVF/ICSI, whether singletons or multiples,4,5 have been widely reported at greater risks of adverse neonatal outcomes in terms of preterm birth (PTB), low birthweight (LBW), small-for-gestational age (SGA) and congenital malformations. Some studies suggest that the risks are attributable to the etiology of infertility,6 while others speculate the potential effects of the assisted reproduction technology itself, such as the controlled ovarian hyperstimulation (COH) protocol, embryo culture condition and cryopreservation technique.7–9
The process of COH is considered to be a key determinant in IVF/ICSI success by recruiting multiple dominant follicles and thereby increasing the quantity of oocytes retrieved within a single cycle. Routine COH regimens, using gonadotropin-releasing hormone (GnRH) agonists and antagonists, have been generally accepted as safe protocols based on the initial reassuring results of numerous prospective or retrospective follow-up studies on obstetrical complications and congenital malformations.10,11 Pituitary down-regulation with GnRH agonists promotes antral follicle synchronization, but poses a higher chance of ovarian hyperstimulation syndrome (OHSS) by human chorionic gonadotropin (hCG) trigger. While GnRH antagonists could suppress luteinizing hormone (LH) in a rapid and reversible manner without initial flare effects, a varied proportion (0.34–38%) of patients experienced premature LH surge, especially in women with advanced age and diminished ovarian reserve.12,13
In 2015, we proposed a new COH protocol named progestin-primed ovarian stimulation (PPOS), in which exogeneous progesterone, adjuvant to human menopausal gonadotropin (hMG), was used from the early follicular phase to block the estradiol (E2)-induced positive feedback effects.14 Compared with the conventional GnRH-agonist short regimen, the PPOS protocol takes the advantage of an oral administration route to effectively prevent premature LH surge while achieving comparable oocyte retrieval and pregnancy outcomes.14 Coupled with dual trigger (GnRH agonist and a low dose of hCG) for final oocyte maturation and the application of a freeze-all strategy for viable embryos, it also allows for nearly complete avoidance of the OHSS incidence.15 In addition, the safety of PPOS protocol, using medroxyprogesterone acetate (MPA) or micronized progesterone (brand name: Utrogestan), has been demonstrated respectively in our large follow-up studies of 1931 and 855 newborns regarding neonatal outcomes and congenital malformations.16,17
Dydrogesterone (DYG), which has a molecular structure as retroprogesterone, is a selective progesterone receptor agonist with high oral bioavailability.18 It is estimated that about 113 million women and 20 million fetuses have been exposed to DYG since 1960s in the treatment of a variety of conditions such as endometriosis, menstrual disorders and recurrent miscarriage.19 Moreover, we recently showed that DYG could also be applied as an alternative progestin in PPOS protocol with the strength of weaker pituitary suppression, thus leading to a lower dose and shorter duration of hMG stimulation during COH.20
To date, the DYG + hMG protocol has resulted in over 1000 infants born after frozen-thawed embryo transfer (FET) cycles. However, the question remains whether this unique protocol is safe for the newborn population. Therefore, the aim of the present study was to comprehensively assess the neonatal outcomes and congenital malformations in children born after the DYG + hMG protocol in comparison with the conventional GnRH-agonist short protocol.
Materials and methods
Study design and participants
This was a retrospective cohort study performed at the Department of Assisted Reproduction of Shanghai Ninth People’s Hospital affiliated with Shanghai Jiao Tong University School of Medicine. The study protocol was approved by the hospital’s Ethics Committee (Institutional Review Board). Written informed consent was obtained from patients in accordance with the ethics committee protocol. All women who achieved clinical pregnancy after IVF/ICSI and subsequent FET cycles using the DYG + hMG protocol or the GnRH-agonist short protocol were enrolled from January 2014 to December 2017. Clinical pregnancy was defined as the presence of gestational sac with or without fetal heart activity, as measured by ultrasound examination 7 weeks after FET. Patients were excluded from the study if they reported pregnancy-related disorders such as gestational diabetes mellitus, hypertension, thyroid diseases and intrahepatic cholestasis of pregnancy, or adverse environmental exposure including cigarette smoking and alcohol consumption, in consideration of their possible associations with congenital malformations.21 We also excluded donor sperm cycles for their lower risks of LBW compared with partner sperm IVF/ICSI,22,23 as well as cycles with embryo cryopreservation for over 1 year to keep consistency between patient age at oocyte retrieval and at embryo transfer. Other exclusion criteria included core data missing such as fertilization method.
Treatment
A detailed description of the ovarian stimulation protocols has been presented in our previous publications.20 Briefly, in the DYG + hMG protocol, patients were administered daily with 20 mg DYG (Duphaston, Abbott Biologicals, USA) and 150–225 IU hMG (Anhui Fengyuan Pharmaceutical Co., China) from menstrual cycle day 3 (MC3) to the day of trigger. In the GnRH-agonist short protocol, patients were injected daily with 0.1 mg triptorelin (Decapeptyl, Ferring Pharmaceuticals, Germany) from MC2 onward and 150–225 IU hMG from MC3 onward until trigger. The doses of hMG were adjusted according to ovarian response, as assessed by transvaginal ultrasound examination and serum E2 concentration. When at least three follicles reached 18 mm in diameter or one dominant follicle reached 20 mm, final oocyte maturation was co-triggered using 0.1 mg triptorelin and 1000 IU hCG (Lizhu Pharmaceutical Trading Co., China) for the DYG + hMG protocol, or triggered with 5000 IU hCG alone for the GnRH-agonist short protocol.
All follicles with a diameter over 10 mm were aspirated at 34–36 h after trigger. The retrieved oocytes were fertilized by conventional IVF and/or ICSI according to semen parameters. The zygotes were transferred and cultured in the Continuous Single Culture (Irvine Scientific, USA) throughout the entire developmental stage. According to the criteria described by Cummins et al.,24 only embryos classified as top-quality (grade I and II) were cryopreserved via vitrification on day 3 after oocyte retrieval, whereas suboptimal embryos (grade III and IV) were subjected to extended culture. The Gardner and Schoolcraft grade system was then applied to select morphologically good blastocysts (grade ≥3BC) for vitrification on day 5 or 6.25 The vitrification and thawing procedures were performed the same as those presented elsewhere.14
As previously described,14,20 endometrial preparation for FET was performed in natural cycles, mild stimulation cycles or hormone replacement cycles, for women with regular menstruation, irregular menstruation or a history of thin endometrium, respectively. Up to two embryos per patient were transferred in each FET cycle. For luteal phase support, progesterone was administered with both oral DYG (40 mg/d; Fematon-yellow tablets, Abbott Biologicals, USA) and vaginal micronized progesterone (400 mg/d; Utrogestan, Besins Manufacturing, Belgium), and was continued to 10 weeks of gestations for those who achieved a pregnancy.
Follow-up of pregnancy and neonatal outcomes
The newborn follow-up system at our department has been described previously.16,17,26 In brief, the couples completed telephone surveys by trained nurses during each trimester of pregnancy and up to 1 week after delivery. Standardized questionnaires were used to gather information including a wide range of pregnancy exposures, pregnancy complications, gestational weeks, mode of delivery, birth date and locality, birth weight and length, newborn gender as well as neonatal diseases. For the reporting of neonatal diseases, further interviews were conducted on the specific diagnosis, severity, treatment and final outcomes. For babies born in our university hospital, the medical records with detailed physical examination and routine ultrasound examination of the brain, kidney, and heart at the first week after birth were obtained, while written proof was acquired from the pediatrician in charge if the babies were born elsewhere. In cases of failed attempts to contact the couples, information was collected through the local family planning service agencies. Furthermore, for babies born with congenital malformations, a special nurse was designated for thorough review to guarantee their accordance with the case definition of the Chinese Birth Defects Monitoring Program.
Outcome measures and definitions
The primary outcome of the study was the incidence of major congenital malformations. Other analyzed adverse neonatal outcomes included LBW, very LBW, PTB, very PTB, SGA, large-for-gestational age (LGA) and early neonatal death.
Major congenital malformations were defined and coded according to the Q codes (Q00–Q99) of the International Classification of Diseases, 10th Revision (ICD-10). LBW and very LBW were defined as birthweight below 2500 g and 1500 g, respectively. PTB and very PTB were defined as delivery before 37 and 32 completed weeks of gestation, respectively. For the standardization of birthweights, Z-scores were calculated after adjusting for the gestational age and the newborn gender, based on a set of general population reference values for Chinese singletons and twins.27,28 In addition, the SGA and LGA were defined as birthweight <10th and >90th percentiles, respectively. Early neonatal death was defined as the death of a live-born baby within 7 days of birth.
Statistical analysis
For continuous variables, the normality was tested by the graphical use of histograms and Q-Q plots as well as the Shapiro-Wilk test. The data were presented as mean with standard deviation (SD) and differences between groups were compared with the Student’s t-test or Mann-Whitney U-test, as appropriate. Categorical variables were described as frequency with rate, and Chi-square test or Fisher exact test was used for comparison.
The associations between the ovarian stimulation protocols (DYG + hMG versus GnRH-agonist short) and major congenital malformations as well as adverse neonatal outcomes were evaluated by binary logistic regression analysis. All potential cofounders were introduced for adjustment whether or not statistical differences between groups were observed, including maternal age (<30, 30–34, 35–37, 38–40 or ≥41 years), maternal body mass index (BMI) (<18.5, 18.5–24.9, 25.0–29.9 or ≥30 kg/m2), gravidity (0 or ≥1), parity (0 or ≥1), duration of infertility, infertility diagnosis (tubal factor, male factor, unexplained or combined/other), sperm origin (ejaculated, testicular or epididymal), fertilization method (IVF, ICSI, or IVF + ICSI), FET endometrial preparation (natural cycle, mild stimulation or hormone replacement therapy), endometrial thickness, number of embryos transferred (single or double) and embryo stage at transfer (cleavage or blastocyst). Furthermore, to account for the correlation between twin births reported by the same delivery, a generalized estimating equation model was applied to calculate crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs).
Statistical analysis was performed with the Statistical Package for the Social Sciences (version 20.0; SPSS Inc., USA). All P-values were based on two-sided tests and P<0.05 was considered to be statistically significant.
Results
The flow chart of the study design and participant selection was presented in Figure 1. In brief, 1399 clinical pregnancies led to 1429 live-born infants after treatment with the DYG + hMG protocol and 2127 live-born infants were resulted from 2095 clinical pregnancies after the GnRH-agonist short protocol. No significant differences were found between the two groups regarding the rates of ectopic pregnancy, pregnancy loss (including spontaneous and therapeutic abortion) in the first trimester, ongoing pregnancy, pregnancy loss in the second or third trimester, stillbirth, lost to follow-up and livebirth (all P>0.05). There was a total of 17 elective terminations of pregnancy due to fetal malformations: 7 (0.50%) in the DYG + hMG protocol and 10 (0.48%) in the GnRH-a short protocol group (P=0.924).
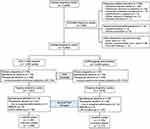 |
Figure 1 Flow chart of the study. Abbreviations: DYG, dydrogesterone; hMG, human menopausal gonadotropin; GnRH, gonadotropin-releasing hormone. |
The baseline characteristics grouped by ovarian stimulation protocols were shown in Table 1. The two groups differed significantly in maternal age, endometrial preparation and number of embryos transferred (all P<0.001). Specifically, the GnRH-agonist short protocol group had a higher age, more natural cycles and more double embryo transfers than the DYG + hMG protocol group. No significant differences were observed when maternal BMI, the proportion of nulliparity and nulligravida, infertility duration, infertility diagnosis, sperm origin, fertilization method, endometrial thickness and embryo stage at transfer were analyzed (all P>0.05).
 |
Table 1 Baseline characteristics grouped by ovarian stimulation protocols |
Table 2 demonstrated the neonatal outcomes in live-born singletons and twins. In total, 873 singletons and 556 twins were born after treatment with the DYG + hMG protocol, while 1321 singletons and 806 twins were born after the GnRH-agonist short protocol. In both singletons and twins, comparisons between the two groups did not reveal any significant difference in the mode of delivery, newborn gender, gestational age, birthweight, length at birth and Z-scores. With regard to adverse neonatal outcomes, the two groups were also similar in the proportion of LBW, very LBW, very PTB, SGA, LGA and early neonatal death among singletons and twins, and remained stable after adjusting for a variety of confounding factors (Figure 2). Notably, the incidence of PTB failed to reach significant difference between the two groups among singletons (8.8% vs 7.3%, P=0.266), but was slightly higher in the GnRH-agonist short protocol group among twins (52.9% vs 47.1%, P=0.038). This higher risk, however, was not maintained after adjustment (adjusted OR=0.85, 95% CI: 0.68–1.07).
 |
Table 2 Neonatal outcome in live-born singletons and twins grouped by ovarian stimulation protocols |
As presented in Table 3, major congenital malformations were observed in 16 out of 1429 live-born infants (1.12%) in the DYG + hMG protocol and in 23 out of 2127 infants (1.08%) in the GnRH-agonist short protocol (P=0.914). No significant differences were found between the two groups when the incidence of major congenital malformations was analyzed according to the category of multiplicity and gender. In both groups, the main type of malformation occurred in the circulatory system (0.56% in the DYG + hMG protocol and 0.71% in the GnRH-agonist short protocol). After adjusting for confounders, no evidently elevated risk of major congenital malformations was observed in infants born after the DYG + hMG protocol in comparison with the GnRH-agonist short protocol in both singletons (adjusted OR=0.98, 95% CI: 0.40–2.39) and twins (adjusted OR=0.90, 95% CI: 0.33–2.41) (Figure 2).
 |
Table 3 Major congenital malformations in live-born infants grouped by ovarian stimulation protocols |
Discussion
In this large retrospective cohort study, we provided the first-time evidence that application of DYG in the PPOS protocol was a safe option for the newborn population without compromising neonatal outcomes or increasing congenital malformation risks.
Oocyte quality is strongly associated with early embryo survival, pregnancy establishment and maintenance, fetal growth and development, neonatal health, and even disease onset in adulthood.29 However, the relationship between oocyte quality and progesterone still remains to be controversial.30–34 Silva et al found that when bovine cumulus-oocyte complexes were exposed to progesterone in vitro, the proportion of blastocyst formation was reduced by approximately 40%.30 This effect could be partially reversed by the progesterone receptor antagonist mifepristone.30 A study conducted by Zavareh et al showed that adding progesterone to the in vitro culture medium significantly inhibited oocyte meiotic resumption in a dose-dependent manner, thus leading to an increase in germinal vesicle arrest and reduction in oocyte maturation.31 In contrast, Carter et al demonstrated a neutral effect of elevated progesterone on the development of in vitro fertilized zygotes to the blastocyst stage.32 Yamashita et al, however, observed that the medium progesterone level was positively correlated with the rate of oocyte germinal vesicle breakdown (GVBD).34 The supplementation of ketoconazole, which suppressed the progesterone production of cumulus cells via demethylation of lanosterol, decreased the GVBD rate but could be overtaken by the addition of progesterone.34 Furthermore, the embryo development was also impaired by either inhibiting progesterone synthesis or blocking its receptor activity.33
Despite the contradictory in vitro data on whether progesterone associates with oocyte quality in a positive or negative way, a consensus seems to be reached that high progesterone levels did not harm the oocyte/embryo developmental potentials in vivo. Several studies have reported that progesterone elevation on hCG trigger day adversely impacted on the rates of implantation and clinical pregnancy in fresh embryo transfer but not in FET or the donor/recipient cycles, suggesting the main detrimental effects of progesterone on endometrial receptivity rather than oocytes.35,36 In addition, a prospective cohort study of 322 pregnant women who used levonorgestrel, an exogenous progesterone for emergency contraception, demonstrated no association with the risks of pregnancy complications, major congenital malformations, or any other adverse neonatal outcomes.37 More directly, luteal-phase ovarian stimulation (LPS), where high levels of endogenous progesterone persisted during the entire process of follicle development with the use of letrozole and hMG, was proposed at our center in 2014.38 This novel regimen not only produced competent oocytes and high-quality embryos,38 but also showed its safety in the follow-up of 587 newborns compared with mild stimulation and GnRH-agonist short protocols.26 Encouraged by this discovery, we further applied exogenous progesterone, either MPA or micronized progesterone (brand name: Utrogestan), in combination with hMG for ovarian stimulation in IVF and achieved comparable outcomes in oocyte retrieval and pregnancy as well as neonatal health including congenital malformations.14,16,17,39
DYG is a synthetic stereoisomer of progesterone with high oral bioavailability.18 Its unique molecular structural features provide high binding specificity and agonistic activity at progesterone receptors, but no or negligible activity at androgen, mineralocorticoid and glucocorticoid receptors, thus minimizing unwanted effects.18 Since the 1960s, the DYG has been applied to treat a variety of conditions related to progesterone deficiency.19 Recently, we also showed that DYG could effectively prevent premature LH surge during COH at a dose 5–10 times lower than micronized progesterone,40 and avoid profound pituitary suppression with a lower hMG dose than MPA.20 With regard to the offspring safety, a retrospective case-control study of 202 children reported a positive association between congenital heart diseases and DYG use in preventing miscarriage during early pregnancy.41 The study finding, however, is limited by its selection, confounding and information bias, and is challenged by the Lotus I and II Phase III studies showing that the incidence of serious adverse events as well as the congenital, familial and genetic disorders were similar in the newborn population between the oral DYG and micronized vaginal progesterone capsule or gel groups for luteal phase support.42,43 Nevertheless, all these studies were conducted in pregnant women when embryos had been implanted in the uterus. Due to the difference in structural and pharmacological characteristics between DYG and other exogenous progesterone,18,19 it remains unclarified whether DYG use for ovarian stimulation would exert any unsuspected influence on the oocyte quality and thereby neonatal health.
In the present study, we demonstrated that the DYG + hMG protocol was comparable to the classic GnRH-agonist short protocol in all neonatal outcomes and major congenital malformations in FET cycles after adjusting for various confounding factors. In line with previous studies,44 cardiovascular malformations were dominant among all types of defects in both protocols. No special type of malformations was observed, suggesting no specific teratogenic effect of DYG.
Notably, the total incidence of congenital malformations in live-birth infants in this study (1.10%) is lower than a data-linkage cohort study of IVF newborns in China (1.97%),44 while in accordance with report of the LPS, MPA + hMG and micronized progesterone (brand name: Utrogestan) + hMG protocols (1.02, 1.04 and 1.52%, respectively).16,17,26 The reasons could be elucidated in the following aspects. Firstly, in order to evaluate precisely the independent effect of ovarian stimulation protocols, we excluded participants with reported pregnancy-related disorders and adverse environmental exposures from data analysis, which were regarded to increase the risk of congenital malformations.21 Secondly, most of our data collections were carried out through paternal questionnaires without direct access to medical records. Therefore, some congenital malformations were likely to be omitted especially in children with minor or multiple defects, thus leading to an underestimation of the actual incidence. Finally, all infants in this study were born from FET cycles, where embryos were transferred into a more physiological endometrial environment than fresh embryo transfers. This could also lower the rate of congenital malformations, as indicated in previous studies.45
The main strength of our study is the large sample size of over 3500 live-born infants at a single center, with 1429 newborns in the DYG + hMG protocol and 2127 in the GnRH-agonist short regimen. During the study period, no changes were made in our routine clinical practice including fertilization methods and vitrification or thawing procedures. The lost to follow-up rate was below 1% thanks to our highly specialized nurses, which further guaranteed the accuracy and reliability of neonatal data. However, the current study is limited by its retrospective and non-randomized design. Some baseline characteristics were inconsistent between the two groups. Although we adopted the binary regression analysis to reassure the robustness of results, possible unknown confounders may not be accounted for in the model.
Conclusion
Our study suggested that DYG could serve as a feasible progestin in the PPOS protocol with satisfied neonatal outcomes and unelevated congenital malformation risk. Prospective randomized control trials with a larger sample size and continuous follow-up from birth to adulthood are needed to further validate the safety of this novel ovarian stimulation protocol.
Acknowledgment
This study was funded by the National Key Research and Development Program of China (SQ2018YFC100163) and the National Natural Science Foundation of China (81571397, 81771533).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366. doi:10.1016/S0140-6736(78)92957-4
2. Sunderam S, Kissin DM, Crawford SB, et al. Assisted reproductive technology surveillance - United States, 2013. MMWR Surveill Summ. 2015;64(11):1–25. doi:10.15585/mmwr.ss6411a1
3. Yang X, Li Y, Li C, Zhang W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril. 2014;101(2):385–391. doi:10.1016/j.fertnstert.2013.10.017
4. Qin JB, Sheng XQ, Wu D, et al. Worldwide prevalence of adverse pregnancy outcomes among singleton pregnancies after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Arch Gynecol Obstet. 2017;295(2):285–301. doi:10.1007/s00404-016-4250-3
5. Qin JB, Sheng XQ, Wang H, et al. Worldwide prevalence of adverse pregnancy outcomes associated with in vitro fertilization/intracytoplasmic sperm injection among multiple births: a systematic review and meta-analysis based on cohort studies. Arch Gynecol Obstet. 2017;295(3):577–597. doi:10.1007/s00404-017-4291-2
6. Pinborg A, Wennerholm UB, Romundstad LB, et al. Why do singletons conceived after assisted reproduction technology have adverse perinatal outcome? Systematic review and meta-analysis. Hum Reprod Update. 2013;19(2):87–104. doi:10.1093/humupd/dms044
7. Sunkara SK, La Marca A, Seed PT, Khalaf Y. Increased risk of preterm birth and low birthweight with very high number of oocytes following IVF: an analysis of 65 868 singleton live birth outcomes. Hum Reprod. 2015;30(6):1473–1480. doi:10.1093/humrep/dev076
8. Vergouw CG, Kostelijk EH, Doejaaren E, Hompes PG, Lambalk CB, Schats R. The influence of the type of embryo culture medium on neonatal birthweight after single embryo transfer in IVF. Hum Reprod. 2012;27(9):2619–2626. doi:10.1093/humrep/des252
9. Alviggi C, Conforti A, Carbone IF, Borrelli R, de Placido G, Guerriero S. Influence of cryopreservation on perinatal outcome after blastocyst- vs cleavage-stage embryo transfer: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2018;51(1):54–63. doi:10.1002/uog.18942
10. Anthony S, Buitendijk SE, Dorrepaal CA, Lindner K, Braat DD, Den Ouden AL. Congenital malformations in 4224 children conceived after IVF. Hum Reprod. 2002;17(8):2089–2095. doi:10.1093/humrep/17.8.2089
11. Bonduelle M, Oberye J, Mannaerts B, Devroey P. Large prospective, pregnancy and infant follow-up trial assures the health of 1000 fetuses conceived after treatment with the GnRH antagonist ganirelix during controlled ovarian stimulation. Hum Reprod. 2010;25(6):1433–1440. doi:10.1093/humrep/deq072
12. Bosch E, Valencia I, Escudero E, et al. Premature luteinization during gonadotropin-releasing hormone antagonist cycles and its relationship with in vitro fertilization outcome. Fertil Steril. 2003;80(6):1444–1449. doi:10.1016/j.fertnstert.2003.07.002
13. Reichman DE, Zakarin L, Chao K, Meyer L, Davis OK, Rosenwaks Z. Diminished ovarian reserve is the predominant risk factor for gonadotropin-releasing hormone antagonist failure resulting in breakthrough luteinizing hormone surges in in vitro fertilization cycles. Fertil Steril. 2014;102(1):99–102. doi:10.1016/j.fertnstert.2014.07.1243
14. Kuang Y, Chen Q, Fu Y, et al. Medroxyprogesterone acetate is an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Fertil Steril. 2015;104(1):62–70.e63. doi:10.1016/j.fertnstert.2015.03.022
15. Lu X, Hong Q, Sun L, et al. Dual trigger for final oocyte maturation improves the oocyte retrieval rate of suboptimal responders to gonadotropin-releasing hormone agonist. Fertil Steril. 2016;106(6):1356–1362. doi:10.1016/j.fertnstert.2016.07.1068
16. Zhang J, Mao X, Wang Y, et al. Neonatal outcomes and congenital malformations in children born after human menopausal gonadotropin and medroxyprogesterone acetate treatment cycles. Arch Gynecol Obstet. 2017;296(6):1207–1217. doi:10.1007/s00404-017-4435-4
17. Wang N, Lin J, Zhu Q, et al. Comparison of neonatal outcomes and live-birth defects after progestin-primed ovarian stimulation versus conventional ovarian stimulation for in vitro fertilization: a large retrospective cohort study. Medicine (Baltimore). 2018;97(34):e11906. doi:10.1097/MD.0000000000011906
18. Rizner TL, Brozic P, Doucette C, et al. Selectivity and potency of the retroprogesterone dydrogesterone in vitro. Steroids. 2011;76(6):607–615. doi:10.1016/j.steroids.2011.02.043
19. Griesinger G, Tournaye H, Macklon N, et al. Dydrogesterone: pharmacological profile and mechanism of action as luteal phase support in assisted reproduction. Reprod Biomed Online. 2019;38(2):249–259. doi:10.1016/j.rbmo.2018.11.017
20. Yu S, Long H, Chang HY, et al. New application of dydrogesterone as a part of a progestin-primed ovarian stimulation protocol for IVF: a randomized controlled trial including 516 first IVF/ICSI cycles. Hum Reprod. 2018;33(2):229–237. doi:10.1093/humrep/dex367
21. Harris BS, Bishop KC, Kemeny HR, Walker JS, Rhee E, Kuller JA. Risk factors for birth defects. Obstet Gynecol Surv. 2017;72(2):123–135. doi:10.1097/OGX.0000000000000442
22. Kamath MS, Antonisamy B, Selliah HY, La Marca A, Sunkara SK. Perinatal outcomes following IVF with use of donor versus partner sperm. Reprod Biomed Online. 2018;36(6):705–710. doi:10.1016/j.rbmo.2018.03.016
23. Gerkowicz SA, Crawford SB, Hipp HS, Boulet SL, Kissin DM, Kawwass JF. Assisted reproductive technology with donor sperm: national trends and perinatal outcomes. Am J Obstet Gynecol. 2018;218(4):
24. Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3(5):284–295. doi:10.1007/BF01133388
25. Gardner DK, Schoolcraft WB. In vitro culture of human blastocyst. In: Jansen R, Mortimer D, editors. Towards Reproductive Certainty: Infertility and Genetics beyond 1999. Carnforth: Parthenon Press; 1999:378–388.
26. Chen H, Wang Y, Lyu Q, et al. Comparison of live-birth defects after luteal-phase ovarian stimulation vs. conventional ovarian stimulation for in vitro fertilization and vitrified embryo transfer cycles. Fertil Steril. 2015;103(5):1194–1201.e1192.
27. Dai L, Deng C, Li Y, et al. Birth weight reference percentiles for Chinese. PLoS One. 2014;9(8):e104779. doi:10.1371/journal.pone.0104779
28. Dai L, Deng C, Li Y, et al. Population-based birth weight reference percentiles for Chinese twins. Ann Med. 2017;49(6):470–478. doi:10.1080/07853890.2017.1294258
29. Krisher RL. The effect of oocyte quality on development. J Anim Sci. 2004;82 E-Suppl:E14–E23.
30. Silva CC, Knight PG. Effects of androgens, progesterone and their antagonists on the developmental competence of in vitro matured bovine oocytes. J Reprod Fertil. 2000;119(2):261–269.
31. Zavareh S, Saberivand A, Salehnia M. The effect of progesterone on the in vitro maturation and developmental competence of mouse germinal vesicle oocytes. Int J Fertil Steril. 2009;3(1):21–28.
32. Carter F, Rings F, Mamo S, et al. Effect of elevated circulating progesterone concentration on bovine blastocyst development and global transcriptome following endoscopic transfer of in vitro produced embryos to the bovine oviduct. Biol Reprod. 2010;83(5):707–719. doi:10.1095/biolreprod.109.082354
33. Aparicio IM, Garcia-Herreros M, O’Shea LC, Hensey C, Lonergan P, Fair T. Expression, regulation, and function of progesterone receptors in bovine cumulus oocyte complexes during in vitro maturation. Biol Reprod. 2011;84(5):910–921. doi:10.1095/biolreprod.110.087411
34. Yamashita Y, Shimada M, Okazaki T, Maeda T, Terada T. Production of progesterone from de novo-synthesized cholesterol in cumulus cells and its physiological role during meiotic resumption of porcine oocytes. Biol Reprod. 2003;68(4):1193–1198. doi:10.1095/biolreprod.102.010934
35. Bosch E, Labarta E, Crespo J, et al. Circulating progesterone levels and ongoing pregnancy rates in controlled ovarian stimulation cycles for in vitro fertilization: analysis of over 4000 cycles. Hum Reprod. 2010;25(8):2092–2100. doi:10.1093/humrep/deq125
36. Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC. Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update. 2013;19(5):433–457. doi:10.1093/humupd/dmt014
37. Zhang L, Chen J, Wang Y, Ren F, Yu W, Cheng L. Pregnancy outcome after levonorgestrel-only emergency contraception failure: a prospective cohort study. Hum Reprod. 2009;24(7):1605–1611. doi:10.1093/humrep/dep076
38. Kuang Y, Hong Q, Chen Q, et al. Luteal-phase ovarian stimulation is feasible for producing competent oocytes in women undergoing in vitro fertilization/intracytoplasmic sperm injection treatment, with optimal pregnancy outcomes in frozen-thawed embryo transfer cycles. Fertil Steril. 2014;101(1):105–111. doi:10.1016/j.fertnstert.2013.09.007
39. Zhu X, Zhang X, Fu Y. Utrogestan as an effective oral alternative for preventing premature luteinizing hormone surges in women undergoing controlled ovarian hyperstimulation for in vitro fertilization. Medicine (Baltimore). 2015;94(21):e909. doi:10.1097/MD.0000000000000874
40. Zhu X, Ye H, Fu Y. Duphaston and human menopausal gonadotropin protocol in normally ovulatory women undergoing controlled ovarian hyperstimulation during in vitro fertilization/intracytoplasmic sperm injection treatments in combination with embryo cryopreservation. Fertil Steril. 2017;108(3):505–512.e502.
41. Zaqout M, Aslem E, Abuqamar M, Abughazza O, Panzer J, De Wolf D. The impact of oral intake of dydrogesterone on fetal heart development during early pregnancy. Pediatr Cardiol. 2015;36(7):1483–1488. doi:10.1007/s00246-015-1190-9
42. Tournaye H, Sukhikh GT, Kahler E, Griesinger G, Phase A. III randomized controlled trial comparing the efficacy, safety and tolerability of oral dydrogesterone versus micronized vaginal progesterone for luteal support in in vitro fertilization. Hum Reprod. 2017;32(5):1019–1027. doi:10.1093/humrep/dex023
43. Griesinger G, Blockeel C, Sukhikh GT, et al. Oral dydrogesterone versus intravaginal micronized progesterone gel for luteal phase support in IVF: a randomized clinical trial. Hum Reprod. 2018;33(12):2212–2221.
44. Yu HT, Yang Q, Sun XX, et al. Association of birth defects with the mode of assisted reproductive technology in a Chinese data-linkage cohort. Fertil Steril. 2018;109(5):849–856. doi:10.1016/j.fertnstert.2018.01.012
45. Blockeel C, Drakopoulos P, Santos-Ribeiro S, Polyzos NP, Tournaye H. A fresh look at the freeze-all protocol: a SWOT analysis. Hum Reprod. 2016;31(3):491–497.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

