Back to Journals » Journal of Experimental Pharmacology » Volume 15
Moringa oleifera Leaves Extract Ameliorates Doxorubicin-Induced Cardiotoxicity via Its Mitochondrial Biogenesis Modulatory Activity in Rats
Authors Patintingan CG, Louisa M , Juniantito V, Arozal W, Hanifah S, Wanandi SI, Thandavarayan R
Received 19 March 2023
Accepted for publication 12 July 2023
Published 26 July 2023 Volume 2023:15 Pages 307—319
DOI https://doi.org/10.2147/JEP.S413256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Cyntia Gracesella Patintingan,1 Melva Louisa,2 Vetnizah Juniantito,3 Wawaimuli Arozal,2 Silmi Hanifah,1 Septelia Inawati Wanandi,4 Rajarajan Thandavarayan5
1Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 2Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 3Department of Veterinary Clinic Reproduction and Pathology, Faculty of Veterinary Medicine, Agriculture Institute of Bogor, Bogor, Indonesia; 4Department of Biochemistry and Molecular Biology, Faculty of Medicine Universitas Indonesia, Jakarta, Indonesia; 5Department of Cardiovascular Sciences Houston Methodist Research Institute, Houston, TX, USA
Correspondence: Melva Louisa, Department of Pharmacology and Therapeutics Faculty of Medicine, Universitas Indonesia, Jakarta, 10430, Indonesia, Tel +62-21-31930481, Email [email protected]
Background: Doxorubicin, an anthracycline class of anticancer, is an effective chemotherapeutic agent with serious adverse effects, mainly cardiotoxicity. Several possible causes of doxorubicin cardiotoxicity are increased oxidative stress, nucleic acid and protein synthesis inhibition, cardiomyocyte apoptosis, and mitochondrial biogenesis disruptions. Moringa oleifera (MO), a naturally derived medicine, is known for its antioxidative properties and activity in alleviating mitochondrial dysfunction. To determine the potency and possible cardioprotective mechanism of MO leaves aqueous extract via the mitochondrial biogenesis pathway in doxorubicin-induced rats.
Methods: Twenty-four Sprague-Dawley rats were divided into four groups of six. The first group was normal rats; the second group was treated with doxorubicin 4 mg/kg BW intraperitoneally once weekly for four weeks; the third and fourth groups were treated with doxorubicin 4 mg/kg BW intraperitoneally once weekly, and MO leaves extract at 200 mg/kg BW or 400 mg/kg BW orally daily, for four weeks. At the end of the fourth week, blood and cardiac tissues were obtained and analyzed for cardiac biomarkers, mitochondrial DNA copy number, mRNA expressions of peroxisome-activated receptor-gamma coactivator-1 alpha (PGC-1α), the nuclear factor erythroid 2-related factor 2 (Nrf2), superoxide dismutase 2 (SOD2), caspase 3, the activity of glutathione peroxidase (GPx), levels of 8-hydroxy-2-deoxyguanosine (8-OH-dG), and malondialdehyde.
Results: MO leaves extract was shown to decrease biomarkers of cardiac damage (LDH and CK-MB), malondialdehyde levels, and GPx activity. These changes align with the reduction of mRNA expressions of caspase-3, the increase of mRNA expressions of PGC-1α and Nrf2, and the elevation of mitochondrial DNA copy number. MO leaves extracts did not influence the mRNA expressions of superoxide dismutase 2 (SOD2) or the levels of 8-OH-dG.
Conclusion: Moringa oleifera leaves extract ameliorates doxorubicin-induced cardiotoxicity by reducing apoptosis and restoring gene expression of PGC-1α and Nrf2, a key regulator in mitochondrial biogenesis.
Keywords: anthracycline, DNA damage, mitochondrial DNA, Moringa oleifera Lam, oxidative stress
Introduction
Doxorubicin, an anthracycline anticancer drug, is highly efficacious and successful in treating a broad range of solid tumors and hematological malignancies. Even though doxorubicin is a very efficient anticancer drug, most patients experience cardiotoxicity as a side effect.1,2 Cardiotoxicity due to doxorubicin can be characterized by the elevation of several cardiac enzyme markers, electrocardiogram abnormalities, and left ventricular systolic dysfunction that might progress to cardiomyopathy and congestive heart failure.2,3 The previous study had shown that the incidence of patients with congestive heart failure could increase dose-dependently with the cumulative dose of doxorubicin.4,5
Several hypotheses regarding the mechanism of doxorubicin-induced cardiomyopathy include impaired adrenergic regulation of the myocardium, cardiac cell hyperinflammation, and the generation of excessive oxygen free radicals and lipid peroxidation. Doxorubicin is also known to induce mitochondrial dysfunction, including impaired calcium regulation and apoptosis in cardiomyocytes.6–11
Doxorubicin has a strong affinity for the myocardium, which can induce reactive oxygen species (ROS) production and mitochondrial damage. Mitochondria are the most prominent organelles damaged in the heart by drugs that induce cardiotoxicity.6 Compared with other tissues, the number of mitochondria in cardiomyocytes is 35–40% higher, so doxorubicin tends to cause toxicity in the heart.8,12,13 Following mtDNA damage, the copy number level of mtDNA varies, resulting in mitochondrial malfunction, which plays an essential part in doxorubicin-induced cardiotoxicity.14 In addition, doxorubicin also substantially lowered the expression of mitochondrial superoxide dismutase 2, mitochondria (SOD2) in cardiomyocytes. SOD2 removes superoxide radicals from mitochondria, producing hydrogen peroxide and oxygen.15 The considerable reduction in SOD2 mRNA and protein expression in PGC-1 knockout mice suggests that PGC-1 is required for SOD2 expression in the cardiomyocytes.16 Thus, agents that alter mitochondrial dysfunction and have antioxidative activity are hypothesized to have cardioprotective effects in doxorubicin-induced cardiotoxicity.
To date, no standard treatment is available to combat doxorubicin cardiotoxicity. The selection of natural compounds as cardioprotectors is a future opportunity in drug development. One of the plants that can be developed is Moringa oleifera Lam. A previous study showed that Moringa oleifera aqueous extract could restore cardiac dysfunction in potassium bromate-induced rats via its anti-inflammatory and antioxidant activities.17 Another study showed that isothiocyanates, a compound isolated from Moringa oleifera, caused a decrease in mitochondrial superoxide and restoration of mitochondrial membrane potential in lipopolysaccharide-induced macrophages.18 Several studies have been carried out to examine the impact of Moringa oleifera on the cardiotoxicity induced by short-term doxorubicin.19,20 The research elucidated the potential mechanism of action of Moringa oleifera through its anti-inflammatory19 and antioxidant20 pathways. The potential benefits of Moringa oleifera extract on extended periods of doxorubicin-induced cardiotoxicity and whether mitochondrial biogenesis pathways mediate the effects remain uncertain. Thus, this study aimed to determine the potency and possible cardioprotection mechanism of Moringa oleifera leaves aqueous extract via the mitochondrial biogenesis pathway in a longer duration of doxorubicin-induced rats.
Materials and Methods
Materials
Moringa oleifera leaves extract was obtained commercially from PT Javaplant (Solo, Indonesia). The extraction method and detailed results of the phytochemical analysis of the extract have been provided in the previous manuscript.21 Doxorubicin-HCl pro injection 2 mg/mL was purchased from Otto Pharmaceutical, Indonesia. Spectrophotometric kits for Lactate dehydrogenase (LDH) and creatine phosphokinase-MB (CK-MB) were from Diasys Diagnostic Systems, USA. Reagents for quantitative reverse-transcription Polymerase-Chain Reaction (qRT-PCR) consist of Quick-DNA Miniprep Plus Kit (Zymo Research), Quick-RNA Miniprep Plus Kit (Zymo Research), ReverTra Ace™ qPCR RT Master Mix with gDNA Remover (Toyobo), ThunderbirdTM SYBR qPCR Mix (Toyobo), and primers are from Integrated DNA TechnologiesTM (IDT, Singapore). Rat 8-hydroxy-2-deoxyguanosine (8-OHdG) ELISA kits were purchased from MyBioSource, USA.
Animals
The experiment was done on 24 male Sprague-Dawley rats aged eight weeks, weighing 200–250 grams. The rats were housed at the Animal Research Facility in the Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jakarta. Conditions used for animal housing: 22 ± 2°C, with approximately 60% relative humidity, 12-hour dark-light cycle. Animals were kept with free water and standard food access. We implemented the institution’s standard for animal research. Ethical clearance was approved before the commencement of the study by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia (approval number: KET-39/UN2.F1/ETIK/ PPM.00.02/2022, January 17, 2022).
Experimental Procedure
Twenty-four Sprague-Dawley rats were divided into four groups of six. Group 1 (normal) was injected with NaCl once weekly for four (4) weeks and given the vehicle (1% CMC) every day for 28 days, except on the injection day. Group 2 (Dox) was a negative control group, injected with doxorubicin once weekly for four (4) weeks (the total dose was 16 mg/kg BW) and given the vehicle (1% CMC) every day for 28 days, except on the injection day. Group 3 (Dox-MO 200) was injected with doxorubicin once weekly for four (4) weeks (the total dose was 16 mg/kg BW) and given the Moringa oleifera aqueous leaves extract at 200 mg/kg BW every day for 28 days, except on the injection day. Group 4 (Dox-MO 400) was injected with doxorubicin once weekly for four (4) weeks (the total dose was 16 mg/kg BW) and given the Moringa oleifera aqueous leaves extract at 400 mg/kg BW every day for 28 days, except on the injection day (Figure 1). At the end of the fourth week (or day 29), the rats were anesthetized with ketamine and xylazine. Afterward, blood samples were taken by direct puncture of the heart. The rats were necropsied, and the heart organs were removed.
Markers of Cardiac Enzymes Analysis
Blood samples collected in the EDTA tube were centrifuged at 3000 g, 4°C, for 10 minutes. Plasma was then separated and stored at −70°C before analysis. Afterward, the activities of LDH and CK-MB were analyzed spectrophotometrically using the procedure provided by the manufacturer (CK-MB FS kit and LDH kit from Diasys Diagnostic Systems, USA).
Histopathological Analysis
The cardiac tissues were fixed with a 10% neutral buffered formalin solution. Histopathological analysis of the cardiac tissue with a 3–5 μm thickness was performed with HE (hematoxylin-eosin) staining for morpho-pathological observations. A pathologist evaluated abnormalities on cardiac tissues using ordinal scoring of Gibson-Corley et al’s modified scoring criteria.22 Before determining the scoring criteria, observations were made on each histopathology slide, then slides with subjectively similar levels of myocarditis were grouped into four scoring criteria groups, which are 0 (none, very little), +1 (mild), +2 (moderate), and +3 (severe) (Supplementary Material Table 1). Scoring was carried out by two pathologists who did not know the identity of the sample group (blind scoring).
qRT-PCR Assay
Homogenization of 50 mg of cardiac tissues was done in a micro pestle with 800 μL of DNA and RNA shield (provided in the isolation kit). Total RNA was isolated from cardiac homogenates using the Quick-RNA Miniprep Plus Kit (Zymo Research). Then, cDNA synthesis was performed from total RNA using the ReverTra Ace™ qPCR RT master mix with gDNA remover. Subsequently, mRNA expressions were determined from the cDNA template by adding a qRT-PCR reaction mixture (Thunderbird SYBR® qPCR Mix). The primers used for amplifying β-actin and Peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α) were according to the study by Barinda et al,23 Nrf2 according to Louisa et al,24 SOD2 and Caspase-3 were following the primers used by Zhao25 and Khan,26 respectively. The full sequences of the primers are provided in Supplementary Table 2. The ratio of PGC-1α, SOD2, and Caspase-3 mRNA expressions compared to β-actin as a reference gene was then calculated using the Livak method.27
Mitochondrial DNA Copy Number Analysis
Twenty-five (25) mg of heart tissue was added to 95 µL nuclease-free water (NFW), 95 µL solid tissue buffer, and 10 µL proteinase K. The mixture was incubated for 90 minutes, and the tissue was homogenized using a micro pestle. The determination of the mitochondrial DNA (mtDNA) copy number was carried out in a qRT-PCR machine. Total DNA was isolated from cardiac homogenates using the Quick-DNA Miniprep Plus Kit (Zymo Research). The primers were designed to detect cytochrome c oxidase subunit II (COII) for mitochondrial DNA (mtDNA) and β-actin for nuclear DNA (nDNA), according to Santos et al.5 A total of 50 ng of template DNA was added to the qPCR reaction mixture consisting of 10 µL of ThunderbirdTM SYBR qPCR mix. The reactions were done following the manufacturer’s methods. The cycle threshold value (Ct) of COII and β-actin in each sample was used to obtain the ratio of mtDNA to nDNA. The full sequences of the primers are provided in Supplementary Table 3.
Oxidative Stress Markers Analysis
As much as 100 mg of heart tissue was added to a mixture of 1 mL of PBS 0.01 M pH 7.4 solution and 0.1% protease inhibitor. It was then homogenized in a cold state using an Ultra Turrax homogenizer, followed by centrifugation at 10,000 rpm for 10 minutes. The supernatant was taken to measure oxidative stress markers. The ELISA method was utilized to determine the levels of 8-hydroxy-2’-deoxyguanosine (8-OH-dG) and glutathione peroxidase activity (GPx) in homogenized cardiac tissue samples. The Rat 8-hydroxy-2-deoxyguanosine (8-OH-dG) and glutathione peroxidase activity kits were utilized following the manufacturer’s protocol, as MyBioSource (USA) provided. The extent of lipid peroxidation was assessed by quantifying Malondialdehyde (MDA) levels in cardiac tissues through the utilization of the thiobarbituric acid (TBA) method, as previously outlined by Pomierny-Chamioo et al.28
Statistical Analysis
All the results were provided as means ± SEM and were subjected to normality and homogeneity tests. Differences between groups were analyzed using One-way ANOVA Tukey’s multiple comparisons for data that met parametric requirements. Otherwise, the data were analyzed with Kruskal–Wallis followed by Dunn’s method. The significance level was set at p<0.05. All statistical analysis and figure creation in the manuscript were performed on GraphPad Prism version 9.4.0.
Results
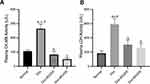
Moringa oleifera Leaves Extract Reduces the Activity of Cardiac Enzymes CK-MB and LDH
Doxorubicin increased the activity of cardiac enzymes CK-MB and LDH by up to 300% compared to the normal group. However, at both doses, Moringa oleifera leaf extract brought the enzymes back to a level close to the normal group’s (Figure 2).
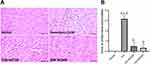
Moringa oleifera Leaves Extract Normalizes the Morphology of Cardiomyocytes
The histological examination in the normal or control group revealed intact and densely packed striated myocardial fibers, demonstrating the preservation of the normal myocardial architecture. In contrast, the Doxorubicin (Dox) group exhibited a significantly different histological profile, characterized by extensive myocarditis, with a mean score of 3.17. The tissues showed excessive infiltration of inflammatory cells, indicating an inflammatory response and the observation of necrotic myocardial fibers indicated tissue damage. However, in both the Dox-MO200 and Dox-MO400 groups, with the score of 0.55 and 0.33, able to mitigate the effects of Dox, the histological analysis revealed the presence of densely striated myocardial fibers, resembling the histological features observed in the normal control group (Figure 3).
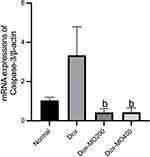
Moringa oleifera Leaves Extract Improves the Changes in the mRNA Expression of Caspase 3
Caspase-3 is known as an apoptosis executor.29 Doxorubicin significantly enhanced the mRNA expression caspase-3 up to 3 times vs normal. Co-treatment with Moringa oleifera leaves extract resulted in the restoration of mRNA expression of caspase-3 like the normal group. However, both dosages resulted in a similar level of expression (Figure 4).
Moringa oleifera Leaves Extract Does Not Alter Cardiac Tissue Concentrations of 8-Hydroxy-2’-Deoxyguanosine, However Lowering Malondialdehyde Concentration and Glutathione Peroxidase Activity
8-hydroxy-2’-deoxyguanosine (8-OH-dG) is a marker of DNA damage associated with the loss of mtDNA.30 Our results showed no significant difference in the cardiac tissue concentrations of 8-hydroxy-2’-deoxyguanosine after treatment with doxorubicin or doxorubicin with Moringa oleifera leaves extract in both dosages. However, there is a significant reduction of malondialdehyde levels up to −37% in Dox-MO200 and −33% in Dox-MO400 compared to the Dox group. There is a tendency toward a decrease in glutathione peroxidase activity in both Dox-MO200 (−31%) and Dox-MO400 (−31%), yet the reduction does not reach statistical significance (Figure 5).
Moringa oleifera Leaves Extract Affects the mRNA Expressions of PGC-1α, SOD2, and the Copy Number of Mitochondrial DNA
Doxorubicin was consistently shown to repress the mRNA expressions of PGC-1α (up to −70% vs normal), Nrf2 (−28% vs normal), SOD2 (−58% vs normal), and the copy number of mtDNA (−55% vs normal). Co-treatment with Moringa oleifera leaves extract improves the mRNA expression of PGC-1α and Nrf2 back to the normal value but not SOD2. Mitochondrial DNA was shown to escalate with the treatment of Moringa oleifera leaves extracts. However, no dose dependency was displayed (Figure 6).
Discussion
This study aimed to determine the potential of Moringa oleifera leaves aqueous extract as a cardioprotective agent in Sprague Dawley rats induced by doxorubicin through the regulation of mitochondrial biogenesis. Our results showed that co-treatment of doxorubicin with Moringa oleifera leaves extract alleviates doxorubicin-induced toxicity by improving cardiac enzyme activities, increasing mRNA expressions of PGC-1α and SOD, and restoring mtDNA copy number and caspase-3 mRNA expressions.
Mitochondrial damage, including deficiency of mitochondrial biogenesis and oxidative phosphorylation systems, decreased fatty acid oxidation and ATP production, and DNA damage, was identified as the leading causes of doxorubicin-induced cardiotoxicity.13,31 Decreased mitochondrial function due to doxorubicin can lead to apoptosis and necrosis, which are fatal to the heart and cause left ventricular dysfunction, cardiomyopathy, and heart failure.8,31 Chronic doxorubicin has been shown in both in vitro and in vivo investigations to cause cardiotoxicity through oxidative DNA damage and activation of the p53-apoptosis pathway, mainly in the mitochondria.32,33 Therefore, the maintenance of the regulation of mitochondrial biogenesis may be a potential solution to doxorubicin-induced cardiotoxicity.
In this study, the Moringa oleifera leaves extract, as determined in the previous research, is rich in triterpenoids, polyphenols, saponins, tannins, and flavonoids.21 In an earlier study, the same extract at 500 mg/kg BW showed neuroprotective activity in mice with a scopolamine-induced memory impairment model.21 Polyphenols in Moringa oleifera have been investigated to modulate mitochondrial biogenesis, mitochondrial membrane potential, and electron transport chains, oxidative status, and mitochondrial (intrinsic) pathway apoptosis. Polyphenols can protect against mitochondrial damage caused by various xenobiotics.34
Doxorubicin induces cardiotoxicity characterized by increased plasma activity of CK-MB and LDH.35 CK-MB. LDH can leak out of the myocardium due to the disintegration of the contractile apparatus and increased sarcoplasmic permeability. Increased circulating levels of LDH and CK-MB indicate leakage from damaged cardiomyocytes.35 Treatment of Moringa oleifera leaves extract in both dosages reduced the activity of these two enzymes. Our findings align with previous research results, which showed a significant decrease in CK-MB and LDH activity in isoproterenol-induced rat heart tissue compared to the normal group of rats.36
Doxorubicin is known to induce apoptosis in cardiomyocytes via caspase-3 activation. Doxorubicin induces oxidative stress, opening the mitochondrial permeability transition pore and releasing pro-apoptotic proteins, including cytochrome C, from the mitochondrial matrix. Cytochrome C activates caspase-3 through interaction with apoptotic peptidase activating factor-1 (Apaf-1) and caspase-9, leading to apoptosis.37 Therefore, it is known that doxorubicin might interfere with mitochondrial function and induce apoptosis mediated through the activation caspase-3 via the mitochondrial pathway.38
Our study displayed that the co-treatment of doxorubicin and Moringa oleifera leaf extract can prevent apoptosis in the myocardium by reducing caspase-3 mRNA expression. However, no dose-dependency effect was observed. Caspase-3 is secreted as a procaspase that can only be activated after further modification during the apoptotic process by caspase-8 and caspase-9. The mRNA level of caspase-3 can only reflect the amount of procaspase. However, it cannot reflect the level of biologically active caspase.39 However, a study by Chen et al showed a positive correlation between the level of mRNA expression and protein expression.40 Despite the close correlation, the level of cleaved caspase-3 should also be studied.
Apoptosis in the myocardium can also be induced by 8-OH-dG, which is a marker of DNA damage, via the activation of initiator and executor caspases.30 The imbalance between ROS formation and antioxidant defense system activity can induce the oxidation of biological macromolecules such as DNA, one of the oxidative product is 8-OH-dG.41 Doxorubicin is known to induce oxidative stress and increase 8-OH-dG levels. 8-OHdG is formed from an attack by hydroxyl radicals at the C-8 position of deoxyguanosine DNA residues.42 Doxorubicin caused an increase in 8-OH-dG levels, and co-treatment with Moringa oleifera leaves extract resulted in an improvement in oxidative stress. However, no difference was observed in the tissue 8-OH-dG levels.
The accumulation of 8-OH-dG was twofold higher than the copy number of mtDNA. The distinction may be due to doxorubicin causing the formation of more hydroxyl radicals and 8-OH-dG in the mitochondria or a slower repair rate in the mitochondria than in the nucleus. Since mitochondrial genome expression is critical for the integrity of the electron transport chain, high oxidation of mtDNA can disrupt the mitochondrial structure and decrease bioenergetic function. The cardioselective accumulation and persistence of 8-OH-dG and mtDNA reflect mitochondrial dysfunction in cardiotoxicity that was observed clinically in patients receiving doxorubicin. 8-Oh-dG results from DNA damage due to an imbalance of the antioxidant defense system and ROS formation.43 Moreover, detoxification of reactive oxygen species is regulated by PGC-1α.44
Even though MO leaves extract does not diminish the content of 8-OH-dG, there is a considerable reduction in lipid peroxidation following treatment with MO at both dosages. As previously stated, doxorubicin may cause oxidative stress and DNA damage, as seen by the high degree of lipid peroxidation. In contrast, we noticed a trend for MO to reduce glutathione levels. It is still being determined how this phenomenon occurs. However, it might be explained by the ambiguous responses of antioxidant enzymes in response to accumulating reactive oxygen species, either increased or diminished.45
PGC-1α is a transcription factor that functions as the cardiovascular system’s primary regulator of mitochondrial biogenesis.11 Further, PGC-1α regulates the expression of myocardial antioxidants, including SOD2. Moreover, PGC-1α will lead to mitochondrial DNA (mtDNA) synthesis and mitochondrial biogenesis.46,47
Doxorubicin causes a decrease in PGC-1α mRNA expression. Our results align with research conducted by Guo et al that doxorubicin can inhibit PGC-1α, which disrupts cardiac mitochondrial biogenesis.48 PGC-1α interacts with receptors and nuclear transcription factors to activate the transcription of downstream target genes, including mitochondrial transcription factor A. (TFAM), which is essential for mtDNA transcription and preservation and mtDNA nucleoid formation.44 In our study, administration of Moringa oleifera extracts restored PGC-1α mRNA expression. Qualitative phytochemical analysis showed that Moringa oleifera leaves extract contains secondary metabolites: triterpenoids, polyphenols, saponins, tannins, and flavonoids.31 Polyphenols, such as resveratrol, quercetin, and flavonoids, have been investigated in vivo to increase PGC-1α expression and mitochondrial biogenesis.43 PGC-1α regulates the expression of the mitochondrial antioxidant superoxide dismutase 2 (SOD2) and protects the heart from oxidative stress.9
According to a study, PGC-1α regulates mitochondrial biogenesis through the transcriptional machinery to increase the bulk of the mitochondria. Oxidative stress is one of the stressors that increase PGC-1α activity and cause it to move from the cytoplasm to the nucleus. Nrf1 and Nrf2 expression is increased because of activated PGC-1α.16,47 As shown in our findings, the reduction of PGC-1α aligns with those of Nrf2 mRNA expressions.
Of the three isoforms of superoxide dismutase (SOD) known, SOD2 (or manganese SOD, MnSOD) is localized in the mitochondrial matrix. Mitochondrial localization control and the activity of SOD2 are essential to remove ROS in mitochondria.44 In our study, doxorubicin caused a significant decrease in SOD2 mRNA expression compared to the normal group. However, the addition of Moringa oleifera leaves extracts failed to restore the expression of SOD2 mRNA to normal.
Despite an increase in PGC1 and Nrf2 expression, SOD2 expression remains unaltered after treatment with MO. One potential reason is that MO might induce epigenetic modifications that may influence the PGC-1α levels. A study by Monraz-Mendez et al reported that MO improved Moringa inhibited the progression of liver damage in a non-alcoholic steatohepatitis (NASH) model by modulating miRNAs important in NASH development, one of which is AMPK, hence disrupting the AMPK/PGC1α axis.49 However, whether the same process occurred in cardiomyocytes is yet unknown.
Mitochondrial biogenesis, including mitochondrial copy number, is regulated by PGC-1α TFAM activation. It is known that inhibition of PGC-1α significantly reduces the copy number of mtDNA, and methylation of the promoter of PGC-1α is associated with an increase in the copy number of mtDNA.50,51 This study showed that induction of doxorubicin causes a decrease in mtDNA copy number. Doxorubicin causes mtDNA oxidation, inhibiting its ability to replicate and express. It is followed by a reduction in the mitochondria in the copy number of mtDNA, a decrease in ATP production, and an increase in the production of reactive oxygen species (ROS) due to reduced transcription of oxidative phosphorylation subunits, which ultimately can develop into cardiomyopathy.31,52 Though insignificant, administration of Moringa oleifera extract in both doses increased the copy number of mtDNA. Moringa oleifera may affect other stages of mitochondrial biogenesis. Moringa oleifera can potentially increase mitochondrial biogenesis by increasing the expression of mitochondrial subunit complex proteins I, II, III, and V in the skeletal muscle of rats treated with endurance training.53,54
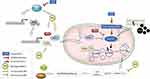
Based on the analysis of various parameters above, there is a tendency to improve the regulation of mitochondrial biogenesis in rats induced by cardiotoxicity due to doxorubicin after co-treatment with Moringa oleifera leaves extract. Based on our findings, the proposed mechanism of Moringa oleifera in doxorubicin induced cardiotoxicity is described in Figure 7.
Conclusion
Moringa oleifera leaves extract showed a protective effect on doxorubicin-induced cardiotoxicity in the rats. The effect of Moringa oleifera leaves extract is mediated by its activity to normalize the level of apoptosis and regulate mitochondrial biogenesis, as shown by the normalization of mitochondrial DNA copy number and mRNA expressions of PGC-1α and Nrf2.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be account for all aspects of the work.
Funding
This study was funded by the Grant from Universitas Indonesia Contract No. NKB-1405/UN2.RST/HKP.05.00/2022.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: the good, the bad, and the ugly effect. Curr Med Chem. 2009;16(25):3267–3285. doi:10.2174/092986709788803312
2. Pugazhendhi A, Edison T, Velmurugan BK, Jacob JA, Karuppusamy I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018;200:26–30. doi:10.1016/j.lfs.2018.03.023
3. Murabito A, Hirsch E, Ghigo A. Mechanisms of anthracycline-induced cardiotoxicity: is mitochondrial dysfunction the answer? Front Cardiovasc Med. 2020;7:35. doi:10.3389/fcvm.2020.00035
4. Kalyanaraman B. Teaching the basics of the mechanism of doxorubicin-induced cardiotoxicity: have we been barking up the wrong tree? Redox Biol. 2020;29:101394. doi:10.1016/j.redox.2019.101394
5. Santos D, Goldenberg R. Doxorubicin-induced cardiotoxicity: from mechanisms to development of efficient therapy; 2018.
6. Ichikawa Y, Ghanefar M, Bayeva M, et al. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest. 2014;124(2):617–630. doi:10.1172/JCI72931
7. Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes are pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367(Pt 3):729–740. doi:10.1042/bj20020752
8. Ferreira A, Cunha-Oliveira T, Simões RF, et al. Altered mitochondrial epigenetics associated with subchronic doxorubicin cardiotoxicity. Toxicology. 2017;390:63–73. doi:10.1016/j.tox.2017.08.011
9. Osataphan N, Phrommintikul A, Chattipakorn SC, Chattipakorn N. Effects of doxorubicin-induced cardiotoxicity on cardiac mitochondrial dynamics and mitochondrial function: insights for future interventions. J Cell Mol Med. 2020;24(12):6534–6557. doi:10.1111/jcmm.15305
10. Wei S, Ma W, Li X, et al. Involvement of ROS/NLRP3 Inflammasome Signaling Pathway in Doxorubicin-Induced Cardiotoxicity. Cardiovasc Toxicol. 2020;20(5):507–519. doi:10.1007/s12012-020-09576-4
11. Cappetta D, De Angelis A, Sapio L, et al. Oxidative stress and cellular response to doxorubicin: a common factor in the complex milieu of anthracycline cardiotoxicity. Oxid Med Cell Longev. 2017;2017:1521020. doi:10.1155/2017/1521020
12. Gorini S, De Angelis A, Berrino L, Malara N, Rosano G, Ferraro E. Chemotherapeutic drugs and mitochondrial dysfunction: focus on doxorubicin, trastuzumab, and sunitinib. Oxid Med Cell Longev. 2018;2018:7582730. doi:10.1155/2018/7582730
13. Wang DD, Cheng RK, Tian R. Combat doxorubicin cardiotoxicity with the power of mitochondria transfer. JACC CardioOncol. 2021;3(3):441–443. doi:10.1016/j.jaccao.2021.08.001
14. Yue P, Jing S, Liu L, et al. Association between mitochondrial DNA copy number and cardiovascular disease: current evidence based on a systematic review and meta-analysis. PLoS One. 2018;13(11):e0206003. doi:10.1371/journal.pone.0206003
15. Cheung KG, Cole LK, Xiang B, et al. Sirtuin-3 (SIRT3) protein attenuates doxorubicin-induced oxidative stress and improves mitochondrial respiration in H9c2 cardiomyocytes. J Biol Chem. 2015;290(17):10981–10993. doi:10.1074/jbc.M114.607960
16. Lu Z, Xu X, Hu X, et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13(7):1011–1022. doi:10.1089/ars.2009.2940
17. Oseni O, Ogunmoyole T, Idowu K. Lipid profile and cardio-protective effects of aqueous extract of moringa oleifera (lam) leaf on bromate-induced cardiotoxicity on Wistar albino rats. Eur J Adv Res Biol Life Sci. 2015;3(2):1–15.
18. Sailaja BS, Aita R, Maledatu S, Ribnicky D, Verzi MP, Raskin I. Moringa isothiocyanate-1 regulates Nrf2 and NF-κB pathway in response to LPS-driven sepsis and inflammation. PLoS One. 2021;16(4):e0248691. doi:10.1371/journal.pone.0248691
19. Quagliariello V, Basilicata MG, Pepe G, et al. Combination of spirulina platensis, ganoderma lucidum, and moringa oleifera improves cardiac functions and reduces pro-inflammatory biomarkers in preclinical models of short-term doxorubicin-mediated cardiotoxicity: new frontiers in cardiology? J Cardiovasc Dev Dis. 2022;9(12). doi:10.3390/jcdd9120423.
20. Cheraghi M, Namdari M, Daraee H, Negahdari B. Cardioprotective effect of magnetic hydrogel nanocomposite loaded N,α-L-rhamnopyranosyl vincosamide isolated from Moringa oleifera leaves against doxorubicin-induced cardiac toxicity in rats: in vitro and in vivo studies. J Microencapsul. 2017;34(4):335–341. doi:10.1080/02652048.2017.1311955
21. Arozal W, Purwoningsih E, Lee HJ, Barinda AJ, Munim A. Effects of moringa oleifera in two independents formulation and as neuroprotective agent against scopolamine-induced memory impairment in mice. Front Nutr. 2022;9. doi:10.3389/fnut.2022.799127
22. Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol. 2013;50(6):1007–1015. doi:10.1177/0300985813485099
23. Barinda AJ, Arozal W, Sandhiutami NMD, et al. Curcumin prevents epithelial-to-mesenchymal transition-mediated ovarian cancer progression through NRF2/ETBR/ET-1 axis and preserves mitochondria biogenesis in kidney after cisplatin administration. Adv Pharm Bull. 2022;12(1):128. doi:10.34172/apb.2022.014
24. Melva Louisa AMP, Sidqi AA, Kirana Mahaputra D, et al. Attenuation of cisplatin-induced hepatotoxicity by nanocurcumin through modulation of antioxidative and anti-inflammatory pathways. J Appl Pharm Sci. 2023;13:3.
25. Zhao H, Liu J, Pan S, et al. SOD mRNA and MDA expression in rectus femoris muscle of rats with different eccentric exercise programs and time points. PLoS One. 2013;8(9):e73634. doi:10.1371/journal.pone.0073634
26. Khan V, Sharma S, Bhandari U, Sharma N, Rishi V, Haque SE. Suppression of isoproterenol-induced cardiotoxicity in rats by raspberry ketone via activation of peroxisome proliferator-activated receptor-α. Eur J Pharmacol. 2019;842:157–166. doi:10.1016/j.ejphar.2018.10.034
27. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
28. Pomierny-Chamioło L, Moniczewski A, Wydra K, Suder A, Filip M. Oxidative stress biomarkers in some rat brain structures and peripheral organs underwent cocaine. Neurotox Res. 2013;23(1):92–102. doi:10.1007/s12640-012-9335-6
29. Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol. 2018;36(1):489–517. doi:10.1146/annurev-immunol-042617-053010
30. Torres-Gonzalez M, Gawlowski T, Kocalis H, Scott BT, Dillmann WH. Mitochondrial 8-oxo guanine glycosylase decreases mitochondrial fragmentation and improves mitochondrial function in H9C2 cells under oxidative stress conditions. Am J Physiol Cell Physiol. 2014;306(3):C221–C229. doi:10.1152/ajpcell.00140.2013
31. Wallace KB, Sardão VA, Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126(7):926–941. doi:10.1161/CIRCRESAHA.119.314681
32. Yoshida M, Shiojima I, Ikeda H, Komuro I. Chronic doxorubicin cardiotoxicity is mediated by oxidative DNA damage-ATM-p53-apoptosis pathway and attenuated by pitavastatin through the inhibition of Rac1 activity. J Mol Cell Cardiol. 2009;47(5):698–705. doi:10.1016/j.yjmcc.2009.07.024
33. Hu C, Zhang X, Wei W, et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway. Acta Pharm Sin B. 2019;9(4):690–701. doi:10.1016/j.apsb.2019.03.003
34. Sandoval-Acuña C, Ferreira J, Speisky H. Polyphenols and mitochondria: an update on their increasingly emerging ROS-scavenging independent actions. Arch Biochem Biophys. 2014;559:75–90. doi:10.1016/j.abb.2014.05.017
35. Warpe VS, Mali VR, A S, Bodhankar SL, Mahadik KR. Cardioprotective effect of ellagic acid on doxorubicin-induced cardiotoxicity in Wistar rats. J Acute Med. 2015;5(1):1–8. doi:10.1016/j.jacme.2015.02.003
36. Nandave M, Ojha SK, Joshi S, Kumari S, Arya DS. Moringa oleifera leaf extract prevents isoproterenol-induced myocardial damage in rats: evidence for an antioxidant, antiperoxidative, and cardioprotective intervention. J Med Food. 2009;12(1):47–55. doi:10.1089/jmf.2007.0563
37. Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2: Bax ratio. Cancer Res. 2002;62(16):4592–4598.
38. Michihiko U, Yoshihiko K, Koh-ichi Y, et al. Doxorubicin induces apoptosis by activating caspase-3 in cultured cardiomyocytes in vitro and rat cardiac ventricles in vivo. J Pharmacol Sci. 2006;101(2):151–158. doi:10.1254/jphs.FP0050980
39. Vandaele L, Goossens K, Peelman L, Van Soom A. mRNA expression of Bcl-2, Bax, caspase-3, and −7 cannot be used as a marker for apoptosis in bovine blastocysts. Anim Reprod Sci. 2008;106(1–2):168–173. doi:10.1016/j.anireprosci.2007.12.016
40. Chen Q, Kao X, Gao Y, Chen J, Dong Z, Chen C. Increase in NO causes osteoarthritis, and chondrocyte apoptosis and chondrocyte ERK play a protective role in the process. Mol Biol Rep. 2021;48(11):7303–7312. doi:10.1007/s11033-021-06731-0
41. El-Agamy DS, El-Harbi KM, Khoshhal S, et al. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Cancer Manag Res. 2019;11:47–61. doi:10.2147/CMAR.S186696
42. Hassanien RT, Shoukry HS, Moataz MK, Rabab AR, Ibrahim HS, Ibrahim ER. Vitamin E improves doxorubicin induced nephrotoxicity; possible underlying mechanisms. Med J Cairo Univ. 2018;86:651–657. doi:10.21608/mjcu.2018.55380
43. Chodari L, Dilsiz Aytemir M, Vahedi P, et al. Targeting mitochondrial biogenesis with polyphenol compounds. Oxid Med Cell Longev. 2021;2021:4946711. doi:10.1155/2021/4946711
44. Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 2019;51(12):1–13. doi:10.1038/s12276-019-0355-7
45. Kong C-Y, Guo Z, Song P, et al. Underlying the mechanisms of doxorubicin-induced acute cardiotoxicity: oxidative stress and cell death. Int J Biol Sci. 2022;18(2):760–770. doi:10.7150/ijbs.65258
46. Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30(4):145–151. doi:10.1152/advan.00052.2006
47. S-i O, Sabry AD, Cawley KM, Warren JS. Multiple levels of PGC-1α dysregulation in heart failure. Front Cardiovasc Med. 2020;7:2. doi:10.3389/fcvm.2020.00002
48. Guo Q, Guo J, Yang R, et al. Cyclovirobuxine D attenuates doxorubicin-induced cardiomyopathy by suppression of oxidative damage and mitochondrial biogenesis impairment. Oxid Med Cell Longev. 2015;2015:151972. doi:10.1155/2015/151972
49. Monraz-Méndez CA, Escutia-Gutiérrez R, Rodriguez-Sanabria JS, et al. Moringa oleifera improves MAFLD by inducing epigenetic modifications. Nutrients. 2022;14(20):4225. doi:10.3390/nu14204225
50. Chaudhary S, Ganguly S, Palanichamy JK, et al. PGC1A driven enhanced mitochondrial DNA copy number predicts outcome in pediatric acute myeloid leukemia. Mitochondrion. 2021;58:246–254. doi:10.1016/j.mito.2021.03.013
51. Bam S, Buchanan E, Mahony C, O’Ryan C. DNA methylation of PGC-1α is associated with elevated mtDNA copy number and altered urinary metabolites in autism spectrum disorder. Front Cell Dev Biol. 2021;9. doi:10.3389/fcell.2021.696428
52. Popov LD. Mitochondrial biogenesis: an update. J Cell Mol Med. 2020;24(9):4892–4899. doi:10.1111/jcmm.15194
53. Muhammed RE, El-Desouky MA, Abo-Seda SB, Nahas A, Elhakim HK, Alkhalaf MI. The protecting role of Moringa oleifera in cypermethrin-induced mitochondrial dysfunction and apoptotic events in rats brain. J King Saud Univ Sci. 2020;32(6):2717–2722. doi:10.1016/j.jksus.2020.06.006
54. Sánchez-Muñoz M A, Valdez-Solana MA, Campos-Almazán MI, et al. Streptozotocin-induced adaptive modification of mitochondrial supercomplexes in the liver of Wistar rats and the protective effect of Moringa oleifera Lam. Biochem Res Int. 2018;2018. doi:10.1155/2018/5681081
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.